Abstract
Peroxisomes are organelles that carry out diverse biochemical processes in eukaryotic cells, including the core pathways of β-oxidation of lipid molecules and detoxification of reactive oxygen species. In multicellular organisms defects in peroxisome assembly result in multiple biochemical and developmental abnormalities. As peroxisomes do not contain genetic material, their protein content, and therefore function, is determined by the import of nuclearly encoded proteins from the cytosol and, presumably, removal of damaged or obsolete proteins. Import of matrix proteins can be broken down into four steps: targeting signal recognition by the cycling import receptors; receptor-cargo docking at the peroxisome membrane; translocation and cargo unloading; and receptor recycling. Import is mediated by a set of evolutionarily conserved proteins called peroxins that have been identified primarily via genetic screens, but knowledge of their biochemical activities remains largely unresolved. Recent studies have filled in some of the blanks regarding receptor recycling and the role of ubiquitination but outstanding questions remain concerning the nature of the translocon and its ability to accommodate folded, even oligomeric proteins, and the mechanism of cargo unloading and turnover of peroxisomal proteins. This review seeks to integrate recent findings from yeast, mammalian and plant systems to present an up to date account of how proteins enter the peroxisome matrix.
Introduction: importance of peroxisomes
Peroxisomes are metabolic organelles found in all eukaryotes except the Archaezoa Citation[1] and are associated with a wide range of processes that are key to the survival of most organisms. Examples of such processes are summarized in .
Table I. Examples of peroxisomal function in mammals, yeast and plants.
The importance of peroxisome-specific processes is reflected by the human disorders associated with peroxisomal defects Citation[2]. These include disorders resulting from a defect in a single peroxisomal enzyme and disorders that result from a deficiency in the biogenesis of the peroxisome, referred to as peroxisome biogenesis disorders (PBDs). PBDs result in multiple biochemical and developmental abnormalities which are generally fatal. When the genes responsible for plant peroxisome biogenesis are impaired, the phenotypes are also severe, ranging from sucrose dependency during early seedling development to embryo lethality Citation[3], Citation[4]. The difference in the severity of peroxisome deficiency between multi-cellular organisms like mammals and plants and unicellular organisms such as yeasts indicates that peroxisomes play important roles in the integration of developmental processes in multi-cellular organisms.
Peroxisomal metabolic plasticity
Peroxisomes exhibit metabolic plasticity as their enzymatic content can vary depending on the organism, cell/tissue-type and environmental conditions. Yeast cells modulate the enzymatic content of their peroxisomes according to the available carbon source Citation[5]. The functional inter-conversion of plant peroxisomes has been extensively studied in oil seed plants, where a light-induced transition of glyoxysomes (primarily fat degrading organelles) to leaf-type peroxisomes (which play an important role in photorespiration) occurs during greening of cotyledon cells. During leaf senescence the reverse process is observed, whereby leaf peroxisomes are converted into glyoxysomes Citation[6–10].
Peroxisome biogenesis
The study of peroxisomal biogenesis was previously hindered due to the extreme fragility and the low abundance of peroxisomes in many tissues. However, forward genetic screens have identified a set of evolutionarily conserved genes needed for peroxisome assembly in yeasts, (notably Saccharomyces cerevisiae Citation[11], Citation[12], Hansenula polymorpha Citation[5] and Yarrowia lipoytica Citation[13]), in Chinese hamster ovary cell lines Citation[14] and more recently in the model plant Arabidopsis thaliana Citation[15], Citation[16]. These genes are referred to as PEX genes and their protein products are called peroxins. In yeasts the nomenclature is PEXN (gene) PexNp (protein), in plants and mammals it is PEXN (gene) PEXN (protein). As this causes some difficulty with nomenclature when discussing cross-species similarities and differences, the yeast nomenclature is used here, unless the text refers specifically to the plant or mammalian proteins.
The complete genome sequences of various yeasts, mouse, human, and Arabidopsis has also allowed the identification of homologues of peroxins previously known from other species and their characterization by reverse genetics. It has also allowed in silico prediction of novel peroxisomal proteins, based on possession of consensus peroxisomal targeting motifs Citation[17–19]. To date 32 PEX genes have been identified in various organisms Citation[20], which are involved in three key stages of peroxisome development: (i) Import of peroxisomal matrix proteins; (ii) membrane biogenesis (import of peroxisomal membrane proteins); and (iii) organelle proliferation. However, most PEX genes are required for the sorting and translocation of peroxisomal matrix proteins across the peroxisomal membrane. This process is the focus of this review. For details of the import of membrane proteins and the proliferation of peroxisomes, the reader is referred elsewhere Citation[21–23].
Peroxisomal matrix protein import
Peroxisomal matrix protein import can be divided into four steps: receptor-cargo interaction, docking at the peroxisome membrane, translocation and cargo release, and receptor recycling. These are reviewed below.
Receptor-cargo interaction
Matrix proteins are synthesized on free polyribosomes in the cytosol Citation[24]. They are imported into the organelle via one of two pathways requiring conserved peroxisomal targeting signal (PTS) sequences. The most common of these targeting signals is PTS1, a carboxyl-terminal tripeptide motif which generally complies to the consensus sequence (S/A/C)(K/R/H)(L/M) Citation[25]. The predominately cytosolic protein, Pex5p, is the receptor for PTS1 and structurally, it can be divided into two main domains. Within the carboxyl-terminal half there is a high affinity PTS1-binding site that contains seven tetratricopeptide repeats (TPRs) and a helix bundle, which together form a ring like structure upon ligand binding Citation[26], Citation[27]. The crystal structure of human Pex5p co-crystallized with a PTS1-containing peptide revealed two clusters of three TPRs (TPR1-3 and TPR5-7) almost completely enclosing the peptide Citation[26], whilst TPR4 formed a hinge region and was concluded to not be directly involved in PTS1 binding Citation[28]. The amino-terminal region of Pex5p contains only a few strictly conserved residues (typically in the multiple pentapeptide WXXXF/Y repeats Citation[29]) and it is in this half that the peroxisomal targeting information is contained Citation[29], Citation[30]. Recently it has been shown that a N526K mutation in the carboxyl terminus results in conformational alterations in the amino-terminus which mimic the ones induced by binding of a PTS1 peptide to wild type PEX5 Citation[31]. As the N526K mutant imports into peroxisomes without any cargo, it appears that the triggering mechanism for docking/translocation emerges from PEX5.
The quaternary structure of PEX5 is disputed. Electron microscopy studies point to a tetrameric conformation of both the human Citation[32] and H. polymorpha Citation[33] receptor, which is capable of binding the PTS1 protein catalase Citation[33]. However another study of human PEX5 has led to the proposal that it is a momomeric protein with an abnormal, non-globular shape and that the tetrameric forms of the protein represent a non physiological state Citation[34].
The second peroxisomal targeting signal (PTS2) is located near the amino-terminus and consists of the consensus sequence RLXXXXX(H/Q)L Citation[35]. Only a small number of matrix proteins in yeasts contain a PTS2, although there are many more PTS2 proteins in plants Citation[19]. The PTS2 import pathway has been lost in Caenorhabditis elegans Citation[36]. Pex7p is the receptor for PTS2 proteins and is predicted to fold as a seven-bladed β-propeller domain, where each blade comprises a WD40 repeat Citation[37], Citation[38]. Like Pex5p, Pex7p shuttles between the cytosol and peroxisome during cargo translocation Citation[39]. However, accessory proteins are required in conjunction with Pex7p for delivery of PTS2-containing proteins to the peroxisome Citation[40]. In S. cerevisiae, the structurally related (and partially redundant) peroxins, Pex18p and Pex21p, are crucial for the import of proteins with PTS2 sequences Citation[41] and in N. crassa, P. pastoris, H. polymorpha and Y. lipolytica, Pex20p is required for PTS2-mediated import Citation[42–45]. P. pastoris Pex7p does not require Pex20p to enter the peroxisome, but Pex20p plays an essential synergistic role that allows Pex7p to carry PTS2 cargo into the peroxisomes Citation[43].
In mammals, there are two splice variants of PEX5; a short form (PEX5S) and a long form (PEX5L) Citation[46]. These two proteins differ by a 37-residue insertion in PEX5L and although both forms bind PTS1-containing proteins, only PEX5L interacts with PEX7. PTS2-mediated targeting requires not only binding of PTS2 to PEX7, but also binding of PEX7 and the long isoform of PEX5 Citation[47]. In Arabidopsis, PEX5 and PEX7 form a PTS1/PTS2 receptor complex by interaction between the amino-terminal domain of PEX5 and the carboxyl-terminal domain of PEX7 Citation[48]. A pex5 mutant that abolishes this interaction has a PTS2 protein import defect but is still competent to import PTS1 proteins Citation[49]. Down regulation of PEX5 also results in a PTS2 import defect Citation[50], suggesting that the PTS1 and PTS2 import pathways are coupled in plants as they are in mammals ().
Figure 1. PTS1 and PTS2 receptor recognition in various organisms. (A) In Arabidopsis PEX5 is required for both PTS1 and PTS2 mediated import. (B) In mammals two splice variants of PEX5 exist, a short isoform, PEX5S and a long isoform, PEX5L. PEX5L is required with PEX7 for PTS2 mediated import. Note: Both PEX5L and PEX5S function as the PTS1 receptor. (C) In S. cerevisiae the co-receptors PEX18 and PEX21 function with PEX7 for PTS2 import. (D) In N. crassa, P. pastoris, Y. lipolytica and H. polymorpha the co-receptor PEX20 functions with PEX7 for PTS2 import. PEX5 is not involved in PTS2 mediated import in yeast. In addition to receptor recognition, molecular chaperones may be required during import. These include members of the Hsp70-family Citation[115], Citation[116] and DnaJ-like proteins Citation[117], although the exact role of these chaperones remains to be determined. This Figure is reproduced in colour in Molecular Membrane Biology online.
![Figure 1. PTS1 and PTS2 receptor recognition in various organisms. (A) In Arabidopsis PEX5 is required for both PTS1 and PTS2 mediated import. (B) In mammals two splice variants of PEX5 exist, a short isoform, PEX5S and a long isoform, PEX5L. PEX5L is required with PEX7 for PTS2 mediated import. Note: Both PEX5L and PEX5S function as the PTS1 receptor. (C) In S. cerevisiae the co-receptors PEX18 and PEX21 function with PEX7 for PTS2 import. (D) In N. crassa, P. pastoris, Y. lipolytica and H. polymorpha the co-receptor PEX20 functions with PEX7 for PTS2 import. PEX5 is not involved in PTS2 mediated import in yeast. In addition to receptor recognition, molecular chaperones may be required during import. These include members of the Hsp70-family Citation[115], Citation[116] and DnaJ-like proteins Citation[117], although the exact role of these chaperones remains to be determined. This Figure is reproduced in colour in Molecular Membrane Biology online.](/cms/asset/c7fbf622-c2ee-4396-97c2-b2835c7bda2e/imbc_a_313224_f0001_b.jpg)
In addition to a common function, PTS2 co-receptors share several structural similarities with Pex5p: a common Pex7p-binding motif; common ‘docking motifs’ made of diaromatic pentapeptide repeats (WXXXF/Y); and a common amino-terminal domain of approximately 30 residues Citation[42], Citation[43], Citation[51]. Could PTS1 and PTS2 pathways also be coupled in fungi? In H. polymorpha Pex20p levels are low in pex5 mutants (and vice versa) and Pex20p has been shown to bind directly to Pex5p and alter its conformation Citation[33]. The significance of this is currently unknown.
Anomalies in protein import into the peroxisome
Not all proteins that enter peroxisomes carry a PTS1 or PTS2 signal. Acyl CoA oxidase (AOX) from S. cerevisiae is one example. Although no specific signals have been found which can direct AOX to the peroxisome, Pex5p and AOX interact via novel contact sites. The interaction region in Pex5p is within the amino-terminal part of the protein but outside of the domain involved in PTS1 recognition; whilst the interaction site in AOX is located internally, away from the carboxyl-terminus where a PTS1 can normally be found Citation[52]. It has therefore been suggested that a not yet identified internal PTS (putative PTS3, most likely a conformational epitope) of AOX is recognized by Pex5p.
Peroxisomes have the capacity to translocate folded and even oligomeric proteins, and peroxisomal matrix proteins that lack a PTS can be sorted to peroxisomes when in complex with PTS-containing subunits. This ‘piggy back’ import has been presented as possible explanation for Pex5p dependent import of proteins that lack a PTS. For example, subunits of the bacterial trimeric protein chloramphenicol acetyltransferase (CAT) which lacked a PTS1, were able to form heterotrimers with CAT-PTS1 in the cytosol and then be co-transported with these PTS1-bearing CAT subunits Citation[53]. This has also been demonstrated in the PTS2 pathway, as an amino-terminal truncated variant of peroxisomal thiolase, which lacked a PTS2, was shown to mislocalize to the cytosol unless full-length, PTS2 containing thiolase subunits were co-expressed Citation[54].
Receptor-cargo docking to the peroxisome membrane
The docking complex is thought to comprise of Pex13p and Pex14p, and also Pex17p in S. cerevisiae Citation[55]. Pex17p is a peripheral peroxisomal membrane protein (PMP) which associates with Pex14p in a tight core complex.
Pex13p is an integral PMP whose amino and carboxyl-termini both extend into the cytosol. In S. cerevisiae the amino-terminal domain binds the PTS2 receptor (Pex7p). The interaction between the amino-terminus of PEX13 and PEX7 is conserved in Arabidopsis Citation[56]. The carboxy-terminal region contains a Src-homology-3 (SH3) domain which directly binds the PTS1 receptor (Pex5p) as well as the other docking protein, Pex14p Citation[57].
Pex14p is a membrane protein thought to be involved in the docking of Pex5p, indeed in mammals and plants, the PEX5 pentapeptide repeat motifs (WXXXF/Y) bind to PEX14 with high affinity Citation[29], Citation[30], Citation[48]. Pex20p also contains WXXXF/Y motifs that are required for binding to the docking machinery Citation[43].
Levels of Pex5p and Pex7p found in peroxisomal membrane remnants are dramatically reduced in pex14 mutant cells in comparison to pex13, pex12 or wild-type membranes Citation[46]. Indeed Pex20p is exclusively cytosolic in the absence of Pex14p Citation[43]. In addition, Pex5p loaded with cargo exhibits a higher binding affinity to Pex14p than to Pex13p Citation[58]. The interaction with Pex14p is therefore believed to be the docking-site for Pex5p to interact with the complex network of protein-protein interactions at the peroxisomal membrane. Arabidopsis mutants in PEX14 (ped2, Citation[59]) and PEX13 (apm2, Citation[56]), like their mammalian and yeast counterparts, show deficiency in both PTS1 and PTS2 import.
Receptor-cargo translocation and cargo release
The translocation step for the PTS receptors is operationally defined as a peroxisome-associated and protease-resistant state. This means that the receptor-cargo complex can either enter the peroxisome lumen (extended shuttle hypothesis, ), or be embedded in the peroxisome membrane to such an extent that the cargo can be released into the peroxisome matrix with the receptor remaining protease protected (simple shuttle hypothesis). At steady-state, a pool of Pex5p, Pex7p and Pex20p is peroxisome-associated and protease protected Citation[43].
Figure 2. Overview of peroxisomal matrix protein import. Matrix proteins have either a PTS1 or PTS2 targeting signal that binds to a cytosolic receptor, PEX5 and PEX7 respectively (with or without co-receptors, see ) which targets the protein to the docking complex (PEX13, PEX14 and also PEX17 in yeast). The receptors translocate with their cargo into the peroxisome lumen, release their cargo and are recycled back into the cytosol. The RING-finger complex (PEX2, PEX10 and PEX12) associates with the docking complex via PEX8 (in yeast) and is required for receptor recycling. PEX10 is linked to the E2 ubiquitin conjugating enzyme, PEX4, which is anchored to the membrane by PEX22. PEX5 release from the membrane requires PEX4-dependent monoubiquitination, which is ATP-dependent. The RING-finger peroxins are the putative E3 ligases for this process. The AAA-proteins PEX1 and PEX6 are also involved in receptor release and are attached to the peroxisomal membrane via PEX15 in yeast and PEX26 in mammals. Little is known about the recycling of PEX7, although two of its co-receptors, PEX18 and PEX20, have been shown to be ubiquitinated. This Figure is reproduced in colour in Molecular Membrane Biology online.
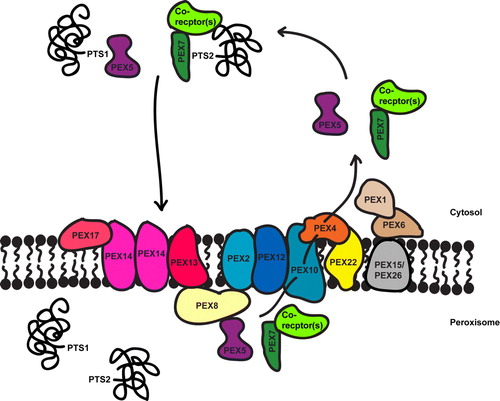
Until recently, there was much debate in the peroxisome field over the ‘extended-shuttle’ and ‘simple-shuttle’ hypothesizes Citation[60–62]. However, in mammals, PEX5 has been shown to traverse the peroxisome membrane (which is a cargo protein dependent step), participate in multiple rounds of entry into the peroxisome matrix and export back to the cytosol Citation[63], Citation[64]. Also, in S. cerevisiae Pex5p was found to interact with Pex8p, the only known peroxin bound to the trans side of the membrane Citation[65]. This shows that Pex5p can traverse the membrane or at least be inserted into it to such an extent that it is exposed to the trans side.
Pex7p also behaves like a cycling receptor. When GFP is fused to the carboxyl-terminus of Pex7p, the intracellular distribution shifts from predominantly cytosolic to predominantly peroxisomal. Cleavage of a linker between GFP and Pex7p within peroxisomes resulted in GFP remaining in the peroxisome whilst Pex7p was seen to exit to the cytosol Citation[39]. Recently, it has emerged that Pex20p also cycles, as Pex20p can be found in both cytosolic and peroxisomal cell fractions, but when various peroxins thought to be involved in receptor recycling are deleted, there is an increase in peroxisome-associated Pex20p Citation[43]. It is not yet clear whether Pex18p and Pex21p enter the peroxisome during the import cycle Citation[41].
summarizes current understanding of protein translocation into the peroxisomes. However, it should be noted that although Pex5p and Pex7p are shown as being fully translocated into the peroxisomal matrix, it is possible that they remain membrane associated with the cargo binding site exposed on the matrix side of the membrane.
In mammals and plants, the PTS2 is usually proteolytically removed once the protein enters the peroxisome. This removal of the targeting sequence does not appear to be tightly coupled to the import process in mammals, as both cleaved and un-cleaved thiolase are observed after in vitro import into rat liver peroxisomes Citation[66]. In mammals some PTS1 proteins are also processed. The enzyme responsible for both thiolase and Acyl CoA oxidase processing (Tysnd1) has recently been identified Citation[67], and a related enzyme DEG15 carries out PTS2 processing in watermelon and Arabidopsis Citation[68]. Both proteins possess PTS1 signals.
The mechanistic details underlying translocation and the components of the translocon are still lacking. It is possible that components of the docking complex are also part of the translocon. The multiple binding sites for Pex5p at the peroxisomal membrane may point towards the existence of an import cascade, where the cargo-loaded receptor interacts with different components of the import machinery Citation[3]. Interestingly, Pex5p changes its membrane association during the protein import cascade, as it behaves like an integral membrane protein when interacting with Pex14p Citation[69]. This, together with the observation that Pex5p spontaneously inserts into lipid membranes Citation[70], has lead to the suggestion that a population of Pex5p itself forms an import pore via protein-lipid interactions, thus opening the membrane dynamically for a cargo-loaded second Pex5p species Citation[71]. This idea draws parallels to pore forming toxins and is referred to as the ‘transient pore model’.
Little is known about the mechanism of cargo release from the receptor-cargo complex. In vitro experiments with H. polymorpha Pex8p suggests that it is involved in cargo release Citation[72]. In addition to interacting with Pex5p, Pex8p also interacts with Pex20p. Pex8p contains both a PTS1 and a PTS2 sequence, thus indicating a cargo-displacement model of receptor-release. However, mutations in both PTS1 and PTS2 sequences do not affect function Citation[73] and not all homologues of Pex8p contain both PTSs Citation[74]. Also, no homologues for Pex8p have been found in species other than yeast.
Receptor-release to the cytosol
Once the cargo is unloaded, Pex5p, Pex7p and Pex20p return to the cytosol for further rounds of import. This process is thought to require the RING-finger peroxins, Pex2p, Pex10p and Pex12p, and a receptor recycling complex. The RING finger peroxins are associated with the docking complex via Pex8p in yeast Citation[55].
The RING sub-complex proteins have been shown to be required for the export/release of receptors on the peroxisome membrane Citation[43], Citation[51], Citation[74], Citation[75]. In human fibroblast lines pex10 mutants Citation[75], or pex2 or pex12 mutants Citation[76] have increased levels of PEX5 associated with peroxisomes. In Arabidopsis, the complete lack of any one of the RING-finger peroxins results in embryo lethality Citation[77–80]. Weaker alleles result in PTS1 and PTS2 import defects Citation[80], Citation[81]. Genetic interaction studies place PEX12 downstream of PEX13, and PEX5 accumulates in the membrane in pex12 and pex13 point mutants Citation[56].
The dislocation/recycling of the receptors from the peroxisome to the cytosol requires the action of a receptor recycling complex. This is comprised of: the E2-like ubiquitin-conjugating enzyme (UBC) Pex4p (anchored by Pex22p); two AAA ATPases, Pex1p and Pex6p (which interact with each other in an ATP-dependent manner); and a peroxisomal membrane protein (Pex15p in S. cerevisiae or PEX26 in mammals), which provides a docking site for Pex6p Citation[43], Citation[82–84]. An epistasis analysis placed Pex4p and Pex22p at the end of the Pex5p receptor cycle, together with the AAA peroxins Pex1p and Pex6p Citation[85]. Deletion of these components leads to an accumulation of Pex5p at the peroxisomal membrane, and also impairs Pex5p export in vitro in cells of S. cerevisiae Citation[82] and human patients Citation[83]. In Arabidopsis a homologue of PEX26/Pex15p has not been discovered but pex1, pex4 and pex6 mutants also result in matrix protein import defects Citation[81], Citation[86], Citation[87].
Ubiquitination and peroxisome protein import
Ubiquitination has been implicated in peroxisome biogenesis ever since Pex4p was identified as a ubiquitin conjugating enzyme (UBC) Citation[88]. Recent work has also identified the E2 Ubc1p/Ubc4p/Ubc5p-familiy to be involved in peroxisomal processes Citation[89–92]. Three peroxins identified as targets for ubiquitination are Pex5p, the PTS1 receptor Citation[89–91] and Pex18p/Pex20p, the PTS2 co-receptors in yeast Citation[43], Citation[92]. Most work has been done on Pex5p and two distinct forms of ubiquitination have been reported: polyubiquitination of Pex5p, which is dependent on the E2 enzyme Ubc4p Citation[89–92] and monoubiquitination of Pex5p, which is Pex4p dependent Citation[93], Citation[94]. The two forms of ubiquitination appear to serve different roles, with the former in receptor regulation, and the latter in receptor release from the peroxisome membrane.
Pex5p polyubiquitination and regulation
The Ubc4p-dependent ubiquitination of Pex5p has been shown to occur in pex4, pex22, pex1, pex6 and pex15 mutants Citation[89–91]. These deletion strains are blocked at a stage where Pex5p is normally recycled from the peroxisomal membrane to the cytosol. Such a scenario results in the ubiquitination of Pex5p, which accumulates at the peroxisomal membrane Citation[89], Citation[91] and it has been suggested that Ubc4p-dependent ubiquitination provides a ‘quality control’ function, also known as ‘RADAR’ (Receptor Accumulation and Degradation in the Absence of Recycling). Here it is thought that Pex5p trapped in the peroxisomal membrane is primed for proteasomal degradation Citation[43], Citation[89], Citation[91]. In support of this model, the over-expression of UbK48R (which prevents polyubiquitin chains from forming) in H. polymorpha results in defects in protein import Citation[95]. In ubc4 mutants, polyubiquitination occurs via Ubc5p Citation[91] and Ubc1p and ubc4/ubc5 double mutants show a partial import defect Citation[89]. Recent work with S. cerevisiae has shown that when the targets of Ubc4p-dependent ubiquitination (K18 and K24) are substituted with an arginine, the function of Pex5p is not inhibited Citation[94]. This is in line with previous work, where K21 in H. polymorpha Pex5p was shown to be essential for Ubc4p-dependent ubiquitination, but its mutation had no effect on protein function Citation[95].
The efficiency of proteasomal degradation of the PTS1 receptor varies considerably between species. Polyubiquitinated Pex5p in S. cerevisiae has been shown to accumulate at the membrane of pex4, pex22, pex1, pex6 and pex15 mutants Citation[90], Citation[91], although single deletions in the same set of proteins results in a dramatic decrease in steady state Pex5p in human cell lines Citation[76], Citation[96], Arabidopsis Citation[87], H. polymorpha Citation[97], Citation[98] and P. pastoris Citation[85]. This suggests inefficient Pex5p removal in S. cerevisiae but degradation via the 26S proteasome in other species. Therefore, it appears that Ubc4p-dependent ubiquitination of Pex5p is a conserved, but non-essential process that is activated in mutants which are blocked in receptor recycling.
Pex18p/Pex20p polyubiquitination and regulation
In addition to regulating the PTS1-receptor pathway, ubiquitination is thought to play an important role in regulation of the PTS2-receptor pathway. Pex18p is constitutively degraded by the 26S proteasome pathway during the import cycle. This is mediated by Ubc4p and Ubc5p Citation[92], although the constitutive degradation of Pex18p is abolished in several pex mutants where matrix protein import is impaired, including mutants in receptor docking (pex13 or pex14), pex4, and pex1 Citation[92]. This suggests that Pex18p is deficient in recycling back to the cytosol and that similarily to Pex5p, may be removed from the peroxisome by the RADAR pathway. Pex20p is intriguingly similar to Pex5p in terms of its UBC-based regulation. Pex20p is more stable than Pex18p and it is polyubiquitinated with K48-branched chains in pex4, pex1 and pex6 mutants in P. pastoris Citation[43]. This suggests that when import is impaired, the co-receptor is degraded.
Receptor recycling and the role of ubiquitination
Ubiquitination of the import receptors is not merely a quality control mechanism. Evidence points to a distinct form of ubiquitination of Pex5p, monoubiquitination on a cysteine by the UBC Pex4p (as opposed to polyubquitination on lysines, by Ubc1/Ubc4/Ubc5), as being important for receptor recycling from the peroxisome. Therefore, the role of ubiquitination in receptor release will be discussed in greater detail below, as will the components of the RING-finger and recycling complexes.
Pex4p-dependent ubiquitination
Pex4p is anchored to the cytosolic face of the peroxisome via its interaction with the integral membrane peroxin Pex22p in yeast and plants Citation[84], Citation[86]. Its association with Pex22p is required for function, as Pex4p is mislocalized to the cytosol in pex22 mutants. Investigations of monoubiquitination and the role of Pex4p have been hindered in the past due to the accumulation of polyubiquitinated Pex5p Citation[82], Citation[89]. However, polyubiquitination of Pex5p in S. cerevisiae can be prevented by either fusing three myc epitopes to the amino-terminus of Pex5p or by substituting the first conserved lysine and the adjacent lysine (K18 and K24, respectively) in Pex5p with arginine Citation[93]. The modified myc-PEX5 and Pex5pK18/24R behave as wild-type Pex5p, as they import PTS1 targeted proteins and are released from the peroxisomal membrane as normal, despite the lack of polyubiquitination. This agrees with the idea that polyubiquitination is part of a quality control system that primes membrane-accumulated Pex5p for proteasomal degradation Citation[71], Citation[82], Citation[95]. However, myc-PEX5 and Pex5pK18/24R are monoubiquitinated. This has enabled the UBC to be identified by testing monoubiquitination in various E2 UBC mutant backgrounds (ubc1, ubc2, ubc4, ubc5, ubc6, ubc7, ubc8, ubc10, ubc11, and ubc13). It was found that the only UBC required for monoubiquitination was Pex4p (Ubc10p) Citation[93].
To confirm that the catalytic activity of Pex4p is required for Pex5p monoubiquitination, an inactive Pex4p mutant protein was used. This contained a C115S mutation, removing the cysteine essential for the activity of ubiquitin-conjugating enzymes Citation[99]. The mutant protein was expressed in a pex4 background. Although Pex4pC115S was targeted to peroxisomes correctly, monoubiquitination of myc-PEX5 and Pex5pK18/24R was completely abolished Citation[93]. To determine that Pex5p ubiquitination is a prerequisite for release from the peroxisomal membrane, in vitro export assays were performed with membranes from pex1, pex4, or pex1/pex4 cells expressing wild-type Pex5p, myc-Pex5p or Pex5pK18/24R and cytosol fractions. It was found that Pex5pK18/24R still exported from the peroxisomal membrane in an AAA peroxin and ATP-dependent manner but in a Pex4p-deficient system (pex4 membranes incubated with pex4/pex5 cytosol) release of the receptor was completely blocked. Therefore, a loss of both poly- and monoubiquitination prevents Pex5p release; hence demonstrating ubiquitination is required for its dislocation from the membrane. As pex4 cells also exhibit a PTS2 import defect, there may be a similar role of Pex4p in the cycling of the PTS2 receptor. It remains to be seen however, if the export of Pex7p depends on the ubiquitination of Pex7p itself, or if the PTS2 import pathway relies on the ubiquitination of Pex5p/Pex18p/Pex20p coupled to Pex7p.
Further work has shown that the two forms of Pex5p ubiquitination target different amino acid residues within the amino-terminal region of the protein. Ubc4p-dependent ubiquitination (polyubiquitination) occurs on two lysines, K18 and K24 in S. cerevisiae Citation[93], Citation[94], whilst Pex4p-dependent ubiquitination (monoubiquitination) occurs on a conserved cysteine residue in the amino-terminal domain. Mutation of this cysteine not only blocks Pex4p-dependent ubiquitination, but also results in a non-functional Pex5p Citation[94]. This strongly suggests that the conserved cysteine in Pex5p serves as the ubiquitin conjugation site.
As there are no PEX4 or PEX22 orthologues in mammals, it was not known whether mammalian PEX5 is ubiquitinated. However, like Pex5p in yeast, two forms of ubiquitination have been recently reported in mammalian PEX5. The first involves conjugation of ubiquitin molecules to PEX5 via thiol-resistant bonds and the second involves Cys11 of PEX5 Citation[100]. Alkylation or mutation of this residue results in a PEX5 protein unable to recycle back into the cytosol Citation[31]. This provides strong evidence for the existence of PEX4- and PEX22-like proteins in mammals.
Although ubiquitination has not been demonstrated in plants, all the components (PEX4, PEX1, PEX6 and the RING complex) are conserved. A role for PEX4 in matrix protein turnover has been invoked in Arabidopsis as pex4/22 mutants retain a glyoxysome specific enzyme (isocitrate lyase) after they have differentiated into leaf peroxisomes (which would not normally contain this enzyme) Citation[86]. Thus regulation of receptor recycling via ubiquitination appears to be a common theme of peroxisome matrix protein import.
RING-finger peroxins
The RING domain Citation[101] binds two Zn2 + ions through its conserved cysteine and histidine residues in a ‘cross-brace’ arrangement Citation[102–104]. Various structures of RING-domains have been solved by X-ray and NMR. In contrast with the known DNA-binding zinc finger domains, the RING domain appears to function as a protein-protein interaction domain Citation[103], Citation[105]. The majority of RING-finger-containing proteins fall into two subclasses, C3HC4 or C3H2C3 (referred to here RING-HC and RING-H2, respectively) and have been found to be essential for catalyzing E3 ligase activity of RING-containing proteins Citation[106].
Pex2p, Pex10p and Pex12p are integral proteins that expose their RING-finger domains to the cytosolic-side of the peroxisomal membrane. These RING-finger peroxins are thought to form a heteromeric complex. Mammalian PEX10 has been shown to interact with PEX2 and PEX12 Citation[75], Citation[107]. In P. pastoris cells lacking any of these components, the other two components are unstable and the RING subcomplex is not assembled efficiently Citation[74], Citation[108]. All of these RING-finger peroxins belong to the RING-HC family, although Pex2p and Pex12p contain substitutions for the conserved cysteine and histidine residues in the second Zn2 + coordination site.
The RING-finger complex is required for receptor release from the peroxisome, as Pex5p Citation[74–76] and Pex20p Citation[43], accumulate inside the peroxisomal lumen in cells with a disrupted RING-complex. The molecular function of the peroxisomal RING-finger peroxins is still a matter of debate, although it has been hypothesized that they function as the E3 ligases in Pex5p/Pex20p/Pex18p ubiquitination. Evidence for this is that both mono- and polyubiquitination of Pex5p depends on the presence of the RING finger peroxins Citation[89–91] and there is a Pex22p-dependent interaction between Pex4p and Pex10p Citation[109]. Therefore it may be possible that Pex4p and Ubc4p exhibit their E2-activity, together with the putative E3 RING-finger peroxins, for the mono- and/or polyubiquitination of Pex5p and Pex20p.
Pex1p and Pex6p
Pex1p and Pex6p are peroxisomal membrane associated proteins that belong to the AAA (ATPase Associated with diverse cellular Activities) family. Pex6p interacts with the integral membrane protein Pex15p in yeast (PEX26 in humans) and it has been shown that ATP hydrolysis in the conserved domain of Pex6p contributes to the disassembly of the Pex6p-Pex15p complex; indicating that the AAA peroxins interact dynamically with Pex15p Citation[110].
Protein import into peroxisomes is an ATP-dependent process and as Pex1p and Pex6p are the only known ATPase peroxins, they are assumed to provide the majority of the ATP requirement. ATP is mainly required for the recycling of Pex5p and the in vitro reconstitution of the complete Pex5p cycle has revealed that ATP-binding and hydrolysis in the conserved domains of both Pex1p and Pex6p is needed for this reaction Citation[82]. Although a direct interaction between Pex5p and Pex1p or Pex6p has not been demonstrated to date, the first 20 amino acids of the PTS-receptors contain a conserved motif of unknown function C(Xn)N(A/G)(L/A) Citation[82], Citation[83], Citation[111]. It is tempting to speculate that this is the putative binding site for Pex1p or Pex6p, although it could be involved in other protein interactions, such as the RING finger peroxins or Pex4p.
Recent studies have shown that in S. cerevisiae Pex4p-dependent monoubiquitination occurs independently of the AAA peroxins, as in pex1 and pex6 mutants ubiquitination is undisturbed Citation[93], Citation[94]. Therefore, monoubiquitination of Pex5p takes place before the protein is released from the peroxisomal membrane, in an AAA peroxin- and ATP-dependent manner. The mechanistic role of the AAA peroxins has not yet been established. However, AAA proteins are mechanoenzymes that manipulate the structure of substrate proteins; hence the binding and consumption of ATP may result in conformational changes that could generate the driving force to pull the receptor(s) out of the membrane. Another potential function of the AAA proteins is that they may be involved in the de-ubiquitination of Pex5p (via recruiting the necessary complexes), which in turn results in receptor release from the membrane. This idea is supported by the fact that the monoubiquitination of Pex5p is transient and exclusive to the peroxisomal membrane Citation[93]; indicating that ubiquitin is released during the export step. presents a model summarizing current thinking regarding the role of ubiquitination in the Pex5p import cycle.
Figure 3. Model of PEX5 and PEX7 ubiquitination in receptor recycling and degradation. PEX5 recognizes PTS1 proteins in the cytosol and brings them to the docking complex (not shown). The receptor-cargo complex translocates to the luminal side of the peroxisomal membrane and disassociates, freeing the cargo. The receptor is then returned to the cytosol, likely through the monoubiquitination of PEX5 mediated by the UBC PEX4 and the putative E3 ligases PEX10/PEX2/PEX12 (RING). The AAA peroxins, PEX1 and PEX6 have been proposed to recognize the monoubiquitinated PEX5 and facilitate in its release from the membrane. PEX5 has also been shown to be polyubiquitinated by Ubc4p (and Ubc5p), although the E3 ligase responsible for polyubiquitination has not yet been determined. Polyubiquitinated PEX5 may again be recognized by the AAA peroxins and directed to the proteasome for degradation as a part of a quality control system. Asterisks = ubiquitin. Redrawn with permission from Citation[93]. This Figure is reproduced in colour in Molecular Membrane Biology online.
![Figure 3. Model of PEX5 and PEX7 ubiquitination in receptor recycling and degradation. PEX5 recognizes PTS1 proteins in the cytosol and brings them to the docking complex (not shown). The receptor-cargo complex translocates to the luminal side of the peroxisomal membrane and disassociates, freeing the cargo. The receptor is then returned to the cytosol, likely through the monoubiquitination of PEX5 mediated by the UBC PEX4 and the putative E3 ligases PEX10/PEX2/PEX12 (RING). The AAA peroxins, PEX1 and PEX6 have been proposed to recognize the monoubiquitinated PEX5 and facilitate in its release from the membrane. PEX5 has also been shown to be polyubiquitinated by Ubc4p (and Ubc5p), although the E3 ligase responsible for polyubiquitination has not yet been determined. Polyubiquitinated PEX5 may again be recognized by the AAA peroxins and directed to the proteasome for degradation as a part of a quality control system. Asterisks = ubiquitin. Redrawn with permission from Citation[93]. This Figure is reproduced in colour in Molecular Membrane Biology online.](/cms/asset/dc64cb29-af4d-4f79-8081-c76f79a4bcd8/imbc_a_313224_f0003_b.jpg)
Conclusion and perspectives
In the past 21 years since the discovery of the first peroxisome targeting signal Citation[112], the field of peroxisome biogenesis has come of age. Many, but probably not all of the players in protein import into the peroxisome have been discovered. A lot is known about their interactions with one another and insights into the molecular structure of some of the components, especially of Pex5p have been obtained. The past two or three years have led to an understanding of the roles of ubiquitination in import receptor regulation and recycling. However there remain a number of important questions.
What is the quarternary structure of Pex5p? Are the tetrameric structures seen in several studies really the physiologically relevant species? If this is the case, the capacity of a tetrameric protein to bind oligomeric cargo proteins raises the possibility that large hetromeric oligomers assemble at the peroxisome surface prior to translocation; the ‘pre-implex’ model of Gould Citation[113]. Since PEX7 and PEX5 interact in mammals and plants, the possibilities become truly staggering. How are these multimers disassembled and translocated?
We still know very little of the actual translocation step. Since no nuclear pore like structure has ever been observed in peroxisome membranes, and peroxisomes are sealed compartments in vivo, the translocation channel must be assembled on demand. Does Pex5p do the job alone or are other components recruited to form a flexible translocon that can accommodate large, and diversely shaped, folded protein complexes? How is cargo unloaded from the receptors on the trans side of the membrane?
The type of ubiquitin modification that Pex5p undergoes appears to determine its fate. How is ubiquitination on cysteine by Pex4p, as opposed to ubiquitination on lysine by Ubc4/Ubc5, selected? Are the RING finger peroxins the E3 ligases for recycling, RADAR or both? Is Pex7p also a target for ubiquitination or is its recycling controlled by modification of its co-receptors? What are the de-ubquitiation enzymes (DuBs) for the receptors? Are proteins, other than the receptors, exported from peroxisomes? If so, are they exported to remodel the protein content, or is protein turnover accomplished by peroxisomal proteases, such as the Lon type protease found in H. polymorpha and predicted to be present in peroxisomes of other species Citation[114]? One thing is for certain, the next 21 years are likely to be full of surprises.
Acknowledgements
Work in the authors’ laboratory is funded by the BBSRC. Thanks to Kate Johnson and Sarah Gunn for their comments on the manuscript. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts and microbodies. Ann NY Acad Sci 1987; 503: 55–71
- Wanders RJA. Metabolic and molecular basis of peroxisomal disorders: A review. Am J Med Genetics Part A 2004; 126A: 355–375
- Baker A, Sparkes IA. Peroxisome protein import: some answers, more questions. Curr Opin Plant Biol 2005; 8: 640–647
- Hayashi M, Nishimura M. Arabidopsis thaliana – a model organism to study plant peroxisomes. Biochimica et Biophysica Acta-Molec Cell Res 2006; 1763: 1382–1391
- Veenhuis M. Peroxisome biogenesis and function in Hansenula polymorpha. Cell Biochem Funct 1992; 10: 175–184
- Debellis L, Tsugeki R, Nishimura M. Glyoxylate cycle enzymes in peroxisomes isolated from petals of pumpkin (Cucurbita Sp.) during senescence. Plant Cell Physiol 1991; 32: 1227–1235
- Debellis L, Nishimura M. Development of enzymes of the glyoxylate cycle during senescence of pumpkin cotyledons. Plant Cell Physiol 1991; 32: 555–561
- Titus DE, Becker WM. Investigation of the glyoxysome peroxisome transition in germinating cucumber cotyledons using double-label immunoelectron microscopy. J Cell Biol 1985; 101: 1288–1299
- Nishimura M, Yamaguchi J, Mori H, Akazawa T, Yokota S. Analytical studies on microbody transition. 5. Immunocytochemical analysis shows that glyoxysomes are directly transformed to leaf peroxisomes during greening of pumpkin cotyledons. Plant Physiol 1986; 81: 313–316
- Nishimura M, Takeuchi Y, Debellis L, Haranishimura I. Leaf peroxisomes are directly transformed to glyoxysomes during senescence of pumpkin cotyledons. Protoplasma 1993; 175: 131–137
- Erdmann R, Wiebel FF, Flessau A, Rytka J, Beyer A, Frohlich KU, Kunau WH. Pas1, a yeast gene required for peroxisome biogenesis, encodes a member of a novel family of putative ATPases. Cell 1991; 64: 499–510
- Lazarow PB. Genetic approaches to studying peroxisome biogenesis. Trends Cell Biol 1993; 3: 89–93
- Nuttley WM, Brade AM, Gaillardin C, Eitzen GA, Glover JR, Aitchison JD, Rachubinski RA. Rapid identification and characterization of peroxisomal assembly mutants in Yarrowia lipolytica. Yeast 1993; 9: 507–517
- Fujiki Y. Peroxisome biogenesis and peroxisome biogenesis disorders. FEBS Lett 2000; 476: 42–46
- Zolman BK, Yoder A, Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 2000; 156: 1323–1337
- Hayashi M, Toriyama K, Kondo M, Nishimura M. 2,4-dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. Plant Cell 1998; 10: 183–195
- Emanuelsson O, Elofsson A, von Heijne G, Cristobal S. In silico prediction of the peroxisomal proteome in fungi, plants and animals. J Mol Biol 2003; 330: 443–456
- Neuberger G, Maurer-Stroh S, Eisenhaber B, Hartig A, Eisenhaber F. Motif refinement of the peroxisomal targeting signal 1 and evaluation of taxon-specific differences. J Mol Biol 2003; 328: 567–579
- Reumann S, Ma CL, Lemke S, Babujee L. AraPerox. A database of putative Arabidopsis proteins from plant peroxisomes. Plant Physiol 2004; 136: 2587–2608
- Kiel J, Veenhuis M, van der Klei IJ. PEX genes in fungal genomes: common, rare or redundant. Traffic 2006; 7: 1291–1303
- Fujiki Y, Matsuzono Y, Matsuzaki T, Fransen M. Import of peroxisomal membrane proteins: The interplay of Pex3p- and Pex19p-mediated interactions. Biochimica et Biophysica Acta-Molec Cell Res 2006; 1763: 1639–1646
- Van Ael E, Fransen M. Targeting signals in peroxisomal membrane proteins. Biochimica et Biophysica Acta-Molecular Cell Research 2006; 1763: 1629–1638
- Fagarasanu A, Fagarasanu M, Rachubinski RA. Maintaining peroxisome populations: a story of division and inheritance. Annu Rev Cell Dev Biol 2007; 23: 321–344
- Lazarow PB, Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol 1985; 1: 489–530
- Lametschwandtner G, Brocard C, Fransen M, Van Veldhoven P, Berger J, Hartig A. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J Biol Chem 1998; 273: 33635–33643
- Gatto GJ, Geisbrecht BV, Gould SJ, Berg JM. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat Struct Biol 2000; 7: 1091–1095
- Stanley WA, Filipp FV, Kursula P, Schuller N, Erdmann R, Schliebs W, Sattler M, Wilmanns M. Recognition of a functional peroxisome type 1 target by the dynamic import receptor Pex5p. Mol Cell 2006; 24: 653–663
- Klein ATJ, Barnett P, Bottger G, Konings D, Tabak HF, Distel B. Recognition of peroxisomal targeting signal type 1 by the import receptor Pex5p. J Biol Chem 2001; 276: 15034–15041
- Otera H, Setoguchi K, Hamasaki M, Kumashiro T, Shimizu N, Fujiki Y. Peroxisomal targeting signal receptor Pex5p interacts with cargoes and import machinery components in a spatiotemporally differentiated manner: Conserved Pex5p WXXYF/Y motifs are critical for matrix protein import. Mol Cell Biol 2002; 22: 1639–1655
- Saidowsky J, Dodt G, Kirchberg K, Wegner A, Nastainczyk W, Kunau WH, Schliebs W. The di-aromatic pentapeptide repeats of the human peroxisome import receptor PEX5 are separate high affinity binding sites for the peroxisomal membrane protein PEX14. J Biol Chem 2001; 276: 34524–34529
- Carvalho AF, Grou CP, Pinto MP, Alencastre IS, Costa-Rodrigues J, Fransen M, Sa-Miranda C, Azevedo JE. Functional characterization of two missense mutations in Pex5p-C11S and N526K. Biochimica et Biophysica Acta-Molec Cell Res 2007; 1773: 1141–1148
- Schliebs W, Saidowsky J, Agianian B, Dodt G, Herberg FW, Kunau WH. Recombinant human peroxisomal targeting signal receptor PEX5 – structural basis for interaction of PEX5 with PEX14. J Biol Chem 1999; 274: 5666–5673
- Moscicka KB, Klompmaker SH, Wang DY, van der Klei IJ, Boekema EJ. The Hansenula polymorpha peroxisomal targeting signal 1 receptor, Pex5p, functions as a tetramer. FEBS Lett 2007; 581: 1758–1762
- Costa-Rodrigues J, Carvalho AF, Fransen M, Hambruch E, Schliebs W, Sa-Miranda C, Azevedo JE. Pex5p, the peroxisomal cycling receptor, is a monomeric non-globular protein. J Biol Chem 2005; 280: 24404–24411
- Lazarow PB. The import receptor Pex7p and the PTS2 targeting sequence. Biochimica et Biophysica Acta-Molec Cell Res 2006; 1763: 1599–1604
- Motley AM, Hettema EH, Ketting R, Plasterk R, Tabak HF. Caenorhabditis elegans has a single pathway to target matrix proteins to peroxisomes. EMBO Rep 2000; 1: 40–46
- Zhang JW, Lazarow PB. Peb1 (Pas7) in Saccharomyces cerevisiae encodes a hydrophilic, intra-peroxisomal protein that is a member of the WD repeat family and is essential for the import of thiolase into peroxisomes. J Cell Biol 1995; 129: 65–80
- Marzioch M, Erdmann R, Veenhuis M, Kunau WH. Pas7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-Oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J 1994; 13: 4908–4918
- Nair DM, Purdue PE, Lazarow PB. Pex7p translocates in and out of peroxisomes in Saccharomyces cerevisiae. J Cell Biol 2004; 167: 599–604
- Stein K, Schell-Steven A, Erdmann R, Rottensteiner H. Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol Cell Biol 2002; 22: 6056–6069
- Purdue PE, Yang XD, Lazarow PB. Pex18p and Pex21p, a novel pair of related peroxins essential for peroxisomal targeting by the PTS2 pathway. J Cell Biol 1998; 143: 1859–1869
- Einwachter H, Sowinski S, Kunau WH, Schliebs W. Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway. EMBO Rep 2001; 2: 1035–1039
- Leon S, Zhang L, McDonald WH, Yates J, Cregg JM, Subramani S. Dynamics of the peroxisomal import cycle of PpPex20p: ubiquitin-dependent localization and regulation. J Cell Biol 2006; 172: 67–78
- Sichting M, Schell-Steven A, Prokisch H, Erdmann R, Rottensteiner H. Pex7p and Pex20p of Neurospora crassa function together in PTS2-dependent protein import into peroxisomes. Mol Biol Cell 2003; 14: 810–821
- Otzen M, Wang DY, Lunenborg MGJ, van der Kiel IJ. Hansenula polymorpha Pex20p is an oligomer that binds the peroxisomal targeting signal 2 (PTS2). J Cell Sci 2005; 118: 3409–3418
- Otera H, Harano T, Honsho M, Ghaedi K, Mukai S, Tanaka A, Kawai A, Shimizu N, Fujiki Y. The mammalian peroxin Pex5pL, the longer isoform of the mobile peroxisome targeting signal (PTS) type 1 transporter, translocates the Pex7p-PTS2 protein complex into peroxisomes via its initial docking site, Pex14p. J Biol Chem 2000; 275: 21703–21714
- Matsumura T, Otera H, Fujiki Y. Disruption of the interaction of the longer isoform of Pex5p, Pex5pL, with Pex7p abolishes peroxisome targeting signal type 2 – study with a novel PEX5-impaired Chinese hamster ovary cell mutant. J Biol Chem 2000; 275: 21715–21721
- Nito K, Hayashi M, Nishimura M. Direct interaction and determination of binding domains among peroxisomal import factors in Arabidopsis thaliana. Plant Cell Physiol 2002; 43: 355–366
- Woodward AW, Bartel B. The Arabidopsis peroxisomal targeting signal type 2 receptor PEX7 is necessary for peroxisome function and dependent on PEX5. Mol Biol Cell 2005; 16: 573–583
- Hayashi M, Yagi M, Nito K, Kamada T, Nishimura M. Differential contribution of two peroxisomal protein receptors to the maintenance of peroxisomal functions in Arabidopsis. J Biol Chem 2005; 280: 14829–14835
- Dodt G, Warren D, Becker E, Rehling P, Gould SJ. Domain mapping of human PEX5 reveals functional and structural similarities to Saccharomyces cerevisiae Pex18p and Pex21p. J Biol Chem 2001; 276: 41769–41781
- Klein ATJ, van den Berg M, Bottger G, Tabak H, Distel B. Saccharomyces cerevisiae acyl-CoA oxidase follows a novel, non-PTS1, import pathway into peroxisomes that is dependent on Pex5p. J Biol Chem 2002; 277: 25011–25019
- McNew JA, Goodman HM. An oligomeric protein is imported into peroxisomes in vivo. J Cell Biol 1994; 127: 1245–1257
- Glover JR, Andrews DW, Rachubinski RA. Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc Natl Acad Sci USA 1994; 91: 10541–10545
- Agne B, Meindl NM, Niederhoff K, Einwachter H, Rehling P, Sickmann A, Meyer HE, Girzalsky W, Kunau WH. Pex8p: An intraperioxisomal organizer of the peroxisomal import machinery. Mol Cell 2003; 11: 635–646
- Mano S, Nakamori C, Nito K, Kondo M, Nishimura M. The Arabidopsis pex12 and pex13 mutants are defective in both PTS1- and PTS2-dependent protein transport to peroxisomes. Plant J 2006; 47: 604–618
- Pires JR, Hong XJ, Brockmann C, Volkmer-Engert R, Schneider-Mergener J, Oschkinat H, Erdmann R. The ScPex13p SH3 domain exposes two distinct binding sites for Pex5p and Pex14p. J Mol Biol 2003; 326: 1427–1435
- Urquhart AJ, Kennedy D, Gould SJ, Crane DI. Interaction of Pex5p, the type 1 peroxisome targeting signal receptor, with the peroxisomal membrane proteins Pex14p and Pex13p. J Biol Chem 2000; 275: 4127–4136
- Hayashi M, Nito K, Toriyama-Kato K, Kondo M, Yamaya T, Nishimura M. AtPex14p maintains peroxisomal functions by determining protein targeting to three kinds of plant peroxisomes. EMBO J 2000; 19: 5701–5710
- Kunau WH. Peroxisomes: the extended shuttle to the peroxisome matrix. Curr Biol 2001; 11: R659–R662
- Rachubinski RA, Subramani S. How proteins penetrate peroxisomes. Cell 1995; 83: 525–528
- Smith MD, Schnell DJ. Peroxisomal protein import: the paradigm shifts. Cell 2001; 105: 293–296
- Gouveia AM, Guimaraes CP, Oliveira ME, Reguenga C, Sa-Miranda C, Azevedo JE. in Peroxisomal Disorders and Regulation of Genes 2003; 544: 219–220
- Dammai V, Subramani S. The human peroxisomal targeting signal receptor, Pex5p, is translocated into the peroxisomal matrix and recycled to the cytosol. Cell 2001; 105: 187–196
- Rehling P, Skaletz-Rorowski A, Girzalsky W, Voorn-Brouwer T, Franse MM, Distel B, Veenhuis M, Kunau WH, Erdmann R. Pex8p, an intraperoxisomal peroxin of Saccharomyces cerevisiae required for protein transport into peroxisomes binds the PTS1 receptor Pex5p. J Biol Chem 2000; 275: 3593–3602
- Miura S, Miyazawa S, Osumi T, Hashimoto T, Fujiki Y. Post-translational import of 3-ketoacyl-CoA thiolase into rat-liver peroxisomes in vitro. J Biochem (Tokyo) 1994; 115: 1064–1068
- Kurochkin IV, Mizuno Y, Konagaya A, Sakaki Y, Schonbach C, Okazaki Y. Novel peroxisomal protease Tysnd1 processes PTS1- and PTS2-containing enzymes involved in beta-oxidation of fatty acids. EMBO J 2007; 26: 835–845
- Helm M, Luck C, Prestele J, Hierl G, Huesgen PF, Froehlich T, Arnold GJ, Adamska I, Gorg A, Lottspeich F, et al. Dual specificities of the glyoxysomal/peroxisomal processing protease Deg15 in higher plants. Proc Natl Acad Sci USA 2007; 104: 11501–11506
- Gouveia AMM, Reguenga C, Oliveira MEM, Sa-Miranda C, Azevedo JE. Characterization of peroxisomal Pex5p from rat liver – Pex5p in the Pex5p-Pex14p membrane complex is a transmembrane protein. J Biol Chem 2000; 275: 32444–32451
- Kerssen D, Hambruch E, Klaas W, Platta HW, de Kruijff B, Erdmann R, Kunau WH, Schliebs W. Membrane association of the cycling peroxisome import receptor Pex5p. J Biol Chem 2006; 281: 27003–27015
- Erdmann R, Schliebs W. Peroxisomal matrix protein import: The transient pore model. Nature Rev Molec Cell Biol 2005; 6: 738–742
- Wang DY, Visser NV, Veenhuis M, van der Klei IJ. Physical interactions of the peroxisomal targeting signal 1 receptor Pex5p, studied by fluorescence correlation spectroscopy. J Biol Chem 2003; 278: 43340–43345
- Waterham HR, Titorenko VI, Haima P, Cregg JM, Harder W, Veenhuis M. The Hansenula polymorpha Per1 gene is essential for peroxisome biogenesis and encodes a peroxisomal matrix protein with both carboxy-terminal and amino-terminal targeting signals. J Cell Biol 1994; 127: 737–749
- Zhang L, Leon S, Subramani S. Two independent pathways traffic the interperoxisomal peroxin PpPex8p into peroxisomes: mechanism and evolutionary implications. Mol Biol Cell 2006; 17: 690–699
- Chang CC, Warren DS, Sacksteder KA, Gould SJ. PEX12 interacts with PEX5 and PEX10 and acts downstream of receptor docking in peroxisomal matrix protein import. J Cell Biol 1999; 147: 761–773
- Dodt G, Gould SJ. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: Evidence that PTS1 protein import is mediated by a cycling receptor. J Cell Biol 1996; 135: 1763–1774
- Hu JP, Aguirre M, Peto C, Alonso J, Ecker J, Chory J. A role for peroxisomes in photomorphogenesis and development of Arabidopsis. Science 2002; 297: 405–409
- Schumann U, Wanner G, Veenhuis M, Schmid M, Gietl C. AthPEX10, a nuclear gene essential for peroxisome and storage organelle formation during Arabidopsis embryogenesis. Proc Natl Acad Sci USA 2003; 100: 9626–9631
- Sparkes IA, Brandizzi F, Slocombe SP, El-Shami M, Hawes C, Baker A. An Arabidopsis pex10 null mutant is embryo lethal, implicating peroxisomes in an essential role during plant embryogenesis. Plant Physiol 2003; 133: 1809–1819
- Fan JL, Quan S, Orth T, Awai C, Chory J, Hu JP. The Arabidopsis PEX12 gene is required for peroxisome biogenesis and is essential for development. Plant Physiol 2005; 139: 231–239
- Nito K, Kamigaki A, Kondo M, Hayashi M, Nishimura M. Functional classification of Arabidopsis peroxisome biogenesis factors proposed from analyses of knockdown mutants. Plant Cell Physiol 2007; 48: 763–774
- Platta HW, Grunau S, Rosenkranz K, Girzalsky W, Erdmann R. Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nat Cell Biol 2005; 7: 817–822
- Miyata N, Fujiki Y. Shuttling mechanism of peroxisome targeting signal type 1 receptor Pex5: ATP-independent import and ATP-dependent export. Mol Cell Biol 2005; 25: 10822–10832
- Koller A, Snyder WB, Faber KN, Wenzel TJ, Rangell L, Keller GA, Subramani S. Pex22p of Pichia pastoris, essential for peroxisomal matrix protein import, anchors the ubiquitin-conjugating enzyme, Pex4p, on the peroxisomal membrane. J Cell Biol 1999; 146: 99–112
- Collins CS, Kalish JE, Morrell JC, McCaffery JM, Gould SJ. The peroxisome biogenesis factors Pex4p, Pex22p, Pex1p, and Pex6p act in the terminal steps of peroxisomal matrix protein import. Mol Cell Biol 2000; 20: 7516–7526
- Zolman BK, Monroe-Augustus M, Silva ID, Bartel B. Identification and functional characterization of Arabidopsis PEROXIN4 and the interacting protein PEROXIN22. Plant Cell 2005; 17: 3422–3435
- Zolman BK, Bartel B. An Arabidopsis indole-3-butyric acid-response mutant defective in PEROXIN6, an apparent ATPase implicated in peroxisomal function. Proc Natl Acad Sci USA 2004; 101: 1786–1791
- Wiebel FF, Kunau WH. The Pas2 protein essential for peroxisome biogenesis is related to ubiquitin-conjugating enzymes. Nature 1992; 359: 73–76
- Kiel J, Emmrich K, Meyer HE, Kunau WH. Ubiquitination of the peroxisomal targeting signal type 1 receptor, Pex5p, suggests the presence of a quality control mechanism during peroxisomal matrix protein import. J Biol Chem 2005; 280: 1921–1930
- Kragt A, Brouwer TV, van den Berg M, Distel B. The Saccharomyces cerevisiae peroxisomal import receptor Pex5p is monoubiquitinated in wild type cells. J Biol Chem 2005; 280: 7867–7874
- Platta H, Girzalsky W, Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p. Biochem J 2004; 384: 37–45
- Purdue PE, Lazarow PB. Pex18p is constitutively degraded during peroxisome biogenesis. J Biol Chem 2001; 276: 47684–47689
- Platta HW, El Magraoui F, Schlee D, Grunau S, Girzalsky W, Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. J Cell Biol 2007; 177: 197–204
- Williams C, van den Berg M, Sprenger RR, Distel B. A conserved cysteine is essential for Pex4p-dependent ubiquitination of the peroxisomal import receptor Pex5p. J Biol Chem 2007; 282: 22534–22543
- Kiel J, Otzen M, Veenhuis M, van der Klei IJ. Obstruction of polyubiquitination affects PTS1 peroxisomal matrix protein import. Biochimica et Biophysica Acta-Molec Cell Res 2005; 1745: 176–186
- Yahraus T, Braverman N, Dodt G, Kalish JE, Morrell JC, Moser HW, Valle D, Gould SJ. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J 1996; 15: 2914–2923
- van der Klei IJ, Hilbrands RE, Kiel J, Rasmussen SW, Cregg JM, Veenhuis M. The ubiquitin-conjugating enzyme Pex4p of Hansenula polymorpha is required for efficient functioning of the PTS1 import machinery. EMBO J 1998; 17: 3608–3618
- Kiel J, Hilbrands RE, Van der Klei IJ, Rasmussen SW, Salomons FA, Van der Heide M, Faber KN, Cregg JM, Veenhuis M. Hansenula polymorpha Pex1p and Pex6p are peroxisome-associated AAA proteins that functionally and physically interact. Yeast 1999; 15: 1059–1078
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem 2001; 70: 503–533
- Carvalho AF, Pinto MP, Grou UP, Alencastre IS, Fransen M, Sa-Miranda C, Azevedo JE. Ubiquitination of mammalian pex5p, the peroxisomal import receptor. J Biol Chem 2007; 282: 31267–31272
- Freemont PS, Hanson IM, Trowsdale J. A novel cysteine-rich sequence motif. Cell 1991; 64: 483–484
- Barlow PN, Luisi B, Milner A, Elliott M, Everett R. Structure of the C3hc4 domain by H-1-nuclear magnetic-resonance spectroscopy – a new structural class of zinc-finger. J Mol Biol 1994; 237: 201–211
- Borden KLB. RING domains: master builders of molecular scaffolds?. J Mol Biol 2000; 295: 1103–1112
- Borden KLB, Boddy MN, Lally J, O'Reilly NJ, Martin S, Howe K, Solomon E, Freemont PS. The solution structure of the RING finger domain from the acute promyelocytic leukemia proto-oncoprotein Pml. EMBO J 1995; 14: 1532–1541
- Lovering R, Hanson IM, Borden KLB, Martin S, Oreilly NJ, Evan GI, Rahman D, Pappin DJC, Trowsdale J, Freemont PS. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci USA 1993; 90: 2112–2116
- Lorick KL, Jensen JP, Fang SY, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA 1999; 96: 11364–11369
- Okumoto K, Abe I, Fujiki Y. Molecular anatomy of the peroxin Pex12p – RING finger domain is essential for Pex12p function and interacts with the peroxisome-targeting signal type 1-receptor Pex5p and a ring peroxin, Pex10p. J Biol Chem 2000; 275: 25700–25710
- Hazra PP, Suriapranata I, Snyder WB, Subramani S. Peroxisome remnants in pex3-delta cells and the requirement of Pex3p for interactions between the peroxisomal docking and translocation subcomplexes. Traffic 2002; 3: 560–574
- Eckert JH, Johnsson N. Pex10p links the ubiquitin conjugating enzyme Pex4p to the protein import machinery of the peroxisome. J Cell Sci 2003; 116: 3623–3634
- Birschmann I, Stroobants AK, van den Berg M, Schafer A, Rosenkranz K, Kunau WH, Tabak HF. Pex15p of Saccharomyces cerevisiae provides a molecular basis for recruitment of the AAA peroxin Pex6p to peroxisomal membranes. Mol Biol Cell 2003; 14: 2226–2236
- Rosenkranz K, Birschmann I, Grunau S, Girzalsky W, Kunau WH, Erdmann R. Functional association of the AAA complex and the peroxisomal importomer. FEBS J 2006; 273: 3804–3815
- Gould SJ, Keller GA, Subramani S. Identification of a peroxisomal targeting signal at the carboxy-terminus of firefly luciferase. J Cell Biol 1987; 105: 2923–2931
- Gould SJ, Collins CS. Peroxisomal-protein import: is it really that complex?. Nature Reviews Molecular Cell Biology 2002; 3: 382–389
- Aksam EB, Koek A, Kiel J, Jourdan S, Veenhuis M, van der Klei IJ. A peroxisomal lon protease and peroxisome degradation by autophagy play key roles in vitality of Hansenula polymorpha cells. Autophagy 2007; 3: 96–105
- Walton PA, Wendland M, Subramani S, Rachubinski RA, Welch WJ. Involvement of 70-Kd heat shock proteins in peroxisomal import. J Cell Biol 1994; 125: 1037–1046
- Harano T, Nose S, Uezu R, Shimizu N, Fujiki Y. Hsp70 regulates the interaction between the peroxisome targeting signal type 1 (PTS1)-receptor Pex5p and PTS1. Biochem J 2001; 357: 157–165
- Hettema EH, Ruigrok CCM, Koerkamp MG, van den Berg M, Tabak HF, Distel B, Braakman I. The cytosolic DnaJ-like protein Djp1p is involved specifically in peroxisomal protein import. J Cell Biol 1998; 142: 421–434