Abstract
The toxic metalloid arsenic is an abundant element and most organisms possess transport systems involved in its detoxification. One such family of arsenite transporters, the ACR3 family, is widespread in fungi and bacteria. To gain a better understanding of the molecular mechanism of arsenic transport, we report here the expression and characterization of a family member, So_ACR3, from the bacterium Shewanella oneidensis MR-1. Surprisingly, expression of this transporter in the arsenic-hypersensitive Escherichia coli strain AW3110 conferred resistance to arsenate, but not to arsenite. Purification of a C-terminally His-tagged form of the protein allowed the binding of putative permeants to be directly tested: arsenate but not arsenite quenched its intrinsic fluorescence in a concentration-dependent fashion. Fourier transform infrared spectroscopy showed that the purified protein was predominantly α-helical. A mutant bearing a single cysteine residue at position 3 retained the ability to confer arsenate resistance, and was accessible to membrane impermeant thiol reagents in intact cells. In conjunction with successful C-terminal tagging with oligohistidine, this finding is consistent with the experimentally-determined topology of the homologous human apical sodium-dependent bile acid transporter, namely 7 transmembrane helices and a periplasmic N-terminus, although the presence of additional transmembrane segments cannot be excluded. Mutation to alanine of the conserved residue proline 190, in the fourth putative transmembrane region, abrogated the ability of the transporter to confer arsenic resistance, but did not prevent arsenate binding. An apparently increased thermal stability is consistent with the mutant being unable to undergo the conformational transitions required for permeant translocation.
Introduction
Arsenic is one of the most prevalent toxic metalloids in the environment. In its water-soluble inorganic forms, it occurs in two oxidation states, trivalent arsenite [As(III)] which exists primarily as the neutral compound As(OH)3 at neutral pH, and pentavalent arsenate [As(V)], which exists primarily as H2AsO4−. The latter exerts its toxicity by acting as a non-functional phosphate analogue that disrupts metabolic reactions involving phosphorylation. Arsenite, which is considerably more toxic, interferes with protein function by binding to protein cysteine and histidine side-chains. Consistent with the toxicity of this element, most living organisms have evolved systems for arsenic detoxification Citation[1]. Arsenate and arsenite typically enter cells via phosphate transporters or channels of the Major Intrinsic Protein family respectively Citation[1], Citation[2]. Arsenate is then reduced to arsenite by arsenate reductase, following which arsenite is either sequestered in intracellular compartments or extruded from the cell Citation[1].
In bacteria, arsenite extrusion is catalyzed by members of at least two families of transporters. The best understood is the Arsenite-Antimonite (ArsB) Efflux Family, in which efflux of both arsenite and antimonite is driven either by the electrochemical gradient of proteins or, when the ArsB subunit is coupled to ArsA, by ATP hydrolysis Citation[3]. Members of a second, unrelated family, the Arsenical Resistance-3 (ACR3) family, show similarity to the arsenic compounds resistance (ACR3) transporter of the yeast Saccharomyces cerevisiae, which extrudes arsenite (but not antimonite) in a proton motive force-driven manner Citation[3]. Recently, this transporter family has been shown to be evolutionarily related to the Bile Acid:Na+ Symporter (BASS) and Riboflavin Transporter (RFT) families, these three groups of proteins constituting the BART superfamily Citation[4]. The ACR3 family has been much less well studied than the ArsB family, but its importance is evident from the fact that release 17 of the InterPro database Citation[5] records more than 400 family members (accession IPR004706) from eubacteria, cyanobacteria, archaea, plants and fungi. Moreover, recent studies have revealed that its members are very widespread amongst arsenic-resistant soil bacteria Citation[6].
Given the importance of arsenic resistance, and the relationship between the ACR3 family and the physiologically important bile acid transporters of mammals, we selected an ACR3 family member from Shewanella oneidensis MR-1 as a model for structure/function investigations. In the present study, we describe the over-expression and purification of the protein. The availability of the purified protein enabled investigation of its topology and secondary structure, together with the effects of mutating a conserved residue as a potential means of conformationally stabilising the protein for crystallization. Surprisingly, despite the presence of this gene product, the Shewanella strain exhibits an arsenite sensitivity similar to that of the arsenic-hypersensitive Escherichia coli strain AW3110, which lacks the chromosomally encoded arsenical resistance (ars) operon Citation[6], Citation[7]. In contrast, S. oneidensis MR-1 exhibits a somewhat greater resistance to arsenate Citation[6]. This observation prompted us also to investigate whether the Shewanella ACR3 protein is involved in this phenomenon.
Materials and methods
Materials
Escherichia coli strain AW3110 was obtained from the Coli Genetic Stock Center (CGSC), Yale University, New Haven, USA. Genomic DNA of S. oneidensis MR-1 was obtained from ATCC-LGC Standards, Teddington, UK. Oregon Green® 488 maleimide (OGM) was obtained from Molecular Probes (Invitrogen) and 2-trimethylammonium-ethyl-methanethiosulfonate bromide (MTSET) was purchased from Toronto Research Chemicals Inc.
Construction and mutagenesis of an expression vector encoding So_ACR3
The open reading frame encoding S. oneidensis ACR3 (hereafter designated So_ACR3; GenBank accession AAN53615.1) was amplified by PCR from S. oneidensis MR-1 genomic DNA using the primers MPSIL0156F1 + MPSIL0156R1 (Supplementary , online version only) and inserted as an EcoR1/PstI fragment in place of the corresponding E. coli UraA ORF in the construct pTTQ18-RGSH6-UraA Citation[8]. The resultant plasmid, designated pMPSIL0156A, encoded a form of So_ACR3 in which residues G2 and I3 had been mutated to N and S respectively to introduce the EcoRI site, and which bore a C-terminal SAGGRGSH6 tag. Site-directed mutagenesis of residues C126 to A, P190 to A and S3 to C was performed by overlap extension PCR or the Quikchange (Stratagene) procedure using pMPSIL0156A, or where appropriate the C126A variant of this plasmid, as a template and the primers listed in Supplementary (online version only). The entire So_ACR3 coding region was subjected to DNA sequencing in each case to confirm the presence of the desired mutations and the absence of any other changes.
Supplementary Table 1. Oligonucleotides used in this study.
Arsenic sensitivity assays
E. coli strain AW3110 cells transformed with pMPSIL0156A encoding wild-type So_ACR3, with MPSIL0156A-derived plasmids encoding mutant forms of the protein, or the non-recombinant parental vector pTTQ18-RGSH6 were cultured overnight at 37°C in Luria Bertani (LB) medium. They were then washed in MOPS medium Citation[9] before dilution to an attenuance at 600 nm (D600nm) of 0.1 in MOPS medium supplemented with 100 µg/ml ampicillin, 0.25 mM isopropyl-β-D-thiogalactoside (IPTG) and the indicated concentrations of As(III) (potassium arsenite) or As(V) (potassium arsenate). The cells were incubated at 20°C with shaking (250 rpm) for another 72 h. Growth was estimated from measurements of the D600nm at the end of this period. Samples (20 µg total cellular protein) were also taken for analysis by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 4–12% NuPage polyacrylamide gels (Invitrogen). Western blots of these gels were stained by incubating with a horseradish peroxidase-labelled monoclonal antibody against oligohistidine tags (R&D Systems clone AD1.1.10) followed by SuperSignal® West Pico chemiluminescent substrate (Perbio Science UK Ltd). Signals were detected and quantified using a GeneGnome Detection system and GeneTools software respectively (Syngene Bio Imaging).
Expression and purification of So_ACR3
For biochemical investigations of So_ACR3 and its mutants, E. coli strain BL21-Gold (Stratagene) cells harbouring pMPSIL0156A or pMPSIL0156A:P190A were used as a source of membranes. Auto-induction of expression Citation[10] was achieved by culturing the cells (8×500 ml in 2 l flasks) with shaking (250 rpm) at 37°C for 24 h in medium containing 15 mM Tris-HCl, pH 7.2 plus 3.2% (w/v) yeast extract, 0.8% (w/v) tryptone, 0.05% (w/v) glucose, 0.05% (w/v) lactose, 0.5% (w/v) glycerol, and 0.1 mg/ml carbenicillin. These conditions had been found to be optimal for protein over-expression, using the methods detailed in the accompanying paper in this special issue of Molecular Membrane Biology (Deacon et al.) Citation[11]. All subsequent operations were conducted at 4°C. For cell lysis, pellets were resuspended in 20 mM Tris-HCl, pH 7.5 at 4°C, containing 0.5 mM EDTA and then passed twice through a cell disrupter (Constant Systems Ltd) at 30 kpsi. Unbroken cells and other debris were removed by centrifugation at 10,000 gav for 45 min. The supernatant was then centrifuged for 2 h at 100,000 gav and the pellet was washed twice in the same manner with 20 mM Tris-HCl, 300 mM NaCl, pH 8.5 at 4°C. The resultant mixed inner and outer bacterial membranes were resuspended in 20 mM Tris-HCl, pH 8.5 to give a protein concentration of 30 mg/ml, measured using the bicinchoninic acid (BCA) assay (Perbio Science UK Ltd) with bovine serum albumin as a standard.
For purification of So_ACR3, mixed membranes (50 mg, at a final concentration of 2.5 mg/ml) were solubilized by gentle mixing for 1 h in buffer A (20 mM Tris-HCl, pH 8.5 at 4°C) plus 20% (w/v) glycerol), containing 150 mM NaCl, 5 mM imidazole and 1% (w/v) n-dodecyl-β,D-maltopyranoside (DDM, Melford Laboratories Ltd., Ipswich, UK). Following centrifugation at 40,000 gav for 45 min the supernatant was incubated for 2 h with 1 ml of Ni2 + -nitrilotriacetate (Ni-NTA) agarose beads (Qiagen Ltd., UK) that had been pre-equilibrated with buffer A containing 150 mM NaCl, 5 mM imidazole and 0.05% (w/v) DDM. The protein-bound beads were then washed in batch six times with 20 resin volumes of buffer A containing 300 mM NaCl, 20 mM imidazole and 0.05% DDM. Proteins were eluted by incubating the beads in batch with three resin volumes of buffer A containing 200 mM imidazole and 0.05% DDM, 200 mM imidazole. Finally, the imidazole was removed by dialysis against buffer A. The purified protein was characterized by SDS-PAGE as detailed above, followed by staining with Imperial™ Protein Stain (Pierce).
Spectroscopy
Measurements of protein intrinsic fluorescence were made in 96-well black-walled microplates (Greiner) using a FLUOstar OPTIMA microplate reader (BMG LABTECH) with an excitation wavelength of 280 nm and a 340±10 nm emission filter. Each well contained 5 µg protein in 20 mM Hepes, 100 mM NaCl, pH 7.4. The desired concentration of arsenite or arsenate was then added, to give a final volume of 100 µl. Fluorescence quenching by arsenate, ΔF, was calculated as F0-F, where F0 and F, respectively, represent the fluorescence intensity in the absence and presence of the given concentration of arsenate. The maximal extent of quenching, ΔFmax, was calculated as F0-Fmax, where Fmax represents the fluorescence intensity at a saturating concentration of arsenate, estimated from a plot of fluorescence intensity again arsenate concentration. Non-linear fits of the data to a hyperbola were made using the program Origin® version 7.0, and the calculated Kd values for arsenate binding are shown as mean±standard error of the estimate. For Fourier transform infrared (FTIR) spectroscopy, purified protein was concentrated to 1.0 mg/ml using a centrifugal membrane-concentrating device with a molecular weight cut-off of 50,000 (Sartorius Stedim UK Ltd). Measurements were then made using a using a Nicolet 560 FT-IR spectrometer essentially as previously described Citation[12].
Fluorescence assay of protein stability
Protein stability was measured by a modification of the method of Alexandrov et al. Citation[13], using 20 µl protein samples at a concentration of 0.1 mg/ml in 20 mM Tris-HCl buffer, pH 8.0, containing 100 mM NaCl and 0.05% DDM. Before use, OGM (10 mg/ml in dimethyl sulphoxide) was diluted 10-fold in the same buffer. The diluted OGM solution (5 µl) was added to the protein sample and thoroughly mixed. After heating for 5 min at the desired temperature in a PCR machine, 5 µl of 2-mercaptoethanol was then added to each sample to stop the reaction. The protein samples were next resolved by SDS-PAGE. After electrophoresis, the gel was rinsed briefly with distilled water. OGM fluorescence was then detected and quantified as described for western blotting, and the inflection point of the unfolding curve, which was assumed to equal the melting temperature (Tm), was determined by non-linear fitting of the data to a Boltzmann sigmoidal equation using the program Origin® version 7.0.
Site-directed fluorescent labelling
To investigate the topology of So_ACR3 the accessibility of a cysteine residue introduced at position 3 in an otherwise cysteine-less form of the protein (C126A) to the fluorescent thiol-directed probe OGM was examined using the method of Ye et al. Citation[14]. In brief, cultures (100 ml) of E. coli harbouring pMPSIL0156A:S3C/C126A in mid-log phase (OD600nm 0.6) were induced to express the protein by addition of 0.5 mM IPTG and subsequent incubation at 37°C for another 1.5 h. Intact cells were harvested by centrifugation (2500 g, 10 min) and suspended in modified Krebs-Buffer (25 mM Hepes/Tris, pH 7.4, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose), to give a D600nm value of 8. OGM was added to 5 ml of the cell suspension to give a final concentration of 40 µM. After 20 min at 25°C, the labelling reaction was quenched by addition of 10 mM 2-mercaptoethanol and the cells were washed three times with the modified Krebs-Buffer by centrifugation as above. Membranes were then prepared by the water lysis method Citation[15], for subsequent So_ACR3 purification by Ni-NTA agarose affinity chromatography, essentially as detailed above, and SDS-PAGE analysis. After the fluorescence profiles had been recorded, the protein content of each lane was evaluated by staining the gel as described above.
In parallel samples of cell suspensions, the intact cells were first incubated with or without 2 mM MTSET in the modified Krebs-Buffer at 30°C for 15 min. MTSET was then removed by washing the cells three times by centrifugation with the modified Krebs-Buffer and membranes were prepared. The latter were subsequently labelled by incubation with 40 µM OGM for 20 min at 25°C in 20 mM Tris-HCl buffer, pH 7.5, containing 50 mM NaCl. The reaction was quenched with 10 mM 2-mercaptoethanol and the membranes washed three times by centrifugation (25,000 gav) for 20 min in 20 mM Tris-HCl, pH 7.5 before So_ACR3 purification and analysis of fluorescence.
Results
Functional characterization of So_ACR3 in vivo
The amino acid sequence of So_ACR3 shows that it is a member of the ACR3 subfamily of the BART superfamily, more closely related to known arsenite transporters such as ArsB of Streptomyces sp. FR-008 Citation[16] than to bile acid transporters of the BASS subfamily (). However, it exhibits ≤ 36% sequence identity to the Streptomyces protein and other functionally-characterized arsenite transporters from Corynebacterium glutamicum Citation[17], Bacillus subtilis Citation[18] and Saccharomyces cerevisiae Citation[19]. Moreover, S. oneidensis MR-1 is reported to lack resistance to arsenite Citation[6]. It was therefore important to ascertain whether So_ACR3 was indeed an arsenite transporter. Previous functional characterizations of arsenite transporters have taken advantage of the ability of the heterologously expressed proteins to increase the resistance of the E. coli strain AW3110 to arsenic compounds (e.g. Citation[6]). This strain is hypersensitive to arsenite and arsenate because it lacks the chromosomally-encoded arsenite efflux transporter ArsB and the enzyme, ArsC, which is required to reduce arsenate to arsenite prior to efflux Citation[7]. We therefore exploited the availability of this strain to examine the functional properties of So_ACR3.
Figure 1. Relationships between So_ACR3 and other members of the ACR3 and BASS transporter families. The phylogenetic tree was constructed from the sequence alignment shown in Supplementary (online version only), using the Neighbour Joining method of Saitou and Nei [31]. The ACR3 proteins shown are representative of five subfamilies (i–v) identified by phylogenetic analysis of 334 ACR3 sequences obtained from the UniRef90 database [32].
![Figure 1. Relationships between So_ACR3 and other members of the ACR3 and BASS transporter families. The phylogenetic tree was constructed from the sequence alignment shown in Supplementary Figure 1 (online version only), using the Neighbour Joining method of Saitou and Nei [31]. The ACR3 proteins shown are representative of five subfamilies (i–v) identified by phylogenetic analysis of 334 ACR3 sequences obtained from the UniRef90 database [32].](/cms/asset/28a9ba38-527a-40fd-a323-6dfe43f6e604/imbc_a_353761_f0001_b.gif)
The growth properties of E. coli strain AW3110 transformed with pMPSIL0156A or with non-recombinant parental vector were investigated in the presence and absence of arsenite and arsenate as detailed in Materials and methods, using IPTG to induce expression of So_ACR3. As illustrated in A, IPTG-induced cells harbouring pMPSIL0156A, grown in the absence of arsenic, expressed a His-tagged protein of apparent size 30 kDa.
Figure 2. Sensitivity to arsenite and arsenate of E. coli AW3110 expressing wild-type So_ACR3, its P190A mutant or non-recombinant expression vector. Cultures were performed as detailed in Materials and methods. (A) Western blot of samples from cultures harbouring expression vectors encoding the indicated wild-type or P190A mutant forms of So_ACR3 in the presence or absence of 40 mM arsenate. Blots were stained with an antibody against oligohistidine. (B) and (C), growth of cultures, as monitored by D600nm, in the presence of the indicated concentrations of arsenite (As[III]) and arsenate (As[V]), respectively. The cultures were of bacteria expressing wild-type So_ACR3 (○), its P190A mutant (□), or harbouring non-recombinant expression vector (•).
![Figure 2. Sensitivity to arsenite and arsenate of E. coli AW3110 expressing wild-type So_ACR3, its P190A mutant or non-recombinant expression vector. Cultures were performed as detailed in Materials and methods. (A) Western blot of samples from cultures harbouring expression vectors encoding the indicated wild-type or P190A mutant forms of So_ACR3 in the presence or absence of 40 mM arsenate. Blots were stained with an antibody against oligohistidine. (B) and (C), growth of cultures, as monitored by D600nm, in the presence of the indicated concentrations of arsenite (As[III]) and arsenate (As[V]), respectively. The cultures were of bacteria expressing wild-type So_ACR3 (○), its P190A mutant (□), or harbouring non-recombinant expression vector (•).](/cms/asset/640bd6e4-5883-4530-9d5c-f8c88ac05825/imbc_a_353761_f0002_b.gif)
The more rapid migration of this protein than predicted from the size of His-tagged So_ACR3 (38,487 Da) probably reflects the hydrophobic nature of the protein. Despite this expression of So_ACR3, the corresponding E. coli AW3110 transformants exhibited the same hypersensitivity to arsenite as cells harbouring the non-recombinant vector (B). However, the cells expressing So_ACR3 exhibited a significantly greater resistance to As(V) at concentrations up to 40 mM (C). Moreover, Western blotting showed that they continued to express So_ACR3 even in the presence of 40 mM As(V), although the amount of the transporter produced per mg total cell protein was decreased (A).
Functional characterization of purified So_ACR3
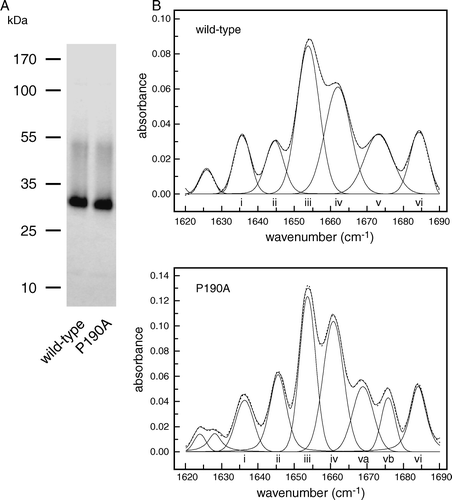
The properties of E. coli AW3110 cells expressing So_ACR3 suggested that, despite being a member of the ACR3 family, the Shewanella protein lacked the ability to transport arsenite. Instead, the increased resistance of the cells to arsenate suggested that if might function as an arsenate exporter. Because the transport characteristics of such exporters are difficult to investigate in vivo, isolation of the transporter was next attempted in order to facilitate its characterization in vitro. To this end, auto-induction was employed for the production of protein on a large scale in E. coli BL21-Gold cells harbouring pMPSIL0156A. The protein was then purified from DDM-solubilized cell membranes by affinity chromatography on Ni-NTA agarose, as detailed in Materials and methods, yielding approximately 0.2 mg protein per litre of culture. The purified protein migrated as a single major band of apparent size 30 kDa, together with a minor band of approximately 50 kDa that may correspond to a dimer (A).
Figure 3. Comparison of wild-type So_ACR3 and its P190A mutant. (A) Imperial™-stained SDS-polyacrylamide gel of the purified proteins. The mobilities of marker proteins of known molecular mass are shown on the left. (B) Amide I region of the FTIR spectra of hydrated films of the wild-type and mutant proteins and bands obtained by deconvolution (solid lines). The latter were assigned to β-sheet (i and vi), unordered structure (ii), α-helix (iii and iv) and β-turns (v, va and vb). The curves fitted using these component bands are shown as dotted lines.
Measurements of transport activity by reconstituted arsenic transporters are complicated by the expense and difficulty in obtaining radioisotopically-labelled arsenic compounds, and the complex nature of alternative assays, such as use of inductively coupled plasma mass spectrometry. However, it has long been known that changes in the environment of aromatic residues, in particular tryptophan, on ligand binding to integral membrane proteins can alter their intrinsic fluorescence. Fluorescence spectroscopy has therefore been widely used to investigate the binding of inhibitors and permeants to transport proteins (e.g. Citation[20]). So_ACR3 contains 10 Trp residues, two of which are highly conserved in the ACR3 transporter family (Supplementary ). The effects of arsenic compounds on the intrinsic fluorescence of the protein were therefore investigated as a potential means of assaying function. The fluorescence emission spectrum of So_ACR3 excited at 280 nm exhibited a λmax of between 330 and 335 nm and was unaffected by arsenite (data not shown). However, the addition of increasing concentrations of arsenate was found to quench the fluorescence of So_ACR3 up to an extent of approximately 10% at 60 mM arsenate ().
Figure 4. Concentration-dependence of the quenching by arsenate of the intrinsic fluorescence of wild-type So_ACR3 (○) and its P190A mutant (•). The fluorescence of samples of the purified proteins was measured in the presence of the indicated arsenate concentrations. The curves represent non-linear fits to the equation of a hyperbola, as detailed in the text.
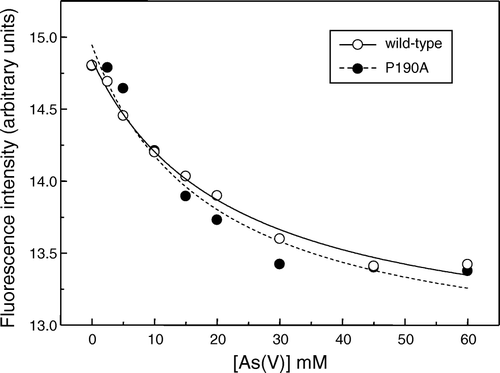
The decrease in fluorescence could be fitted to the hyperbolic relationship ΔF = ΔFmax[arsenate]/(Kd+[arsenate]), yielding an apparent dissociation constant (Kd) for arsenate binding of 22.0±4.1 mM. There was no significant effect of arsenate on the wavelength of maximal fluorescence emission (data not shown).
Secondary structure and topology of So_ACR3
In previous investigations of the membrane topologies and structure/function relationships of BART superfamily transporters the assumption has been made that these proteins are α-helical. The availability of purified So_ACR3 allowed us for the first time to address this assumption directly. FTIR spectroscopic analysis showed that the protein was indeed predominantly α-helical: the amide I region of the spectrum could be reproduced well by a fit of six major components, dominated by peaks at 1654 and 1662 cm-1 indicative of α-helix (B; Supplementary , online version only). It is therefore likely that at least some of the hydrophobic segments identified in the So_ACR3 sequence by transmembrane helix (TM) prediction programs, including as TMHMM2 Citation[21], do represent helices. The latter method identified ten such regions in the protein, and predicted that both termini are cytoplasmic (data not shown). Partial experimental support for such a topology has recently been obtained for the distantly related (31% sequence identity) transporter ArsB (alternatively designated Acr3) from B. subtilis, using translational fusions with alkaline phosphatase and green fluorescent protein Citation[22]. However, a caveat to such an approach is that the majority of fusion proteins lack function, and so the results cannot be unambiguously concluded to reflect the native topology. Moreover, epitope insertion scanning mutagenesis, in which transporter function was retained, has provided strong support for a 7 TM topology with an extracellular N-terminus in the case of a related BART superfamily member, the human apical sodium-dependent bile acid transporter (hASBT, hSLC10A2) Citation[23].
Supplementary Table 2. Analysis of the secondary structure of wild-type and mutant So_ACR3 by FTIR spectroscopy.
To ascertain whether the N-terminus of So_ACR3 is located on the cytoplasmic or periplasmic side of the membrane, the substituted-cysteine accessibility method was employed Citation[14]. This approach had not been possible in the case of ArsB/Acr3 from B. subtilis, because a suitable cysteine-less template could not be generated Citation[22]. In the present study a double mutant of the oligohistidine-tagged form of the So_ACR3 protein was constructed, in which the single endogenous cysteine at position 126 was replaced by alanine, and serine at position 3 was replaced by cysteine. E. coli strain AW3310 cells induced to express the double mutant exhibited the same resistance to 40 mM arsenate as cells expressing the wild-type protein (data not shown). It is therefore likely that the function, and thus topology, of the protein were retained in the mutant. The accessibility of the cysteine residue at position 3 to thiol reagents in intact cells and unsealed membranes was then assessed, as detailed in Materials and methods. shows that the cysteine residue could be labelled with the membrane-impermeant reagent OGM not only in membrane preparations but also in intact cells expressing the mutant.
Figure 5. Accessibility to membrane-impermeant thiol reagents of a cysteine residue introduced at position 3 in So_ACR3. Intact cells expressing a C126A/S3C double mutant of So_ACR3 were incubated directly with OGM (lane 1), or incubated with (lane 3) or without (lane 2) MTSET before isolation of membranes and subsequent incubation with OGM as detailed in Materials and Methods. Following affinity-purification of So_ACR3, samples were subjected to SDS-PAGE followed by detection of protein fluorescence (panel on the right) and subsequent staining for protein (panel on the left).
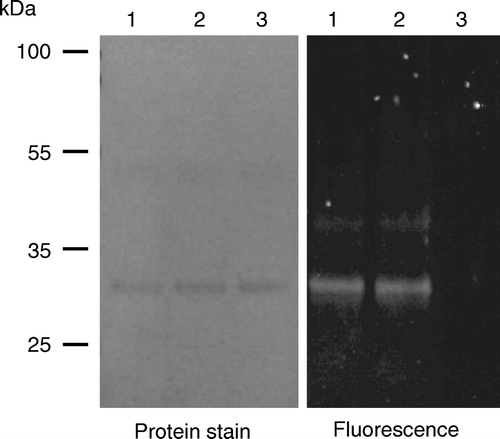
Confirmation of the periplasmic exposure of the residue, and thus of the N-terminal region of the protein, was provided by the observation that prior exposure of intact cells to the non-fluorescent, membrane-impermeant, cysteine-specific reagent MTSET blocked subsequent labelling of the residue by OGM in membrane preparations (, fluorescence panel, lane 3).
Identification of a functionally-important proline residue in So_ACR3
A potential hindrance to the crystallization of membrane transport proteins for structural investigation is their conformational flexibility. It is noteworthy that in several cases, such as that of the lactose transporter LacY of E. coli, crystals of membrane transporters have first successfully been obtained using conformationally-constrained mutants that lack transport activity but retain the ability to bind ligand Citation[24]. In the present study we therefore sought to identify such a mutant of So_ACR3. To this end, proline 190 was mutated to alanine. This residue was chosen because it occupies a highly conserved position in a putative transmembrane region (corresponding to TM4 in human ASBT Citation[23]) where proline is found not only in members of the ACR3 family but also in the BASS family of transporters (Supplementary , online version only). Moreover, proline residues in TM segments have long been proposed to play roles in transporter function by introducing helix distortions and/or providing flexibility Citation[25], and mutation of some TM prolines in proteins such as the sarcoplasmic reticulum Ca2 + -ATPase has been reported to result in profound changes in transport function Citation[26].
Consistent with the proposed functional importance of the proline residue, although the P190A mutant of So_ACR3 could successfully be expressed in E. coli AW3110 cells in the absence of arsenate (A), unlike the wild-type protein it did not confer any resistance to arsenate (C). To investigate whether this lack of apparent functional activity was associated with misfolding of the protein, the mutant transporter was purified (A) and its secondary structure investigated by FTIR spectroscopy. Some differences in spectrum from that of the wild-type protein were observed, in particular in the region attributed to β-turns (B), but the predicted α-helical content of the mutant protein was similar to that for the wild-type protein (Supplementary , online version only). Moreover, arsenate was found to cause concentration-dependent quenching of the intrinsic fluorescence of the protein, suggesting that the mutant retained the ability to bind this ligand (). The calculated apparent Kd for arsenate binding, 18.8±7.2 mM, was not significantly different from that of the wild- type protein.
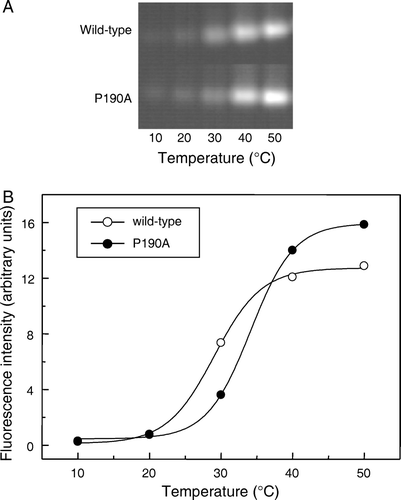
Finally, the thermal stabilities of the mutant and wild-type proteins were compared by monitoring the effect of heating on the reactivity of the single endogenous cysteine residue, C126, towards OGM, using a modification of the method of Alexandrov et al. Citation[13]. In both cases increased reactivity with temperature was observed, as quantified by incorporation of fluorescent label, consistent with protein unfolding (). The calculated apparent Tm value of the mutant, 34°C, was similar to, if slightly higher than, that of the wild type protein (29°C).
Figure 6. Effect of temperature on the reactivity of wild-type and mutant So_ACR3 towards OGM. Following incubation of the purified proteins with OGM at the indicated temperatures, samples were resolved by SDS-PAGE and fluorescence visualized (A). Panel B shows the incorporation of fluorescent label, quantified as described in Materials and methods, into wild-type So_ACR3 (○) and its P190A mutant (•). The curves represent non-linear fits of the data to a Boltzmann sigmoidal equation, performed in order to estimate apparent Tm values.
Discussion
Although So_ACR3 is a member of the Arsenical Resistance-3 transporter family, unlike most other functionally-characterized members, the protein did not confer resistance to arsenite upon the arsenic hypersensitive strain of E. coli AW3110. However, it did confer modest resistance to arsenate, and the quenching of its intrinsic fluorescence by arsenate but not arsenite is consistent with a putative role as an arsenate transporter. The relatively low apparent affinity of the transporter for arsenate probably reflects measurement of binding under non-energized conditions, as has been observed for several other bacterial proton-dependent transporters Citation[12]. Phylogenetic analysis of currently known ACR3 family members showed that these can be grouped into five subfamilies. The members shown for three of the subfamilies illustrated in , (i) Citation[19], (ii) Citation[18] and (iii) Citation[16], Citation[17], have all been demonstrated to exhibit arsenite transport activity, while the function of subfamily (iv) members, represented by a putative transporter from Trichodesmium erythraeum, remains unknown. So_ACR3 belongs to a fifth subfamily (v), most closely related to subfamily (iv). Interestingly, another member of this subfamily, Acr3 from the highly metal-resistant bacterium Ochrobactrum tritici SMII24, has recently been shown to confer both arsenate and arsenite resistance on E. coli AW3110 Citation[27]. The availability of the purified protein from S. oneidensis should in future allow the direct testing of the hypothesis that some members of subfamily (v) of the ACR3 transporters contribute to arsenic resistance by extrusion of arsenate, in addition to/instead of the extrusion of arsenite generated intracellularly by arsenate reduction.
The generation of an apparently functional, cysteine-less form of So_ACR3 also opens up the way to a detailed analysis of transmembrane topology in this transporter family by the substituted-cysteine accessibility method. Such analyses will be required to resolve the discrepancies between published investigations using other methods. The seven TM helices identified by epitope insertion mutagenesis in the human BASS family transporter hASBT Citation[23], illustrated in Supplementary (online version only), align reasonably well with seven of the 10 TM helices predicted by bioinformatic analysis of the sequence of ArsB/Acr3 from B. subtilis and from investigations of this protein using translational fusions Citation[22]. The latter study predicted the presence of an additional N-terminal helix which would be consistent with a cytoplasmic location for the protein N-terminus, in contrast to the extracellular location of the N-terminus in hASBT: this hydrophobic segment (TMA in Supplementary , online version only) is relatively well conserved in ACR3 family members but appears to be absent from hASBT and other mammalian BASS family transporters. In the preliminary study reported here, the accessibility of cysteine at position 3 of So_ACR3 to labelling in intact cells by OGM surprisingly suggests that in this protein the N-terminus may instead by located on, or accessible to labelling from, the periplasmic side of the membrane. However, additional experiments will be required to clarify the topology of this region of the protein: we have recently found that So_ACR3 can be successfully expressed bearing an N-terminal octahistidine tag (data not shown). This observation is inconsistent with a periplasmic location for this region of the protein: we have previously shown for multiple membrane proteins that incorporation of an oligohistidine tag, which is likely to bear a net positive charge, at the N- or C-terminus typically precludes expression if these termini are located on the periplasmic side of the membrane, whereas an effectively neutral tag, such as the Strep-tag II peptide, is tolerated Citation[8]. It is likely that this observation reflects the importance of the charge distribution across transmembrane helices for their correct insertion into the membrane during membrane protein synthesis Citation[8]. Cysteine accessibility studies may also clarify the nature of two additional regions (TMB and TMC in Supplementary , online version only) that are predicted to be membrane-spanning in ArsB/Acr3 from B. subtilis Citation[22] but to represent extracellular loops in the mammalian BASS family transporters. Although the BASS and ACR3 family transporters are only distantly related, the apparent conservation of these regions, in particular that corresponding to the TM2-3 loop or TMB region, respectively, suggests that they are likely to be topologically equivalent Citation[23]. Establishment of their topologies, or possible re-entrant natures, is of importance given the fact that mutagenesis studies have revealed them to play key roles in permeant recognition and translocation in the BASS family Citation[28], Citation[29].
Mutation of conserved So_ACR3 residue proline 190 to alanine was found to abrogate the ability of the protein to confer resistance to arsenate upon E. coli strain AW3110 cells, consistent with an important role for this residue in protein function. Surprisingly, mutation of the corresponding residue in hASBT, P175, to cysteine had no significant effect on the ability of that protein to transport taurocholate at physiological sodium concentrations Citation[30]. In the case of So_ACR3, apparent loss of transport activity did not result from a gross alteration in the secondary structure of the protein, and the ability to bind arsenate was retained. A possible explanation for the phenotype of the mutant may have been loss of conformational flexibility. The apparent slight increase in thermal stability of the mutant is consistent with this hypothesis. The availability of a fluorescent binding assay for arsenate should facilitate the identification of further mutations with similar phenotypes, the use of which may facilitate crystallization of the transporter.
Supplementary Figure 1. Alignment of the amino acid sequences of So_ACR3 and other members of the ACR and BASS transporter families. Sequences are identified by their UniProt accession numbers as follows: Q8EJD3, So_ACR3 from Shewanella oneidensis MR-1; A9X5I5, Acr3 from Ochrobactrum tritici; Q10ZD7, putative arsenical resistance protein from Trichodesmium erythraeum; Q1HW02, ArsB from Streptomyces sp. FR-008; Q8NCQC8, ArsB1 from Corynebacterium glutamicum; Q8NTP4, ArsB2 from C. glutamicum; P45946, ArsB/Acr3 from Bacillus subtilis; Q06598, ACR3 from Saccharomyces cerevisiae; Q8E8Y1, putative bile salt transporter from S. oneidensis MR-1; Q14973, SLC10A1/NTCP from Homo sapiens; Q12908, SLC10A2/ASBT from H. sapiens; Q3KNW5, SLC10A6/SOAT from H. sapiens. The alignment was created using the program MUSCLE [1] followed by manual adjustment where necessary. X, Y and Z respectively indicate residues that are identical in 6–8, in 9–10 and in 11–12 of the 12 sequences. The experimentally determined topology and predicted locations of seven transmembrane segments (TM1-7) in human SLC10A2 shown on the Figure are taken from the work of Banerjee and Swan [2]. The indicated locations of three additional, putative transmembrane regions in ArsB/Acr3 from Bacillus subtilis (TMA-C) are taken from the work of Altonen and Silow [3]. An asterisk (*) shows the location of So_ACR3 residue proline 190.
![Supplementary Figure 1. Alignment of the amino acid sequences of So_ACR3 and other members of the ACR and BASS transporter families. Sequences are identified by their UniProt accession numbers as follows: Q8EJD3, So_ACR3 from Shewanella oneidensis MR-1; A9X5I5, Acr3 from Ochrobactrum tritici; Q10ZD7, putative arsenical resistance protein from Trichodesmium erythraeum; Q1HW02, ArsB from Streptomyces sp. FR-008; Q8NCQC8, ArsB1 from Corynebacterium glutamicum; Q8NTP4, ArsB2 from C. glutamicum; P45946, ArsB/Acr3 from Bacillus subtilis; Q06598, ACR3 from Saccharomyces cerevisiae; Q8E8Y1, putative bile salt transporter from S. oneidensis MR-1; Q14973, SLC10A1/NTCP from Homo sapiens; Q12908, SLC10A2/ASBT from H. sapiens; Q3KNW5, SLC10A6/SOAT from H. sapiens. The alignment was created using the program MUSCLE [1] followed by manual adjustment where necessary. X, Y and Z respectively indicate residues that are identical in 6–8, in 9–10 and in 11–12 of the 12 sequences. The experimentally determined topology and predicted locations of seven transmembrane segments (TM1-7) in human SLC10A2 shown on the Figure are taken from the work of Banerjee and Swan [2]. The indicated locations of three additional, putative transmembrane regions in ArsB/Acr3 from Bacillus subtilis (TMA-C) are taken from the work of Altonen and Silow [3]. An asterisk (*) shows the location of So_ACR3 residue proline 190.](/cms/asset/00343646-474b-49bf-8e10-a37051a1890a/imbc_a_353761_f0007_b.jpg)
Acknowledgements
This work was supported principally by the Biotechnology and Biological Sciences Research Council [grant numbers BBS/B/14418 (Membrane Protein Structure Initiative), 24/REI18440] and by the University of Leeds. We thank Jocelyn M. Baldwin for assistance with bioinformatic analyses. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rosen BP. Biochemistry of arsenic detoxification. FEBS Lett 2002; 529: 86–92
- Bienert GP, Schussler MD, Jahn TP. Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci 2008; 33: 20–26
- Rosen BP. Families of arsenic transporters. Trends Microbiol 1999; 7: 207–212
- Mansour NM, Sawhney M, Tamang DG, Vogl C, Saier MH, Jr. The bile/arsenite/riboflavin transporter (BART) superfamily. FEBS J 2007; 274: 612–629
- Mulder NJ, Kersey P, Pruess M, Apweiler R. In silico characterization of proteins: UniProt, InterPro and Integr8. Mol Biotechnol 2008; 38: 165–177
- Achour AR, Bauda P, Billard P. Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 2007; 158: 128–137
- Carlin A, Shi W, Dey S, Rosen BP. The ars operon of Escherichia coli confers arsenical and antimonial resistance. J Bacteriol 1995; 177: 981–986
- Rahman M, Ismat F, McPherson MJ, Baldwin SA. Topology-informed strategies for the overexpression and purification of membrane proteins. Mol Membr Biol 2007; 24: 407–418
- Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol 1974; 119: 736–747
- Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 2005; 41: 207–234
- Deacon SE, Roach PCJ, Postis VLG, Wright GSA, Xia X, Phillips SEV, Knox JP, Henderson PJF, McPherson MJ, Baldwin SA. 2008. Reliable scale-up of membrane protein over-expression by bacterial auto-induction: from microwell plates to pilot scale fermentations. Mol Membr Biol 25:691–701.
- Xie H, Patching SG, Gallagher MP, Litherland GJ, Brough AR, Venter H, Yao SY, Ng AM, Young JD, Herbert RB, Henderson PJ, Baldwin SA. Purification and properties of the Escherichia coli nucleoside transporter NupG, a paradigm for a major facilitator transporter sub-family. Mol Membr Biol 2004; 21: 323–336
- Alexandrov AI, Mileni M, Chien EY, Hanson MA, Stevens RC. Microscale fluorescent thermal stability assay for membrane proteins. Structure 2008; 16: 351–359
- Ye L, Jia Z, Jung T, Maloney PC. Topology of OxlT, the Oxalate Transporter of Oxalobacter formigenes, Determined by Site-Directed Fluorescence Labeling. J Bacteriol 2001; 183: 2490–2496
- Ward A, Sanderson NM, O'Reilly J, Rutherford NG, Poolman B, Henderson PJF. The amplified expression, identification, purification, assay and properties of histidine-tagged bacterial membrane transport proteins. Membrane transport – a practical approach, SA Baldwin. Oxford University Press, Oxford 2000; 141–166
- Wang L, Chen S, Xiao X, Huang X, You D, Zhou X, Deng Z. arsRBOCT arsenic resistance system encoded by linear plasmid pHZ227 in Streptomyces sp. strain FR-008. Appl Environ Microbiol 2006; 72: 3738–3742
- Ordonez E, Letek M, Valbuena N, Gil JA, Mateos LM. Analysis of genes involved in arsenic resistance in Corynebacterium glutamicum ATCC 13032. Appl Environ Microbiol 2005; 71: 6206–6215
- Sato T, Kobayashi Y. The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J Bacteriol 1998; 180: 1655–1661
- Wysocki R, Bobrowicz P, Ulaszewski S. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J Biol Chem 1997; 272: 30061–30066
- Martin GEM, Rutherford NG, Henderson PJF, Walmsley AR. Kinetics and thermodynamics of the binding of forskolin to the galactose-H+ transport protein, GalP, of Escherichia coli. Biochem J 1995; 308: 261–268
- Cuthbertson JM, Doyle DA, Sansom MS. Transmembrane helix prediction: a comparative evaluation and analysis. Protein Eng Des Sel 2005; 18: 295–308
- Aaltonen EK, Silow M. Transmembrane topology of the Acr3 family arsenite transporter from Bacillus subtilis. Biochim Biophys Acta 2007; 1778: 963–973
- Banerjee A, Swaan PW. Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry 2006; 45: 943–953
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science 2003; 301: 610–615
- Cordes FS, Bright JN, Sansom MS. Proline-induced distortions of transmembrane helices. J Mol Biol 2002; 323: 951–960
- Vilsen B, Andersen JP, Clarke DM, MacLennan DH. Functional consequences of proline mutations in the cytoplasmic and transmembrane sectors of the Ca2 + -ATPase of sarcoplasmic reticulum. J Biol Chem 1989; 264: 21024–21030
- Branco R, Chung AP, Morais PV. Sequencing and expression of two arsenic resistance operons with different functions in the highly arsenic-resistant strain Ochrobactrum tritici SCII24T. BMC Microbiol 2008; 8: 95
- Hussainzada N, Claro da ST, Zhang EY, Swaan PW. Conserved aspartic acid residues lining extracellular loop I of sodium-coupled bile acid transporter ASBT interact with Na+ and 7α-OH moieties on the ligand cholestane skeleton. J Biol Chem 2008; 283: 20653–20663
- Banerjee A, Hussainzada N, Khandelwal A, Swaan PW. Electrostatic and potential cation-π forces may guide the interaction of extracellular loop III with Na+ and bile acids for human apical Na+-dependent bile acid transporter. Biochem J 2008; 410: 391–400
- Khantwal CM, Swaan PW. Cytosolic half of transmembrane Domain IV of the human bile acid transporter hASBT (SLC10A2) forms part of the substrate translocation pathway. Biochemistry 2008; 47: 3606–3614
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987; 4: 406–425
- The UniProt Consortium. 2008. The universal protein resource (UniProt). Nucleic Acids Res 36:D190–195.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004; 32: 1792–1797
- Banerjee A, Swaan PW. Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry 2006; 45: 943–953
- Aaltonen EK, Silow M. Transmembrane topology of the Acr3 family arsenite transporter from Bacillus subtilis. Biochim Biophys Acta 2007; 1778: 963–973
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004; 32: 1792–1797
- Banerjee A, Swaan PW. Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry 2006; 45: 943–953
- Aaltonen EK, Silow M. Transmembrane topology of the Acr3 family arsenite transporter from Bacillus subtilis. Biochim Biophys Acta 2007; 1778: 963–973
