Abstract
As a transmembrane protein family, glycerophosphodiester phosphodiesterase (GDPD/GDE) catalyzes the hydrolysis of deacylated glycerophospholipids to glycerol phosphate and alcohol. To date, seven mammalian GDEs have been virtually cloned or predicted by bioinformatics analysis, however, GDE4 has not been molecular isolated and characterized in mammal. Here we report molecular cloning of human GDE4 encoding cDNA sequence, which is 945 base pairs long encoding a 314-amino acid protein with 2 transmembrane regions and a GDE motif. The human GDE1 gene is located on chromosome 19q22 and contains ten exons and nine introns. A molecular 3-D model provides the first structural information of human GDE4 and suggests a triose-phosphate-isomerase barrel core as typically found in bacterial GDPDs. Furthermore, a model of the putative catalytic residues highlights that the individual core residues Glu72, Asp74, and His87 are crucial to maintaining GDE4 catalytic activity. Western blotting shows that human GDE4 is a 36 kDa protein. Subcellular localization of GDE4 tagged with enhanced green fluorescence protein is in the cytoplasm, especially accumulated in the perinuclear region and the cell periphery. Moreover, over-expression of GDE4 did not induce neurite formation or change cell morphology. These results indicate GDE4 protein is a member of the GDE family and suggest it may play different roles from other members of GDE family.
Introduction
Phospholipid is the most structure component of lipid in cell membrane and proper phospholipid composition at defined membrane locations contributes to vesicle formation, trafficking and function, protein folding and maturation, and lipid signaling. Phospholipid biosynthesis and its regulation are quite well understood but much less is known about their intracellular trafficking and degradation, as well as the molecular mechanisms that coordinate these processes toward phospholipid homeostasis. Identification of the components involved in such complex cellular function and deciphering their roles are necessary steps to fully understand membrane structure and function as well as the molecular basis of many pathophysiological situations where phospholipid homeostasis is altered.
The phospholipase B-mediated production of glycerophosphodiesters is a common, but understudied aspect of phospholipid turnover. Glycerophosphodiester phosphodiesterase (GDPD/GDE, EC3.1.4.46) is a well-characterized enzyme of glycerol metabolism, which catalyzes the hydrolysis of deacylated glycerophospholipids to glycerol phosphate and alcohol, thereby maintaining homeostasis of glycerol phosphate concentration as part of the process of phospholipid remodelling and synthesis. GDE belongs to a family of proteins that have many homologues in prokaryotes and eukaryotes. GDE belongs to a family of proteins that have many homologues in prokaryotes and eukaryotes Citation[1]. Escherichia coli expresses two GDEs with different subcellular localization, the periplasmic GLPQ protein and the cytosolic UGPQ protein Citation[2–4]. Based upon sequence similarity to the E. coli GLPQ gene, GDE encoding genes have been identified in Staphylococcus aureus Citation[5], Treponema pallidium Citation[6], and Borrelia hermsii Citation[7], among others. In these prokaryotic organisms, the enzyme is associated with the outer membrane and may be involved in pathogenesis and host immunity. In yeast, there are only two open reading frames (ORFs) containing the canonical GDE domain, YPL110c and YPL206c ORFs. YPL110c is involved in intracellular glycerophosphocholine recycling to phosphatidylcholine and suggested to play a role in membrane recycling Citation[8], Citation[9]. However, YPL206c controls the amount of phosphatidylglyerol via phospholipase C-degradation mechanism Citation[10].
In mammal, as a partner identified to interact with the regulator of G protein signaling 16 (RGS16), human MIR16 shared significant sequence homology with bacterial glycerophosphodiester phosphodiesterase and then was renamed GDE1 Citation[11], Citation[12]. With the enzymatic activity regulated by G protein signaling, GDE1 plays an important role in linking phosphoinositide metabolism with G protein signal transduction and catalyzes anandamide biosynthesis from glycerophophspho-N-arachidonoylethanolamine precursor Citation[12], Citation[13]. Sequence similarity searches with GDE1 revealed four related human GDE proteins (GDE2–5) Citation[12]. GDE3 was found to specifically express at the stage of matrix maturation during osteoblast differentiation, which was involved in actin cytoskeleton modulation and promoted osteoblast differentiation Citation[14]. Then mouse GDE2 and GDE6 were isolated and their tissue distributions were characterized Citation[15]. Recently, mouse GDE2 was demonstrated to play an important role for growing neuritis and osmotically regulate the osmoprotective organic osmolyte glycerophosphocholine Citation[16], Citation[17]. To date, seven mammalian GDEs have been virtually cloned based on bioinformatics analysis Citation[18].
Characterization of mammalian GDEs is very important in order to understand complex mechanisms controlling phospholipid metabolism and to find pharmacologically significant molecules Citation[18], Citation[19]. Although previous studies indicated that mammalian GDEs are involved in numerous physiological functions, such as signal transduction, osteoblast differentiation, cytoskeletal regulation and neuron differentiation, other GDEs homologs are likely to play distinct physiological roles and need to be identified and characterized. In the present study, human glycerophosphodiester phosphodiesterase 4 (hGDE4) was first cloned and characterized as a novel member of GDE family as well as a molecular 3-D model was constructed. Then hGDE4 was expressed tagged with enhanced green fluorescence protein (EGFP) and its subcellular distribution was observed. In addition, effect of over-expression of hGDE4 on cell morphology and neurite formation was investigated as a transmembrane protein.
Materials and methods
Materials
Plasmid pEGFP-N3 was purchased from Clontech (Palo Alto, CA, USA). Human embryo kidney HEK293 cells and mouse neuroblastma N2a cells were purchased from Cell Center of Chinese Academy of Medical sciences (Beijing, China). Trizol and SuperScriptTM III First-Strand Synthesis System for RT-PCR were purchased from Invitrogen (Groningen, The Netherlands). DEPC was obtained from Sigma (St Louis, MO, USA). Ex TaqTM DNA polymerase was purchase from Takara (Dalian, China). Cell culture reagents were purchased from Gibco BRL (Grand Island, NY, USA) and the transfection reagent Lipofectamine 2000 was purchased from Invitrogen Life Technologies (Groningen, The Netherlands). Mouse anti-GFP monoclonal antibody (B-2) and goat anti-mouse IgG HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence (ECL) reagents were obtained from Pierce Biotechnology (Rockford, IL, USA).
Molecular cloning of hGDE4 encoding cDNA sequence
Total RNA was isolated from HEK 293 cells with Trizol as described in the manual and then 5 µg total RNA was reverse transcripted to cDNA in a 50 µl volume with Oligo(dT)20 as the primer according to the manual of SuperScriptTM III First-Strand Synthesis System for RT-PCR. RNA quality and concentration was previously assessed spectrophotometrically and checked on a 1% agarose gel before reverse transcription. According to the predicted cDNA sequence of human GDE4 gene (Genbank accession number AK094770), two gene specific primers for human GDE4 coding sequence named GSP1 and GSP2, 5′ATGTCGTCCACTGCGGCTTT-3′ and 5′-CTATGCTGAAAAGTTATGTAAAAAATCC-3′, were designed to amplify the sequence of hGDE4. The PCR reaction volume is 50 µl, which combines 5 µl 10×PCR buffer, 3 µl 25 mM MgCl2, 1 µl 10 mM dNTP mixture, 3 µl -synthesized cDNA template, 1 µl 20 µM GSP1 and GSP2, 1 µl Ex TaqTM DNA polymerase (5U/µl), 35 µl -sterilized H2O. The hGDE4 gene sequence was amplified by the following program: an initial denaturation step of 4 min at 94°C, followed by 40 temperature cycles for 30 sec at 94°C, 30 sec at 56°C, and 75 sec at 72°C, lastly an extension step of 10 min at 72°C. The PCR production was cloned into T-vector and sequenced.
Computational sequence analyses
Protein domain in hGDE4 was identified by using the protein family database (PFAM), an online database containing collections of protein domains and families Citation[20]. The potential transmembrane domains were predicted by TMHMM2.0 server (http://www.cbs.dtu.dk/services/TMHMM-2.0/). The molecular mass and isoelectric point of the deduced human GDE4 protein were predicted using the Compute pI/Mw tool at the ExPASy molecular biology WWW server of the Swiss Institute of Bioinformatics (http://www.expasy.org/). The deduced signal peptide was predicted using SignalP Citation[21]. DNA and protein sequence comparisons were carried out by BLAST at the NCBI Web server (http://www.ncbi.nlm.nih.gov/BLAST/). Chromosomal localization and genomic organization prediction were carried out using the UCSC Genome Browser (http://www.genome.ucsc.edu/cgi-bin/hgBlat). Comparison of hGDE4 and its homologues were performed using the Mega 3.1 Citation[22]. Phylogenetic tree analysis of amino acid sequences deduced from hGDE4 DNA sequences was also performed using the Mega 3.1. The clustal method was chosen to correct the distances for multiple substitutions at a single site. GenBank accession numbers of previously known GDE4 protein sequences used for these analysises are XP_548237 (Canis familiaris), XP_001503785 (Equus caballus), NP_079914 (Mus musculus), NP_001037703 (Rattus norvegicus), XP_001107942 (Macaca mulatta), XP_415876 (Gallus gallus), NP_001069868 (Bos taurus), NP_001017323 (Xenopus tropicalis), NP_001004118 (Danio rerio) and XP_396303 (Apis mellifera).
Molecular modeling
The 3-D model of human GDE4 protein was built with SWISS-MODEL Web Server (http://swissmodel.expasy.org/workspace/) Citation[23] using the 3-D structure of E. coli glycerophosphodiester phosphodiesterase (PDB accession code 1t8q) as template and ESyPred3D Web Server 1.0 (http://www.fundp.ac.be/sciences/biologie/urbm/bioinfo/esypred) Citation[24] using the 3-D structure of T. tengcongensis glycerophosphodiester phosphodiesterase (PDB accession code 2PZ0) as the template Citation[25].
Plasmid construction
To generate a hGDE4 tagged with EGFP construct, a forward primer (hGDE4F), 5′-TTCTCGAGGCCATGTCGTCCACTGCGGCTT-3′, was designed to bring in an internal Xho I site, Kozak consensus (underline), and a translation start condon (bold). PCR was used to amplify the hGDE4 fragment, using hGDE4F and the reverse primer (hGDE4R) including a Pst I site, 5′-GCCTGCAGATGCTGAAAAGTTATGTAAAA-3′. PCR production was cloned into the Xho I and Pst I sites of the vector pEGFP-N3 to make phGDE4-EGFP. DNA construct was first identified by restriction endonuclease and then verified by DNA sequencing.
Cell culture and transfection
HEK293 and N2a cells were grown and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 µg/ml each penicillin and streptomycin. Incubations were carried out at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were maintained in the logarithmic phase of growth and sub-cultured at 3–4 days intervals. The control vector, pEGFP-N3, and expression construct of phGDE4-EGFP were transfected using Lipofectamine 2000 according to the manufacturer's protocol.
Immunoblot analysis
After 48-hour transfection, cells were rinsed three times with ice-cold phosphate buffered saline (PBS) and lysed in a lysis buffer (50 mM Tris, pH 7.5, 300 mM NaCl, 5 mM EGTA, 1 mM EDTA, 0.5% Triton X-100, 0.5% NP40, 0.1 mM phenylmethylsulfonyl fluoride, and a 10 µg/ml final concentration of each of aprotinin, leupeptin, and pepstatin) and then sonicated on ice. The lysates were clarified by centrifugation at 10,000 g for 10 min at 4°C and the supernatant were collected for further analysis. The protein concentrations were determined using Lowry assay Citation[26]. Lysates (40 µg) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis with a 4% stacking gel and 10% separating gel and transferred to Hybond ECL nitrocellulose membrane (Amersham Biosciences). Following transfer, membranes were washed in Tris-buffered saline (10 mM Tris, 150 mM NaCl, pH 7.4) and blocked in 5% non-fat milk in TBST (TBS containing 0.1% Tween-20) at room temperature for at least 1 hour. Membranes were incubated in TBST containing 1:1000 monoclonal anti-GFP antibody (B-2) at 4°C for overnight. Membranes were washed three times in TBST and then incubated with horseradish peroxidase-conjugated goat-anti mouse IgG diluted 1:1000 in TBST at 37°C for 1 h. Membranes were washed three times in TBST and then developed using standard ECL method.
Confocal microscopy
Human embryo kidney HEK293 cells and mouse neuroblastma N2a cells were mounted on glass slides in six-well cluster plates for 24 h before transfection with the described constructs. Forty-eight hours after transfection, cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature and imaged by confocal scanning microscopy (LSM510, Carl Zeiss, Jena, Germany).
Results
Cloning and sequence analysis of human GDE4
According to the predicted cDNA sequence of the putative human GDE4 gene, two gene specific primers were designed to clone human GDE4 coding sequence. PCR products were subcloned into the pMD18-T vector and were sequenced. The cloned sequence was then submitted to GenBank (GenBank accession No. EU192951). The coding cDNA sequence of human GDE4 is 945 bp in length and encodes 314 amino acids (). The theoretical molecular weigh of human GDE is 36.2 kDa and human GDE has an isoelectric point of 8.66 which was determined by the Compute pI/Mw tool on the ExPASy server.
Figure 1. Nucleotide and deduced amino acid sequences of human GDE4. Both nucleotide and predicted amino acid sequences are countered on the left side. The accession number is EU192951. The sequences of the transmembrane regions were shaded in black (residues 5–24 and 200–222), and the sequence of GDE domain (residues 42–97) is underlined, in which the deduced active sites (Glu72, Asp74, and His87) are typed in bold. Asterisk represents the stop codon.
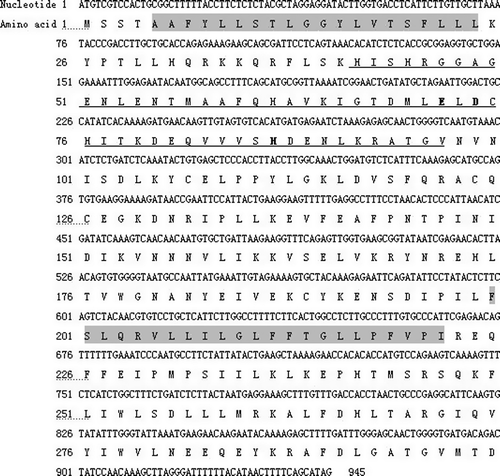
The gene structure and chromosomal localization of human GDE4 was elucidated by Blat (http://genome.ucsc.edu/cgi-bin/hgBlat) searching against the up-to-date human genome assemblies. It was precisely mapped to 17q22 containing 10 exons and 9 introns (B). All exon–intron junctions contain the gt/ag consensus splicing sequence (data not shown) Citation[27]. The translation start codon ATG was in the first exon and the TAG stop codon in the tenth exon.
Figure 2. The protein structure of hGDE4 and the genomic organization of hGDE4 gene. (A) The structure of hGDE4 protein deduced from the cDNA open reading frame. Numbering corresponds to amino acid residues at the N- and C-termini of each domain. The protein sequence contains two transmembrane regions and a GDPD domain. The location of GDPD domain (residues 45–304) is denoted in rectangle, while the transmembrane regions are indicated in ellipses. (B) Genomic organization of hGDE4 gene. Exon (indicated as E) distribution of human GDE4 gene was at chromosome 17q22 by the BLAT program in Human Genome Work Draft database (http://genome.ucsc.edu/).
GDE4 is a novel member of the GDE family
After searching the online database containing collections of protein domains and families database (PFAM), we found that the protein contained a single GDPD domain of 45–304 amino acid (A), the characteristic of the GDE family, which suggested that this protein is a novel member of the GDE family. In the GDPD domain, the sequence of the most conserved region among all identified mammalian GDE proteins and the bacterial GDEs sequence is of about 56 residues (corresponding to amino acids 42–97 of human GDE4), which is shown by underline in . By the most conserved sequence comparison of human GDE4 and GDE1, three amino acid sites (Glu72, Asp74, and His87) in GDE4 as typed in bold was deuced to be essential for its GDE activity (). Two transmembrane regions of 5–24 and 200–222 residues in human GDE4 were predicted by the TMHMM2 program as shaded in black ( and A). In addition, an N-terminal signal peptide was detected from positions 1–19 in human GDE4 using SignalP version 3.0.
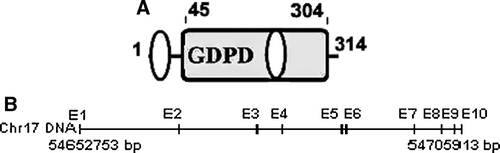
The multiple sequence alignments indicate that human GDE4 shares high identity of 89–96% at amino acid level with members of GDE4 in other mammalian species, house mouse (91%), rat (90%), cattle (95%), horse (94%), dog (96%), rhesus monkey (89%) and middle identity of 56–81% with other non-mammalian species, such as chicken (81%), zebrafish (69%) and honey bee (56%) (A). Moreover, the GDE domain, two transmembrane regions and the deduced three amino acid sites essential for its GDE activity are conserved in all known members of GDE4 (A). We can see the relationship among the GDE4 in different species more clearly from the phylogenetic tree that there are evidently two clusters of mammalian and non–mammalian animal (B).
Figure 3. Multiple alignment and phylogenetic of GDE4. (A) Multiple alignment of the GDE4 amino acid sequence with those of known GDE4. The conserved two transmembrane regions were indicated by black frame, the sequence of GDE domain was underlined and the essential active sits (E, D and H) was shown by bold black dot. (B) A phylogenetic tree constructed by the neighbor-joining method from the amino acid sequences of the GDE4 proteins. The numbers indicate the bootstrap confidence values obtained for each nodes after 1000 replications. (A) and (B) include mammalian and non-mammalian animals, respectively.
Molecular 3-D model of human GDE4
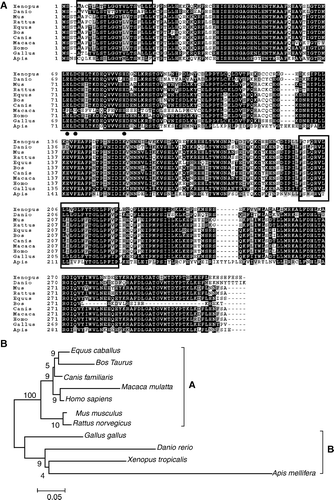
To further study the relationship between structure and function, we generated a molecular 3-D model of human GDE4 using two web server, SWISS-MODEL and ESyPred3D. Previous study showed that the main secondary structural elements were present and that the pattern of tertiary folding was also similar in E. coli, T. maritima, T. thermophilus and A. tumefaciens GDPD Citation[28]. Because of its highest conservation level and sequence signatures as well as its most significant degree of confidence (E-value of 1.00e-35) by comparison, E. coli GDPD (protein database code 1t8q) was selected as the best structural template available at the present time for the construction of a molecular 3-D model for human GDE4. In the predicted structure, the fundamental structural framework, comprising the position and orientations of the secondary structural elements, is inherited from residues 35–356 of E. coli GDPD. The template does not uniquely determine the conformations of the variable regions and side chain packing.
As shown in A by SWISS-MODEL web servers, a 3-D structural model for human GDE4 protein includes a region containing 269 residues around the GDPD consensus domain (residues 42–310). The model consists of a central β sheet made up of eight strands, 16 additional β strands, and 13 surrounding α-helices. Another 3-D model for human GDE4 protein containing 272 residues (residues 39–310) sharing 21.9% identities with the template, was built by ESyPred3D web server (B), which consists of a central β sheet made up of eight strands, six additional β strands, and 10 surrounding α-helices. Two models are similar and the predicted α/β structure resembles a triose-phosphate-isomerase (TIM) barrel domain from which a smaller domain, the putative membrane-binding domain protrudes. A second membrane-binding domain, which was predicted for GDE4 by the TMHMM2 program (A), is not projected in the models, because this region lies outside the range of the modeled sequence at the very N-terminus (residues 5–24 of GDE4). The conservation of residues within the groove in the center of the TIM barrel indicates a possible location for the GDE domain.
Figure 4. Molecular 3-D model of human GDE4 and locations of the deduced catalytic sites. (A) and (B) Molecular 3-D model of human GDE4 by SWISS-MODEL (A) and ESyPred3D (B) Web Server. The colored segments of the backbone structure mark the location of α-helix (red), β sheet (yellow), turn (blue), and coil (white). C and D: Locations of the deduced catalytic sites in 3-D model of human GDE4 by SWISS-MODEL (C) and ESyPred3D (D) Web Server. This Figure is reproduced in colour in Molecular Membrane Biology online.
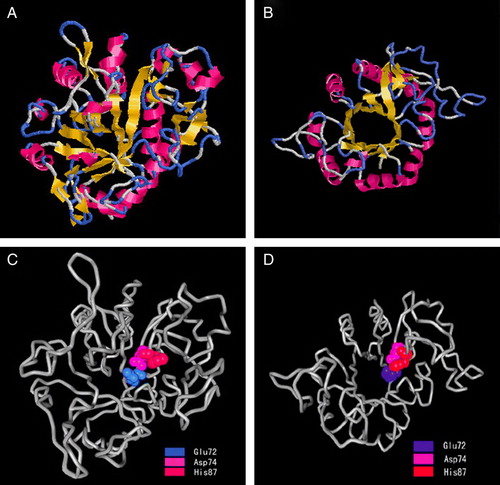
By sequence comparison, three amino acid sites, Glu72, Asp74, and His87, in GDE4 as typed in bold in was deuced to be essential for its GDE activity. We further located the most probable three sites for catalytic activity in the 3-D structure (C and 4D). The proposed catalytic sites lie near a groove in the center of the TIM barrel and is comprised of two strands of the TIM barrel. The location of the binding site suggests that the catalytic reaction takes place in the inner surface of the TIM barrel. Because the positions of the residues at the active site are inherited from the template, the extent to which these residues satisfy the biological criterion of being highly conserved can provide evidence for the validity of the sequence alignment used in the modeling.
Over-expression of hGDE4 in mammalian cells
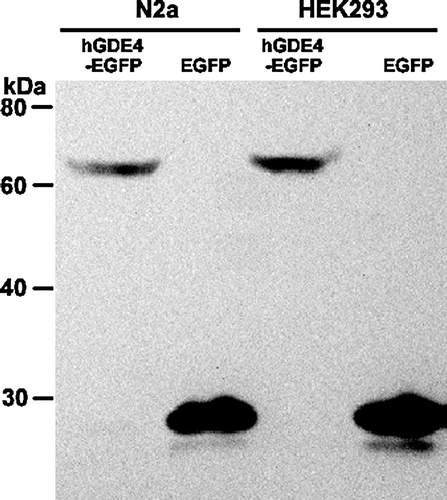
To further study the cellular function of human GDE4, a construct of human GDE4 tagged with GFP is generated and transfected into mammalian cells. At 48-h post-transfection, cells were lysed for hGDE4 expression by western blot analysis using the GFP antibody. As shown in , N2a and HEK293 cells transfected with the control vector pEGFP-N3 expressed a protein of 27 kDa, which correlated to EGFP protein expression; however, cells transfected with phGDE4-EGFP expressed a protein of 63 kDa corresponding to EGFP-tagged hGDE4, because hGDE4 was expressed as a protein of 36 kDa, which was in accordance with the theoretical molecular weigh of hGDE4 protein.
Figure 5. Expression of hGDE4 tagged with EGFP in mammalian cells. N2a and HEK293 cells were transfected with either control vector pEGFP-N3 or phGDE4-EGFP. After 48-hour transfection, the expression of hGDE4 tagged with EGFP was detected with western blotting analysis. Migration of molecular weight standard proteins is indicated to the left of the Figure.
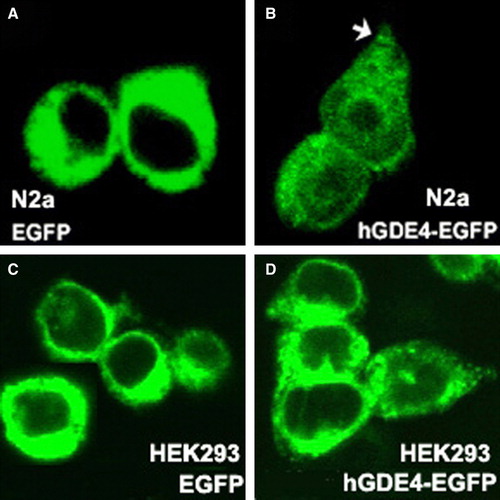
Distribution of hGDE4 protein in mammalian cells
In order to address the intracellular localization of hGDE4 protein, we transfected cells with phGDE4-EGFP construct and observed the distribution of green florescence by confocal microscopy. In N2a and HEK293 cells expressing EGFP itself, intense fluorescence was evenly distributed over the entire cytoplasm (A and 6C). In contrast, hGDE4-fused EGFP was concentrated at the perinuclear region and the cell periphery (B and 6D), and also observed at growth cones in mouse neuroblastma N2a cells, as indicated by the arrow (B). In addition, we found that over-expression of GDE4 did not induce neurite formation in N2a cells or change cell morphology in HEK293 cells.
Figure 6. Distribution of EGFP-tagged hGDE4 protein in N2a and HEK293 cells. N2a and HEK293 cells were transfected with pEGFP-N3 (A, C), phGDE4-EGFP (B, D) and visualized by confocal microscopy as described in Materials and methods section. hGDE4-EGFP accumulated at the perinuclear region and the cell periphery in both cells and growth cones (arrows) in Neuro2A cells. This Figure is reproduced in colour in Molecular Membrane Biology online.
Discussion
To date, seven mammalian GDEs have been virtually cloned or predicted by a search of database of expressed sequence tags (ESTs) based on the highly conserved GDE domain using bioinformatics Citation[18]. The coding cDNA sequence of human GDE4 was first cloned and the highly conserved GDE domain existed in the deduced human GDE4 protein. Based on phylogenetic analysis of the GDE domain, GDE family was proposed to be classified into three distinct α, β, γ groups and GDE4 was alone in the β group Citation[12]. Previous studies showed that GDE1, GDE2, GDE3 and GDE6 had multiple hydrophobic regions, two transmembrane domains were also predicted to be in human GDE4, which may serve as transmembrane regions and may be related to its biological functions as other GDE members Citation[11], Citation[12], Citation[14], Citation[15], Citation[18], Citation[29], Citation[30].
Membrane proteins are core components of many essential cellular processes; however progress in membrane structure has been slow owing to the many bottlenecks associated with membrane protein crystallization Citation[31]. Based on the structure of prokaryotic homologues, molecular modeling may be a fast way to take us towards a structural understanding of the eukaryotic/human membrane proteome(s) Citation[31]. Although the relationship of between structure and catalytic activity in some eukaryotic members of phosphodiesterase family, such as human phosphodiesterase 9, has been revealed Citation[32], little has been known about mammalian GDEs. It is possible that such type of transmembrane proteins also plays roles in keeping a suitable redox state in body Citation[33].Because of the presence of multiple putative transmembrane regions, domain–structure analysis indicated that GDE4 in group β resembled GDE1, 2 and GDE3 in group α Citation[12]. Furthermore, 3-D model of human GDE4 based on homology modeling exhibited the similarity of 3-D model between GDE4 and GDE1 Citation[28], which suggested that GDE members have similar catalytic mechanism. Experimental data and 3-D model showed that the GDE domain of GDE1 represents its catalytic domain and Glu-97/Asp-99 and His-112 are essential for its GDE activity Citation[12], Citation[28]. On the basis of the domain–structure similarity between GDE1 and GDE4, three amino acid sites (Glu72, Asp74, and His87) in GDE4 were deduced to be essential for its GDE activity, which was highlighted by their location in molecular 3-D structure. Therefore, His87 residue may act as a general acid-base catalyst for the formation of the reaction intermediate in the phosphotransfer step. The negatively charged Asp74 and Glu72 residues may interact with a calcium ion that is required for the catalytic activity of phosphatidylinositol phosphodiesterases, and they likely form hydrogen bonds with a hydroxyl group of the substrate.
Using bioinformatics analysis, we found that GDE4 sequences are highly conserved in human, mouse, rat, cattle, horse, dog, monkey, chicken and other vertebrates for more than 80% identical amino acid residues. The GDE domain, two transmembrane regions and the deduced three amino acid sites essential for its GDE activity are conserved in all known members of GDE4. In human, the location of GDE4 is on chromosome 17 and consists of ten exons and nine interval introns. From analysis of the protein sequences of different species, we drew an evolutionary map for GDE4 among these various species. The evolutionary map showed that monkey was the closest species to human; cattle, horse and dog were further from human than monkey, while mouse and rat were further from human than cattle, horse and dog. The results are in accordance with traditional evolution theory. Intriguingly, among the deduced amino acid sites (Glu72, Asp74, and His87) essential for human GDE activity, only His87 was conserved in monkey GDE4, although monkey was the closest evolutionary species to human. Research into the characteristics of human GDE4 can provide new insights into studies of the significance of GDE4 in mammals.
Human GDE4 appears to be a membrane protein because it contains two transmembrane helixes by the sequence analysis. Over-expressing of human GDE4 tagged with EGFP showed it was located in cytoplasm and concentrated at the perinuclear region and the cell periphery, which suggested that GDE4 was anchored to intracellular membranes and plasma membrane. GDE4 was also found in the growth cones in neuroblastma N2a cells as the location of mouse GDE2 Citation[16]. The expression of GDE2 in neuroblastoma Neuro2a cells was significantly upregulated during neuronal differentiation by retinoic acid (RA) treatment and over-expression of GDE2 resulted in neurite formation in the absence of RA Citation[16]. In contrast, we found that over-expression of GDE4 did not induce neurite formation. In addition, over-expression of GDE4 did not change cell morphology in HEK293 cells, while GDE3 induced cell rounding Citation[14]. These results suggested that the function of GDE4 may differ from other members of GDE family.
In summary, we have isolated and characterized a novel human GDE-containing gene GDE4. The protein is composed of 314 amino acids with a GDE domain and two transmembrane regions, which displays structural homology with other GDE proteins. Furthermore, the molecular 3-D model provides the first structural information and highlights that the individual core residues Glu72, Asp74, and His87 are crucial to maintaining its catalytic activity. Human GDE4 is located in the cytoplasm, especially accumulated in the perinuclear region and the cell periphery. In addition, over-expression of hGDE4 has no effect on cell morphology or induces neurite formation as a transmembrane protein. Together, these results suggest that GDE4 protein is a member of the GDE family and may play different roles from other members of GDE family.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (30600329, 30870298) and the Natural Science Foundation Project of CQCSTC (2007BB5443), and by the Science and Technology Project from Chongqing Municipal Education committee (KJ070510).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Patton-Vogt J. Transport and metabolism of glycerophosphodiesters produced through phospholipid deacylation. Biochim Biophys Acta 2007; 1771: 337–342
- Kasahara M, Makino K, Amemura M, Nakata A. Nucleotide sequence of the ugpQ gene encoding glycerophosphoryl diester phosphodiesterase of Escherichia coli K-12. Nucleic Acids Res 1989; 17: 2854–2866
- Tommassen J, Eiglmeier K, Cole ST, Overduin P, Larson TJ, Boos W. Characterization of two genes, glpQ and ugpQ, encoding glycerophosphoryl diester phosphodiesterases of Escherichia coli. Mol Gen Genet 1991; 226: 321–327
- Larson TJ, Ehrmann M, Boos W. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem 1983; 258: 5428–5432
- Nilsson RP, Beijer L, Rutberg B. 1994. The glpT and glpQ genes of the glycerol regulon in Bacillus subtilis. Microbiology, 140:723–730.
- Shevchenko DV, Akins DR, Robinson EJ, Li M, Shevchenko OV, Radolf JD. Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, and glycerophosphodiester phosphodiesterase in outer membrane fractions from Treponema pallidum, the syphilis spirochete. Infect Immun 1997; 65: 4179–4189
- Stebeck CE, Shaffer JM, Arroll TW, Lukehart SA, Van Voorhis WC. Identification of the Treponema pallidum subsp. glycerophosphodiester phosphodiesterase homologue. FEMS Microbiol Lett 1997; 154: 303–310
- Fernandez-Murray JP, McMaster CR. Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the kennedy pathway. J Biol Chem 2005; 280: 38290–38296
- Fisher E, Almaguer C, Holic R, Griac P, Patton-Vogt J. Glycerophosphocholine-dependent growth requires gde1p (ypl110c) and git1p in Saccharomyces cerevisiae. J Biol Chem 2005; 280: 36110–36117
- Simocková M, Holic R, Tahotná D, Patton-Vogt J, Griac P. Yeast Pgc1p (YPL206c) controls the amount of phosphatidylglycerol via a phospholipase C-type degradation mechanism. J Biol Chem 2008; 283: 17107–17115
- Zheng B, Chen D, Farquhar MG. MIR16, a putative membrane glycerophosphodiester phosphodiesterase, interacts with RGS16. Proc Natl Acad Sci USA 2000; 97: 3999–4004
- Zheng B, Berrie CP, Corda D, Farquhar MG. GDE1/MIR16 is a glycerophosphoinositol phosphodiesterase regulated by stimulation of G protein-coupled receptors. Proc Natl Acad Sci USA 2003; 100: 1745–1750
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem 2008; 283: 9341–9349
- Yanaka N, Imai Y, Kawai E, Akatsuka H, Wakimoto K, Nogusa Y, Kato N, Chiba H, Kotani E, Omori K, Sakurai N. Novel membrane protein containing glycerophosphodiester phosphodiesterase motif is transiently expressed during osteoblast differentiation. J Biol Chem 2003; 278: 43595–43602
- Nogusa Y, Fujioka Y, Komatsu R, Kato N, Yanaka N. Isolation and characterization of two serpentine membrane proteins containing glycerophosphodiester phosphodiesterase, GDE2 and GDE6. Gene 2004; 337: 173–179
- Yanaka N, Nogusa Y, Fujioka Y, Yamashita Y, Kato N. Involvement of membrane protein GDE2 in retinoic acid-induced neurite formation in Neuro2A cells. FEBS Lett 2007; 581: 712–718
- Gallazzini M, Ferraris JD, Burg MB. GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC. Proc Natl Acad Sci USA 2008; 105: 11026–1031
- Yanaka N. Mammalian glycerophosphodiester phosphodiesterases. Biosci Biotechnol Biochem 2007; 71: 1811–1818
- Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience 2007; 144: 239–246
- Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR. The PFAM protein families database. Nucleic Acids Res 2004; 32: D138–141
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340: 783–795
- Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 2004; 5: 150–163
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 2006; 22: 195–201
- Lambert C, Leonard N, De Bolle X, Depiereux E. ESyPred3D: Prediction of proteins 3D structures. Bioinformatics 2002; 18: 1250–1256
- Shi L, Liu JF, An XM, Liang DC. Crystal structure of glycerophosphodiester phosphodiesterase (GDPD) from Thermoanaerobacter tengcongensis, a metal ion-dependent enzyme: insight into the catalytic mechanism. Proteins 2008; 72: 280–288
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem:265–275.
- Shapiro MB, Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 1987; 15: 7155–7174
- Bachmann AS, Duennebier FF, Mocz G. Genomic organization, characterization, and molecular 3D model of GDE1, a novel mammalian glycerophosphoinositol phosphodiesterase. Gene 2006; 371: 144–153
- Lang Q, Zhang H, Li J, Yin H, Zhang Y, Tang W, Wan B, Yu L. Cloning and characterization of a human GDPD domain-containing protein GDPD5. Mol Biol Rep 2008; 35: 351–359
- Rao M, Sockanathan S. Transmembrane protein GDE2 induces motor neuron differentiation in vivo. Science 2005; 309: 2212–2215
- Granseth E, Seppälä S, Rapp M, Daley DO, Von Heijne G. Membrane protein structural biology – how far can the bugs take us?. Mol Membr Biol 2007; 24: 329–332
- Liu S, Mansour MN, Dillman KS, Perez JR, Danley DE, Aeed PA, Simons SP, Lemotte PK, Menniti FS. Structural basis for the catalytic mechanism of human phosphodiesterase 9. Proc Natl Acad Sci USA 2008; 105: 13309–13314
- Shao HB, Chu LY, Lu ZH, Kang CM. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 2008; 4: 8–14