Abstract
Metal ion homeostasis is important for healthy cell function and is regulated by metal ion transporters and chaperones. To explore metal ion binding to membrane transport proteins we have used cadmium-113 as a solid state NMR probe of the Escherichia coli zinc exporter ZitB present in native membrane preparations. Competition experiments with other metal ions indicated that nickel and copper are also able to bind to this protein. Metal ion uptake studies were also performed using ZitB-reconstituted into proteoliposomes for a well established fluorescence assay. The results of both the solid state NMR and the uptake studies demonstrate that ZitB is potentially capable of transporting not only zinc but also cadmium, nickel and copper. The solid state NMR approach therefore offers great potential for defining the substrate spectrum of metal ion transporter proteins in their native membrane environments. Further, it should be useful for functional dissection of transporter mechanisms by facilitating the identification of functional residues by mutational studies.
Introduction
Zinc is an essential micronutrient for the development and differentiation of living cells and plays an important role in a range of biological processes including gene expression, DNA synthesis, enzymatic catalysis, hormone storage and release and neurotransmission and is tightly bound to associated proteins Citation[1], Citation[2]. For normal cellular function, micronutrients such as zinc and copper are toxic at abnormal free intracellular concentrations which must therefore be regulated by appropriate import and export transport proteins as well as intracellular chaperones. Metal transporters are integral membrane proteins which constitute a substantial component of the membrane proteome and play a key role in protecting cells from excess essential metals as well as from other metals, such as cadmium, that have no clear biological role and are toxic at very low levels.
Zinc transporters are found in all classes of organism where they play a central role in regulating cellular zinc homeostasis and also transport other metal ions, including cadmium, cobalt, iron, nickel, manganese, copper and mercury Citation[3–8]. The Escherichia coli transporter ZitB belongs to the cation diffusion facilitator (CDF) family of transport proteins, members of which contain six putative transmembrane spanning regions and reduce intracellular cytoplasmic zinc concentrations by promoting zinc efflux from cells or into intracellular vesicles Citation[2], Citation[8], Citation[9]. ZitB also transports Cd2 + Citation[2]. CDF family members have been implicated in a number of diseases Citation[6]. Recently, a mutation in a pancreatic β-cell vesicular CDF transporter has been associated with type 2 diabetes mellitus Citation[7].
A homologue of ZitB named YiiP, also from E. coli, appears to be responsible only for iron detoxification Citation[10] but is capable of in vitro Zn2 + transport. Mutagenesis studies on ZitB and YiiP have identified an extracellular zinc binding site (Z1; comprising H53, D57, H159, D163 in ZitB) likely to be the exit site for zinc efflux, based on a recent 3.8 Å crystal structure of YiiP Citation[10]. Three other zinc binding sites are also suggested by the structural studies and these lie at the interface between the homodimer subunits. Z2 binds to an intracellular loop connecting transmembrane regions two and three while Z3 and Z4 are EDTA-resistant sites that lie at the dimer interface of the soluble intracellular domain Citation[10].
The transport mechanism of CDF family members has been investigated Citation[8], Citation[11], often using detergent-solubilized or reconstituted proteins removed from their native membrane environment, and thus possibly lacking protein cofactors associated with substrate recognition and binding and/or translocation. Calorimetry, equilibrium dialysis, fluorescence and NMR have all previously been used to examine substrate binding to transporters. Here we have investigated the use of cadmium-113 solid state NMR spectroscopy for the direct observation of metal-ion binding to transporters expressed in their native membranes to allow analysis of substrate selectivity and as a prospective tool for the identification of functional residues through mutagenesis experiments. As with other in vitro studies the kinetics of binding or transport may differ from native conditions.
Methods
Expression plasmid construction
The expression plasmids encoding the Escherichia coli zinc exporter, ZitB(Lumio™-His8) and glutamate transporter, GltP(Lumio™-His8) were generated by Gateway™ recombinational cloning (Invitrogen) as previously described Citation[12]. Expression constructs bearing C-terminal Lumio™ and His8 tags (CCPGCCHHHHHHHH) are called pLMP-01-ZitB and pLMP-01-GltP. A version of the ZitB expression vector (pLMP-01-ZitB-1) lacking the His8 tag was produced from pLMP-01-ZitB by using QuikChange™ (Stratagene) mutagenesis and primers ZitB-1-F and ZitB-1-R () to introduce a stop codon (TAA) after the Lumio™ tag sequence. For the cysteineless-ZitB construct, an expression vector containing the ZitB gene (pTTQ18-ZitB) was first constructed by conventional cloning procedures. For this purpose EcoRI and PstI sites introduced at the 5′ and 3′ ends of the ORF were used to insert a PCR product, amplified using the primers ZitB-F and ZitB-R (), into the corresponding sites of pTTQ18. The cysteineless-ZitB construct (pZitB-C230S/C294S/C299S) containing serines in place of cysteines was generated from pTTQ18-ZitB by using QuikChange™ (Stratagene) mutagenesis and primers C230S-F and −R, C294S-F and −R and C299S-F and −R ().
Table I. Primer sequences used in the construction of expression plasmids.
Expression and detection of recombinant transporters
For expression experiments, plasmids were transformed into E. coli strain BL21. Cultures were grown at 37°C in an orbital incubator (200 rpm) in M9 minimal medium supplemented with 0.2% (w/v) casamino acids plus 0.2% (w/v) glycerol, in the presence of carbenicillin (100 µg/ml). When cells harboring ZitB plasmids or pLMP-01-GltP reached an A600nm of 0.5 or 1.0, respectively, expression was induced by the addition of isopropyl-β-d-thiogalactoside (IPTG) to a final concentration of 0.25 mM or 0.05 mM, respectively, and the cells were harvested by centrifugation after growth for a further 3 h.
Small scale membrane preparations were performed by the water lysis method Citation[13]. For large scale membrane preparations cells were resuspended in 20 mM Tris, 1 mM EDTA, pH 7.5 containing 10% glycerol, 1 mM phenylmethylsulfonyl fluoride (PMSF) and Complete protease inhibitor (one tablet per 8 g wet biomass; Roche Diagnostic GmbH, Germany). Cells were lyzed by two to three passages at 4°C through a cell disruptor (Constant Systems Ltd.) at 20–30 kpsi and the resulting mixed membrane vesicles (inner and outer membranes) collected by centrifugation at 100,000 g for 2 h. Following washing in 20 mM Tris pH 7.5 containing 1 mM PMSF by the same procedure, membrane vesicles were stored at -70°C.
Protein concentration was determined using the bicinchoninic acid (BCA) assay (Perbio Science UK Ltd.). Detection of His-tagged proteins on western blots was performed using HisProbe™-HRP (Perbio Science UK Ltd). Lumio™ tagged ZitB protein produced from pLMP-01-ZitB-1 was detected using a Lumio™ in-gel detection kit following the manufacturer's instructions except that samples were dissolved in 9.6 mM Tris-HCl, pH 6.8, containing 0.96% SDS, 2.5 mM EDTA, 15% glycerol, 20 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP) and 0.075% bromophenol blue to ensure complete solubilization of membrane proteins before SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The Lumio™ fluorescence was imaged on a UV transilluminator equipped with a standard camera before subsequent staining of the gel with Coomassie blue for total protein detection. Cysteineless ZitB protein samples were analyzed by SDS-PAGE followed by staining with Coomassie blue.
Sample preparation for NMR experiments
Cross polarization magic angle spinning (CP-MAS) NMR experiments on ZitB were performed at 4C with membranes prepared from IPTG-induced E. coli BL21 cells harboring pLMP-01-ZitB, pLMP-01-ZitB-1 or pZitB-C230S/C294S/C299S as described above. In such membranes ZitB represented 16–22% of total membrane protein. For experiments with ZitB(Lumio™-His8) and ZitB(Lumio™) or ZitB(C230S/C294S/C299S), 48 and 24 mg samples of total membrane protein were used, respectively. For control experiment, membranes containing 24 mg of protein were obtained from cells induced to express the glutamate transporter GltP fused with a Lumio™-His8 tag. In each case membranes were suspended to 3 ml in 20 mM Tris-HCl, pH 7.5 containing 5 mM 113CdCl2. The mixture was equilibrated at 4°C for 30 min. They were then sedimented by ultracentrifugation (100,000 g, 4°C, 30 min) and packed into a MAS sample rotor by centrifuging briefly in a bench top centrifuge only until the speed reached 2000 rpm.
For displacement experiments, competitive divalent metal salts were added in a series of additions into ZitB membranes to displace 113Cd, directly to the MAS NMR sample rotor and mixed with the help of a syringe needle by stirring gently into the membrane suspension. The latter was then collected to the base of the rotor by briefly centrifuging in a bench top centrifuge only until the speed reached 2 000 rpm, and left for equilibration at 4°C for 30 min before the collection of data.
NMR conditions
113Cd CP MAS ssNMR spectra were recorded using a Varian InfinityPlus 300 MHz spectrometer (66.50 MHz for 113Cd and 299.5 MHz for 1H) with a Varian double bearing MAS probe for 6 mm sample rotors. Cadmium acetate was used to set up the CP condition and cadmium carbonate was used as the chemical shift standard, which is at -32.4 ppm. Samples were spun at 2.4 kHz and held at 4°C by passing the bearing gas through a coil immersed in a dry ice bath. A relaxation delay of 1 second with the given CP contact time was used. Each spectrum was averaged over 28,000 acquisitions. Data were processed with 800 Hz exponential line broadening.
Purification of ZitB for proteoliposome reconstitution experiments
Mixed membrane vesicles (18 mg protein) prepared from IPTG-induced bacteria harboring pTTQ18-ZitB were solubilized by incubation on ice for 1.5 h in 6 ml 20 mM HEPES pH 7.5, containing 100 mM NaCl, 20% (v/v) glycerol, 4 mM β-mercaptoethanol, 5 mM imidazole, 1% (w/v) n-dodecyl-β-d-maltopyranoside (DDM) (Melford Laboratories Ltd. UK), 1 mM PMSF and 0.5% protease inhibitor mixture (Sigma). Insoluble material was removed by centrifugation at 100,000 g for 1 h at 4°C and then the supernatant was gently mixed for 2 h at 4°C with 0.8 ml Ni-NTA agarose (50% suspension) (Qiagen UK) which was pre-equilibrated with a wash buffer (20 mM HEPES 7.5, containing 300 mM NaCl, 12.5% (v/v) glycerol, 2 mM β-mercaptoethanol, 30 mM imidazole and 0.05% (w/v) DDM). After packing into a column the agarose was washed with 40 column volumes of wash buffer and then ZitB was eluted with the same wash buffer with additional imidazole added to 500 mM.
The purified protein was placed into a 10 kDa cutoff dialysis cassette (Pierce) and dialyzed overnight against 20 mM HEPES 7.5, containing 100 mM NaCl, 12.5% (v/v) glycerol, 4 mM β-mercaptoethanol and 0.05% (w/v) DDM and then concentrated to 10–15 mg per ml using a 50 kDa cut-off Viva spin concentrator. Before reconstitution the aggregated protein was removed by centrifuging the sample at 100,000 g for 30 min and protein concentration was measured at 280 nm.
Reconstitution, encapsulation of membrane-impermeable Fluozin-1 and metal-ion transport assay
These procedures were performed as reported by Citation[2] with some modifications, as described below. Liposomes were prepared from E. coli polar lipid extract (Avanti Polar Lipids, Inc., Alabaster AL USA). A 5 ml solution of lipids in chloroform containing 50 mg lipids was completely dried under argon and then incubated in a desiccator for 30 min to remove traces of chloroform. The lipids were hydrated in a 20 mM Bis-Tris pH 6.8 containing 2 mM β-mercaptoethanol to a concentration of 50 mg per ml by vortexing and then the suspension was extruded through polycarbonate filters of pore size 400 nm and then 100 nm using a Mini-Extruder (Avanti Polar Lipids, Inc., Alabaster AL, USA). The reconstitution of ZitB was achieved by mixing 130 µl of extruded lipids with 100 µl of 10% n-octyl-β-D-glucopyranoside (β-OG; Sigma) and 770 µl of protein (0.1 or 0.25 mg per ml in 20 mM Bis-Tris pH 6.8 plus 1% β-OG and 2 βME). The mixture was incubated at room temperature for 20 min with gentle shaking and then applied to an Econo-Pac 10 DG desalting column (Bio-Rad), pre-equilibrated with 20 mM Bis-Tris pH 6.8 (assay buffer). A void volume fraction of 2 ml was collected and proteoliposomes were collected by centrifugation at 140,000 g for 45 min. Control liposomes were similarly prepared but no protein was added during the procedure.
Proteoliposome and control liposome pellets were resuspended in 200 µl of assay buffer and Fluozin-1 dye (Molecular Probes, Invitrogen) was added to a final concentration of 200 µM. The samples were subjected to three cycles of freeze-thaw in liquid nitrogen followed by incubation in water at room temperature. The dye exterior to the proteo-/liposomes was removed using an Econo-Pac 10 DG desalting column. The dye-loaded proteo-/liposomes were eluted in the void fraction and the presence of proteo-/liposomes was checked by measuring the turbidity of the solution at 540 nm. To ensure complete removal of the non-entrapped dye the proteo-/liposomes were sedimented by ultracentrifugation at 140,000 g for 30 min. The pellets were gently washed with 2 ml of assay buffer and then resuspended in 200 µl of the same buffer.
The fluorescence measurements were performed on a PTI scanning fluorimeter (Photon Technology International Inc., UK). Excitation and emission slit widths of 3.0 and 4.0 nm were used, respectively. For the transport assays Flouzin-1 loaded proteoliposomes or control liposomes were diluted 50–100 times with assay buffer to a final volume of 1.25 ml in a 2 ml cuvette. The temperature of the cuvette holder was maintained at 8°C. The transport reactions were initiated by the addition ZnCl2, Cd(CH3COO)2, NiCl2 or Cd(CH3OO)2+CuCl2 to a final concentration of 2 mM. The changes in Fluozin-1 fluorescence were monitored by exciting the samples at 490 nm and collecting emission data at 515 nm as a function of time. During measurement, samples were constantly stirred using a magnetic stirrer. The fluorescence traces collected for liposomes were subtracted from proteoliposome traces, as described by Chao and Fu Citation[2], to provide the net fluorescence changes (ΔF) and then normalized to the maximum fluorescence intensity (ΔFmax) obtained by adding 1% β-OG to proteoliposomes samples containing 2 mM of respective metal-ion. For Zn2 + transport, proteoliposomes prepared using a protein to lipid ratio of 1:325 (w/w) and 1:81 (w/w) were tested and subsequent uptake experiments with alternative ions were performed at a protein to lipid ration of 1:325 (w/w). To determine whether Cu2 + ions were transported by the reconstituted ZitB, a competition assay was performed using a mixture of 2 mM CdCl2 and CuCl2 because the sensitivity of Fluozin-1 to Cu2 + ions is poor.
Results and discussion
NMR experiments
There is no naturally occurring spin-½ isotope for zinc, so 113Cd was chosen as an NMR-active probe of metal-ion binding. This isotope has often been used as a tractable NMR-active replacement for Zn2 + and Ca2 + cations in biological samples, where its comparable size allows functional activity to be retained Citation[2], Citation[14–16]. In a magnetic field of 7 T, 113Cd resonates at a frequency of 66.4 MHz; it has a natural abundance of 12.26% with a receptivity compared with 13C of 7.69. The NMR observation of 113Cd usually requires isotopic enrichment and the use of experiments that increase sensitivity. Hence, 1H-113Cd cross-polarization magic-angle spinning (CP-MAS) NMR was used to detect the signal for cadmium-binding in aqueous samples of E. coli membranes that had been incubated with [113Cd]CdCl2. Under these conditions, the CP experiment can distinguish between substrate that has undergone interactions with the membranes from substrate that has remained free in solution Citation[17–19].
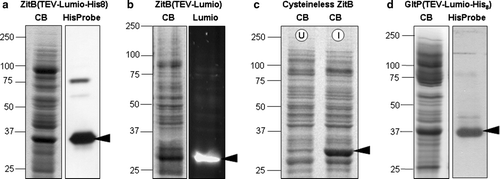
Membrane protein samples were prepared and analyzed for the presence of ZitB variants and for GltP, a glutamate/aspartate transporter from E. coli, as shown in . The differences in migration of the various ZitB samples reflect both differences in cloning strategies, resulting in presence or absence of various C-terminal tags, and differences in methods of sample preparation. ZitB-Lumio™-His8 tag (39950 Da; a) and Cysteineless-ZitB (34840 Da; c) were isolated and treated in a similar manner and both migrate at apparent molecular masses slightly below their calculated values, a common observation for membrane proteins. The migration of these samples cannot be directly compared with the ZitB-Lumio™ sample (38860 Da; b) as the membrane solubilization and sample preparation buffers contained the strong reducing agent TCEP and the samples were then reacted with the biarsenical Lumio detection reagent before fractionation by SDS-PAGE. Such treatment consistently resulted in faster migration of this ZitB-Lumio protein.
Figure 1. Expression levels and tag-based detection of over-expressed proteins in membrane samples. SDS-PAGE fractionation of membrane samples with detection of protein by Coomassie blue staining (CB); biarsenical detection of fluorescence due to the Lumio™ tag (Lumio); India™ probe detection of His8 tag (His-tag). In each case the arrowhead indicates the protein of interest. (a) ZitB(TEV-Lumio™-His8) with detection of the His8 tag; (b) ZitB(TEV-Lumio™) with detection of the Lumio tag; (c) Cysteineless ZitB carrying the mutations C230S, C294S, C299S and lacking the Lumio™-tag shown by Coomassie staining of protein in IPTG induced (I) compared with an uninduced (U) control; (d) GltP(TEV-Lumio™-His8) with detection of the His-tag. In all cases substantial bands are visible in the Coomassie blue stained gel indicating good over-expression of protein.
Membranes containing high levels (∼ 15% total protein) of ZitB bearing a C-terminal Lumio™-His8 tag (a) were prepared from E. coli strain BL21 Citation[12]. Initial 113Cd CP-MAS spectra recorded for these membranes containing 5 mM [113Cd]CdCl2 gave rise to a sharp signal, characteristic of an exchange process, at around 0 ppm (a) using CdCO3 as chemical shift reference. That the observed signal was due to specific binding of 113Cd by ZitB was indicated by its elimination upon addition of a 10-fold excess of ZnCl2 (b). The dynamics of 113Cd-binding in the membranes were probed by measuring the intensity of the 113Cd signal in the undisplaced sample over a range of cross polarization contact times (c). A maximum peak intensity was observed at a contact time of 7 msec, a value greater than that obtained for protein carbonyl and lipid resonances in 13C CP-MAS spectra of E. coli membranes and consistent with a higher mobility of 113Cd in the ZitB binding site compared to these membrane constituents Citation[20].
Figure 2. 113Cd CP-MAS analysis of membranes from E. coli expressing the zinc transporter ZitB(TEV-Lumio™-His8). (a) 113Cd CP-MAS spectrum upon incubation with 5 mM [113Cd]CdCl2 and (b) following preincubation with 50 mM ZnCl2. Spectra were recorded at 66.4 MHz for 113Cd using a MAS frequency 2.4 kHz, CP contact time 7 msec and acquisition delay 1 sec over 28 000 scans at a temperature of 4°C.
![Figure 2. 113Cd CP-MAS analysis of membranes from E. coli expressing the zinc transporter ZitB(TEV-Lumio™-His8). (a) 113Cd CP-MAS spectrum upon incubation with 5 mM [113Cd]CdCl2 and (b) following preincubation with 50 mM ZnCl2. Spectra were recorded at 66.4 MHz for 113Cd using a MAS frequency 2.4 kHz, CP contact time 7 msec and acquisition delay 1 sec over 28 000 scans at a temperature of 4°C.](/cms/asset/4427ec6d-15c4-4caa-bd82-1d3333b5f140/imbc_a_349694_f0002_b.gif)
Control experiments were performed to test whether non-specific binding of 113Cd to the His8 or Lumio™ tags, or to endogenous cysteines, contributed to the NMR signal. Membranes containing ZitB with only a Lumio™ tag (b) exhibited Zn-displaceable 113Cd binding comparable to that observed for ZitB(Lumio™-His8) (a, 3b). Similar results (c, 3d) were obtained for membranes containing ZitB with no tag and in which the three non-essential endogenous cysteine residues (C230, C294, C299) Citation[21], Citation[22] had been mutated to serines (c). In contrast, only a broad, indistinct signal, centered around 0 ppm, probably indicative of non-specific and/or non-exchangeable interactions (e), was seen for membranes with equivalent levels of His8-tagged GltP (d). Taken together these results indicate that the sharp signal at ∼ 0 ppm seen in NMR spectra of ZitB results from specific interaction of 113Cd with a substrate-binding site.
Figure 3. 113Cd CP-MAS analysis of E. coli membranes. 113Cd CP-MAS spectra were obtained by incubation of 5 mM [113Cd]CdCl2 with E. coli membranes containing ZitB(TEV-Lumio™) before (a) and after (b) incubation with 50 mM ZnCl2 or containing cysteineless ZitB (no tag) before (c) and after (d) incubation with 50 mM ZnCl2 or containing (e) GltP(His8). NMR conditions as in .
![Figure 3. 113Cd CP-MAS analysis of E. coli membranes. 113Cd CP-MAS spectra were obtained by incubation of 5 mM [113Cd]CdCl2 with E. coli membranes containing ZitB(TEV-Lumio™) before (a) and after (b) incubation with 50 mM ZnCl2 or containing cysteineless ZitB (no tag) before (c) and after (d) incubation with 50 mM ZnCl2 or containing (e) GltP(His8). NMR conditions as in Figure 2.](/cms/asset/fc3577a4-bbce-4d1a-a7d9-f89897a7685b/imbc_a_349694_f0003_b.gif)
The signal ascribed to 113Cd binding to ZitB(Lumio™-His8) was also competitively eliminated by increasing concentrations of Cu2 + and Ni2 + , which may therefore represent alternative substrates (data not shown). IC50 values calculated from the ssNMR data were 19±1 mM (Zn2 + ), 20±0.8 mM (Cd2 + ), 5±1 mM (Cu2 + ) and 10±1 mM (Ni2 + ). In the case of Cd2 + and Zn2 + these values are substantially larger than the apparent Km values of ∼ 0.1 mM for transport energized by the electrochemical proton gradient Citation[2], and so do not correspond to in vivo affinities. These differences probably reflect measurement of binding under non-energized conditions and/or lower temperatures as has been observed for several other bacterial proton-dependent transporters Citation[17–20]. In this study it was not possible to perform measurements by ssNMR under energized conditions and the extended time required for single point data collection (∼ 8 h) precluded analysis at higher temperature because of potential problems with protein integrity over an experimental time period of some two days.
Metal transport assays
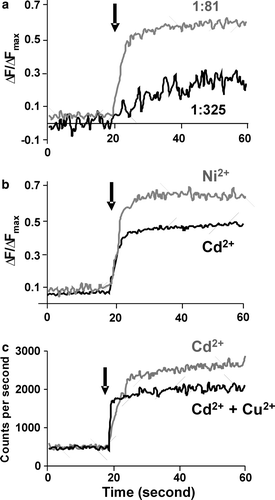
To confirm that Cd2 + , Cu2 + and Ni2 + can also be transported by ZitB we used a proven uptake assay Citation[2]. shows the fluorescence increase upon metal ion transport into proteoliposomes containing Fluozin-1 Citation[23], revealing that Zn2 + uptake is dependent upon the concentration of ZitB incorporated into the proteoliposomes (a) and that Ni2 + and Cd2 + ions are similarly transported (b). Copper uptake can not be measured directly in this assay and so is shown as a competitive assay. Addition of Cd2 + and Cu2 + solution to Fluozin-1 loaded proteoliposomes resulted in a decreased fluorescence signal compared to the addition of Cd2 + alone, indicating that uptake of Cd2 + ions is inhibited (c) and supporting the proposal that Cu2 + is also a transported substrate of ZitB. These uptake results support the ssNMR data indicating that these metals are substrates for ZitB under the conditions tested, and thus potentially under in vivo conditions.
Figure 4. Transport of metal ions by reconstituted ZitB. Changes in Fluozin-1 fluorescence on metal ion addition (arrow) to proteoliposomes compared with control liposomes. ΔF/ΔFmax is relative fluorescence change normalized to maximum fluorescence on proteoliposome dissolution. (a) Zn2 + transport into proteoliposomes at 1:325 (w/w) and 1:81 (w/w) ratio of ZitB to lipid. For (b) and (c) a 1:81 ratio was used. (b) Ni2 + and Cd2 + transport into proteoliposomes (c) competition assay showing that Cu2 + ions inhibit the transport of Cd2 + ions.
We have shown how cadmium-113 CP-MAS NMR can be used to probe the direct binding of metal-ions by the E. coli zinc exporter protein ZitB expressed in native membranes. The method can be used to screen for the binding of alternative substrate ions which are shown to be transportable substrates by uptake assays. Having demonstrated the value of this approach with ZitB we anticipate that the methodology should be applicable to a wide range of divalent-cation binding transporters that belong to different transporter families, including the other members of the zinc efflux family (SLC30), the zinc/iron/manganese permease family (SLC39), the proton coupled metal-ion (Mn2 + /Fe2 + ) transporter family (SLC11), the nickel/cobalt transporter (NiCoT) family, the low affinity cation transporter (LACT) family and the Ca2 + cation antiporter (CaCA) family. In addition, other membrane proteins with affinities for divalent metal-ions may also be amenable to analysis by 113Cd CP-MAS ssNMR. This ssNMR approach could also be used to probe protein-metal interactions directly in dipolar recoupling experiments and to study the role of functionally-important residues in substrate binding by mutagenesis experiments, or by examining naturally occurring disease-associated mutations. For example, the W325R mutation recently identified in the human pancreatic beta cell vesicular zinc transporter SLC30A8 has been associated with an increased propensity of carriers to develop type 2 diabetes mellitus (Sladek et al. 2007) Citation[7].
Acknowledgements
The research was supported by the Biotechnology and Biological Sciences Research Council [grant numbers BBS/B/14418 (MPSI Consortium), 24/17940]; Invitrogen Corporation; the University of Leeds; and the Islamic Development Bank (PhD studentship to MR). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Maret W. Zinc biochemistry, physiology, and homeostasis – recent insights and current trends. Biometals 2001; 14: 187–190
- Chao Y, Fu D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J Biol Chem 2004; 279: 12043–12050
- Hantke K. Bacterial zinc transporters and regulators. Biometals 2001; 14: 239–249
- Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals 2001; 14: 251–270
- Paulsen IT, Saier MH. A novel family of ubiquitous heavy metal ion transport proteins. J Memb Biol 156:99–103.
- Palmiter RD, Huang LP. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Archiv 2004; 447: 744–751
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P., Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007; 445: 881–885
- Haney CJ, Grass G, Franke S, Rensing C. New developments in the understanding of the cation diffusion facilitator family. J Ind Microbiol Biotechnol 2005; 32: 215–226
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutrition 2004; 24: 151–172
- Lu M, Fu D. Structure of the zinc transporter YiiP. Science 2007; 317: 1746–1748
- Wei YN, Fu D. Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J Biol Chem 2006; 281: 23492–23502
- Rahman M, Ismat F, McPherson MJ, Baldwin SA. Topology-informed strategies for the overexpression and purification of membrane proteins. Mol Memb Biol 2007; 24: 407–416
- Ward A, Sanderson NM, O'Reilly J, Rutherford NG, Poolman B, Henderson PJF. The amplified expression, identification, purification, assay and properties of hisitidine-tagged bacterial membrane transport proteins. Membrane transport – a practical approach, SA Baldwin. Oxford University Press, Oxford 2000; 141–166
- Ellis PD. Cd-113 magnetic-resonance spectroscopy. Science 1983; 221: 1141–1146
- Marchetti PS, Ellis PD, Bryant RG. Cd-113 shielding tensors in cadmium-substituted metalloproteins. J Am Chem Soc 1985; 107: 8191–81916
- Kidambi SS, Lee DK, Ramamoorthy A. Interaction of Cd and Zn with biologically important ligands characterized using solid-state NMR and ab initio calculations. Inorg Chem 2003; 42: 3142–3151
- Spooner PJR, O'Reilly WJ, Homans SW, Rutherford NG, Henderson PJF, Watts A. Weak substrate binding to transport proteins studied by NMR. Biophys J 1998; 75: 2794–2800
- Spooner PJR, Rutherford NG, Watts A, Henderson PJF. NMR observation of substrate in the binding-site of an active sugar-H+ symport protein in native membranes. Proc Natl Acad Sci USA 1994; 91: 3877–3881
- Patching SG, Brough AR, Herbert RB, Rajakarier JA, Henderson PJF, Middleton DA. Substrate affinities for membrane transport proteins determined by C-13 cross-polarization magic-angle spinning nuclear magnetic resonance spectroscopy. J Am Chem Soc 2004; 126: 3072–3080
- Xie H, Patching SG, Gallagher MP, Litherland GJ, Brough AR, Venter H, Yao SYM, Ng AML, Young JD, Herbert RB, Henderson PJF, Baldwin SA. Purification and properties of the Escherichia coli nucleoside transporter NupG, a paradigm for a major facilitator transporter sub-family. Mol Memb Biol 2004; 21: 323–336
- Lee SM, Grass G, Haney CJ, Fan B, Rosen BP, Anton A, Nies DH, Rensing C. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol Lett 2002; 215: 273–278
- Anton A, Weltrowski A, Haney CJ, Franke S, Grass G, Rensing C, Nies DH. Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans CH34 and Escherichia coli. J Bacteriol 2004; 186: 7499–7507
- Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium 2002; 31: 245–251