Abstract
Many proteins with pivotal roles in T cell activation are modified by fatty acylation. Examples of these include transmembrane proteins such as the co-receptors CD4 and CD8, the adaptors LAT and Cbp/PAG, the pre-TCR as well as proteins synthesized on free cytosolic ribosomes, such as the Src-related tyrosine kinases Lck and Fyn. The two main types of fatty acylations in eukaryotic cells are N-myristoylation and S-acylation, the latter being more commonly referred to as palmitoylation. N-Myristoylation occurs exclusively on proteins synthesized on soluble ribosomes and provides substrates with an affinity for membranes. Palmitoylation modifies a wide range of substrates that includes both cytosolic and transmembrane proteins, its functions are diverse and in many cases not yet understood. Like myristoylation, palmitoylation promotes membrane-binding of cytosolic proteins, but it has also been implicated in protein targeting, trafficking, stability and activity. In addition, many palmitoylated proteins are insoluble in cold non-ionic detergent, and have therefore been proposed to localize to lipid rafts. The organization of receptors and signaling proteins into microdomains such as lipid rafts provides an attractive model for the initiation and propagation of T cell signaling, although many aspects of this are still poorly understood. This review will discuss the current evidence for the involvement of acylations in the localizations and functions of T cell signaling proteins.
N-Myristoylation and palmitoylation
N-Myristoylation and S-acylation both attach fatty acids to proteins, but in terms of chemistry, enzymology and substrates, these modifications could hardly be more divergent. N-myristoylation involves the cotranslational attachment of myristate, a 14-carbon saturated fatty acid, to an N-terminal glycine via an irreversible amide linkage Citation[1]. This modification is catalysed by N-myristoyltransferases, two of which have been identified in humans (NMT1 and NMT2) Citation[2]. Myristate is attached to glycines in the consensus sequence (M)GXXT/S, which requires the proteolytic removal of the N-terminal methionine. The crystal structure of NMT1 revealed that a myristoyl-CoA binding site restricts the length of the fatty acid that can be bound, and thus determines the preferential use of the short, relatively rare myristate Citation[3].
Although myristoylation bestows an affinity for membranes upon substrates, the presence of myristate alone is not sufficient for stable membrane insertion. Instead, a second signal is required, which can either be a cluster of positively charged amino acids, palmitoylation, or protein-protein interactions Citation[1], Citation[4]. Thus, myristoylation is thought to target proteins to membranes where they are then trapped by a second signal. Myristoylation itself is not reversible, but dynamic regulation of membrane binding can be achieved through, for instance, phosphorylation, which prevents electrostatic interactions between positively charged amino acids and negatively charged phospholipids (myristoyl-electrostatic switch). In addition, for some proteins a myristoyl-conformation switch mechanism, which determines whether the myristate is exposed to the environment or buried within the protein, has been described Citation[1], Citation[4].
In contrast to myristoylation, S-acylation is a posttranslational and reversible modification that attaches fatty acids via a thioester bond to sulfhydryl groups of cysteines Citation[1], Citation[4], Citation[5]. S-acylation was first demonstrated using tritiated palmitate Citation[6], Citation[7], a saturated 16-carbon fatty acid, and has therefore historically been referred to as palmitoylation. However, shorter (C14) and longer (up to C20) fatty acids can also be used, as well as mono- and poly-unsaturated ones. S-palmitoylation should be distinguished from N-palmitoylation, which attaches palmitate to the amide group of N-terminal cysteine residues. This modification has been described for secreted proteins such as Hedgehog and Wnt proteins, and is the topic of another review in this issue.
Two obstacles have stood in the way of rapid progress in the field S-acylation, which was first described almost twenty-five years ago: the absence of a well-defined consensus sequence and, until six years ago, the absence of known enzymes that catalyse this modification. Yet, novel tools have now been developed for large-scale identification of palmitoylated proteins, and a comprehensive picture of the palmitoyl-proteome appears within reach Citation[8], Citation[9]. Palmitoylated proteins known to date are a heterogeneous group that fall into one of four categories: cytosolic proteins with a myristate at the N-terminus; cytosolic proteins with a prenyl group at the C-terminus; cytosolic proteins without other lipid modifications; transmembrane proteins Citation[1], Citation[5], Citation[10].
The purification of palmitoyltransferases (PATs) has proven to be extremely difficult, but genetic approaches in yeast have led to the cloning of two of these enzymes, Erf2p and Akr1p Citation[11], Citation[12]. These proteins have different substrate specificities, but share two features: they are multiple membrane spanning proteins and contain a DHHC motif in a cysteine-rich region (DHHC-CRD). The recognition of the common DHHC-CRD motif has allowed the identification of 23 PATs in mammalian genomes Citation[13], Citation[14]. The specificities of these enzymes, which are discussed in detail in another review in this issue, are still being worked out. The membrane localization of PATs suggests that substrates require some pre-existing membrane affinity. This fits well with the presence of additional lipid-modifications or transmembrane domains in most of these proteins.
Unique among lipid-modifications, is the reversibility of palmitoylation through the activity of palmitoyl-protein thioesterases. Evidence for palmitate cycling exists for numerous substrates. For Ras proteins, the constitutive cycling between depalmitoylation and repalmitoylation was shown to be important for localization and activity Citation[15]. For other substrates such as Gα subunits and G protein coupled receptors, changes in the turnover rate of palmitate in response to specific signals are known to occur Citation[5], Citation[16]. This dynamic palmitoylation is discussed elsewhere in this issue.
Microdomains in T cell signaling
T cells are crucial for the adaptive immune response against pathogens. Activation of T cells is initiated when a pathogen-derived peptide associates with MHC proteins, and is recognized by the T cell receptor (TCR) Citation[17]. This ligand recognition initiates a multitude of signaling pathways that eventually lead to the transcription of genes required for T cell proliferation and differentiation into effector cells ().
Figure 1. Early events in T cell activation. T cell activation is initiated by recognition of MHC/peptide complexes and leads to signaling as described in the main text. Light boxes in the CD3 and ζ chains are Immunoreceptor Tyrosine-based Activation Motifs where phosphorylation by Lck occurs. Thicker boxes in the plasma membrane represent lipid rafts.
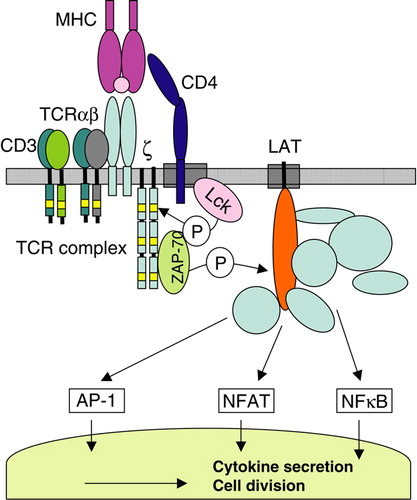
The first detectable event following TCR engagement is the activation of the tyrosine kinase Lck Citation[18]. Lck associates with CD4 and CD8, coreceptors that interact with non-polymorphic regions of MHC molecules in CD4+ and CD8+ T cells, respectively. The activation of Lck leads to the phosphorylation of tyrosines in the cytoplasmic domains of CD3 and TCRζ?chains. Phosphorylated TCRζ chains recruit the tyrosine kinase ZAP-70, which subsequently phosphorylates the transmembrane adaptor LAT. This leads to the assembly of a multiprotein complex that is essential for the propagation of signaling along several pathways, eventually resulting in the activation of transcription factors NFAT, AP-1 and NFκB.
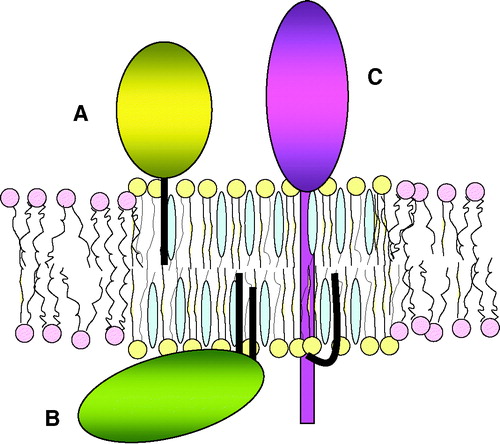
T cell activation has been studied intensely, numerous signaling proteins have been identified and many pathways elucidated. Yet, challenges remain in understanding its initiation, extreme sensitivity and the ability to mount different responses against different ligands. One of the models that have been employed to explain these issues is the lipid raft hypothesis Citation[19–23]. Lipid rafts are defined as membrane microdomains composed of highly-ordered saturated sphingolipids, intercalated with cholesterol, in an environment of less-ordered unsaturated glycerophospholipids Citation[24], Citation[25]. These domains can be isolated from artificial and biological membranes based on their relative insolubility in cold non-ionic detergents Citation[26], Citation[27]. In addition to specific lipids, some proteins are enriched in detergent insoluble fractions as well, including those with lipid anchors, such as GPI-linked proteins that insert into the extracellular leaflet of the plasma membrane and palmitoylated cytosolic proteins that insert into the inner leaflet (). The attached saturated fatty acids of these proteins are thought to preferentially intercalate into the saturated fatty acids of lipid rafts. Transmembrane domains do not have a natural affinity for the highly ordered environment of lipid rafts, but some palmitoylated transmembrane proteins are detergent insoluble nonetheless. Palmitoylation is therefore frequently regarded as a raft-targeting signal. However, the use of detergent insolubility as a criterion for raft localization has come under attack because of its non-physiological nature, and this has opened the challenge to visualize plasma membrane microdomains in intact cells using microscopy-based methods. Results from several studies that apply FRET, single particle tracking, transmission electron microscopy or lipid-sensitive probes are consistent with the existence of microdomains in the plasma membrane, although conflicting reports exist as well. Thus, the debate on the nature and/or existence of these domains is still ongoing and a more extensive application of these imaging techniques will be required to settle this issue in the future. In the meantime, detergent insolubility should not be equated with lipid raft localization, although for historic as well as style reasons, the terms detergent resistant membranes (DRM) and rafts may occasionally be used interchangeably in this review.
Figure 2. Lipid raft-associated proteins. Lipid rafts are characterized by the tightly packed saturated fatty acids with intercalated cholesterol. Three examples of raft-associated proteins are shown: GPI-linked proteins that associate via a lipid anchor with the extracellular membrane leaflet (A), acylated cytosolic proteins that associate via fatty acids with the intracellular leaflet (B), and transmembrane proteins that are acylated in their cytoplasmic domain (C).
The lipid raft model for T cell signaling proposes that the segregation of proteins into specific microdomains is crucial to prevent activation of signaling in resting cells, and to maximize propagation of signaling in activated cells Citation[19–23]. According to this model, lipid rafts in resting T cells are homogeneously distributed and small, but will coalesce upon recognition of MHC/peptide antigens. This raft aggregation is thought to coincide with a re-shuffling of proteins into and out of rafts, recruiting those that promote signaling and excluding those that inhibit it. Two observations stand at the cradle of this model in T cells: (1) Crosslinking of GPI-linked proteins initiates T cell signaling by activating Lck; and (2) like GPI-linked proteins, Lck can be isolated from detergent insoluble membranes Citation[28–30]. Further support for this model has come from the findings that T cell activation can be initiated by crosslinking of the raft lipid GM1 Citation[31], and that the TCR and signaling molecules are recruited to DRM after T cell stimulation Citation[32], Citation[33]. Studies on palmitoylated proteins have further contributed to the shaping of this theory. For some of these, mutant forms deficient in palmitoylation are defective both in DRM localization and T cell signaling (see below). Therefore, a discussion on palmitoylated T cell proteins is inherently linked to the lipid raft theory.
Lck and Fyn
Lck is one of nine Src family proteins that are each composed of a unique domain, followed by SH3, SH2, tyrosine kinase domains and a regulatory tail. Src kinases do not share sequence similarities at the unique domain except for a short region at the very N-terminus, also referred to as the SH4 domain, where acylations occur. All Src kinases are myristoylated and, with the exception of Src and Blk, palmitoylated as well Citation[34].
Lck is myristoylated at Gly2, and palmitoylated at Cys3 and 5 Citation[35], Citation[36]. Mutagenesis of either Gly2 or Ser6 in the consensus sequence (M)GXXT/S abrogates myristoylation of Lck, and as a consequence palmitoylation and membrane-association Citation[35], Citation[37], Citation[38]. However, myristoylation alone is not sufficient for stable membrane-binding: Lck mutants with both palmitoylation sites deleted are myristoylated but do not associate with membranes Citation[37], Citation[39–41]. Hence, myristoylation most likely targets Lck to membranes where PATs are located, and subsequent palmitoylation leads to stable membrane-insertion. Myristoylation has also been shown to be important for the stability of Lck, since treatment of cells with the myristoylation inhibitor 2-hydroxymyristate resulted in a reduced half-life of the protein Citation[42]. Substitution of either Cys3 or Cys5 by mutagenesis does not abolish palmitoylation or membrane-binding, indicating that both sites can be used Citation[37], Citation[38], Citation[40], Citation[41], Citation[43–45]. However, in most studies the absence of Cys5 leads to only a minimal reduction in membrane binding, whereas deletion of Cys3 reduces this by approximately half. Thus, both single and dually palmitoylated Lck are likely to co-exist.
The essential role of Lck in the development and activation of T cells is well established Citation[46]. An absence of Lck in knockout mice leads to a severe reduction in peripheral T cells, and mutant T cell lines that lack Lck are unable to respond to TCR stimulation. What are the roles of acylation in this function? To begin with, the activity of Lck is absolutely dependent on an ability to associate with membranes. Lck mutants with amino acid substitutions at either myristoylation or palmitoylation sites, are unable to reconstitute T cell activation in Lck-deficient cell lines Citation[38], Citation[40], Citation[43], Citation[44]. These soluble proteins do not interact with CD4, phosphorylate TCRζ, recruit ZAP-70 to membranes, or promote downstream signaling. Palmitoylation also appears to play important roles beyond membrane-binding. For instance, an Lck chimera that localizes to the plasma membrane through a transmembrane domain instead of palmitoylation is unable to efficiently reconstitute TCR signaling Citation[44]. Similarly, Lck synthesized in the presence of the palmitate analogue 13-oxy-palmitic acid, cannot induce TCR signaling despite being associated with membranes and CD4 Citation[47]. Oxy-palmitate is likely to be incorporated in place of palmitate since its presence decreases labeling of Lck with iodinated palmitate. This second function of palmitoylation has been postulated to represent a role in targeting Lck to lipid rafts. Indeed, a considerable fraction of Lck is normally insoluble in cold non-ionic detergent Citation[41], Citation[44], Citation[47], but this is not the case for the abovementioned transmembrane Lck chimera or Lck modified with oxy-palmitate. The importance of palmitoylation for detergent insolubility of Lck has further been demonstrated using engineered proteins that contain a six-lysine motif instead of palmitoylation sites Citation[37]. Such proteins can associate with membranes but do not interact with GPI-linked proteins in DRM. In addition, it has been shown that the first 10 amino acids of Lck can target otherwise cytosolic proteins to detergent insoluble membranes Citation[41]. In the absence of detergents, a palmitoylation-dependent localization to specific microdomains was observed by transmission electron microscopy of plasma membrane sheets: a protein composed of the 10 N-terminal Lck residues linked to five lysines and a myc-tag localized to different areas of the membrane than a non-palmitoylated counterpart Citation[48].
Fyn is another Src kinase with roles in T cell activation although its function is not yet completely understood. Experiments on mice and cell lines deficient for Lck have shown that Fyn can replace Lck activity to some extent but cannot sustain full T cell development and activation Citation[49], Citation[50]. Under wildtype conditions, Fyn activation appears to be dependent on and follow that of Lck. In contrast to Lck, Fyn may be required for dampening T cell responses after activation, and play a role in T cell anergy Citation[51], Citation[52]. Like Lck, Fyn is both myristoylated and palmitoylated. Fyn does not contain a myristoylation consensus sequence with a Ser or Thr at position 6, but instead Lys 7 and 9 are important for efficient myristoylation Citation[53]. Myristoylation of Fyn is required for palmitoylation Citation[54], but in contrast to Lck, non-acylated Fyn associates to some extent (∼ 25%) with membranes Citation[55], Citation[56], possibly because N-terminal positively charged amino acids contribute to membrane-binding. This may also explain the faster membrane-binding kinetics of newly synthesized Fyn compared to those of Lck Citation[56], Citation[57]. Although based on mutagenesis studies both Cys3 and Cys6 can be palmitoylated Citation[58], mass spectrometry showed that only a minor fraction of Fyn is dually S-palmitoylated and that the majority is palmitoylated on Cys3 only Citation[59]. This further revealed that in addition to palmitate, mono-unsaturated palmitoyl as well as stearate and oleate are attached by S-acylation. The finding that Cys3 is the main site of Fyn palmitoylation fits well with the importance of this site, in contrast to Cys6, for membrane binding, detergent insolubility, association with GPI-linked proteins and interaction with TCRζ? Citation[45], Citation[53], Citation[60].
While it is obvious that palmitoylation of Lck and Fyn is important for detergent insolubility as well as for function, precisely how targeting to detergent insoluble membranes contributes to the activity of these proteins is not yet clear Citation[19–23]. For instance, it has been reported that in resting murine T cells the majority of Lck is excluded from DRM, whereas Fyn is predominantly present in these domains, leading to the postulate that rafts function to segregate Lck and Fyn in the absence of a T cell stimulatory signal. Lck was demonstrated to have higher activity outside of DRM and to translocate to these domains upon T cell activation, where it then activates Fyn Citation[61]. In this scenario, the activation of Lck may result from its dephosphorylation at the regulatory tail tyrosine by CD45, a phosphatase that is excluded from detergent insoluble domains. Conversely, the tyrosine kinase Csk is to some extent recruited to DRM following phosphorylation of the adaptor Cbp/PAG by Fyn, where it can inhibit Lck by phosphorylating the tail tyrosine Citation[62], Citation[63]. This negative feedback loop fits well with the transient activation of Lck detected with activation-specific antibodies Citation[61], Citation[64]. However, several issues require further clarification. For instance, the importance of Cbp for Csk activity has been disputed (see below), and the role of CD45 is ambiguous since it can also inactivate Lck by dephosphorylating a tyrosine in the kinase domain. Moreover, the very low amount of detergent insoluble Lck is not always observed in resting T cells, although the use of different types and percentages of detergents impedes a direct comparison between different studies.
Other studies have addressed the significance of an association with DRM by following the movements of Lck (and other T cell proteins) upon T cell activation. Several of these analyses used a fusion protein composed of the Lck acylation sites (aa 1–10) coupled to GFP (Lck10-GFP), alongside of Lck. In one report a CD28 costimulation-dependent accumulation of both Lck and Lck10-GFP at the T cell/APC interface was demonstrated by microscopy, which occurred concomitant with an increase in detergent insolubility of Lck Citation[65]. The similar behaviour of Lck10-GFP and Lck points towards a lipid-based rather than protein-based recruitment of this palmitoylated protein to sites of T cell activation. Similarly, following the trajectory of single molecules by TIRF microscopy, the movements of both Lck-GFP and Lck10-GFP molecules were shown to slow down at sites of T cell stimulation Citation[66]. This change in movement has been proposed to result from a trapping of rafts in the reorganized, denser actin cytoskeleton at TCR activation sites. A rearrangement of plasma membrane lipids at T cell activation sites has also been established with the fluorescence membrane probe Laurdan Citation[67], the emission spectrum of which is dependent on the order and condensation of surrounding lipids. These studies provide important evidence for the occurrence of plasma membrane reorganizations at T cell activation sites, yet, what drives the accumulation of proteins and lipids at these sites is not completely clear. Whereas the data on Lck and Lck10-GFP above suggest that lipid-lipid interactions are important for this, other results indicate a predominant role for protein-protein interactions. For instance, Lck was shown to reside in TCR activation-induced CD2 signaling clusters in another TIRF study, whereas Lck10-GFP did not Citation[68]. The formation of CD2 signaling clusters was found to be dependent on protein interactions via LAT tyrosines. Furthermore, in a biochemical analysis, plasma membrane isolates that represent TCR stimulation sites showed an enrichment of LAT, TCRζ and downstream signaling proteins, but not of Lck Citation[69]. This again demonstrates a role for protein rather than lipid interactions in the stability of TCR assemblies. On the other hand, the activity of Lck was required for the formation of these TCR signaling complexes. A current model may therefore be as follows: Lck raft localization is important for the initial signaling that drives subsequent cytoskeletal changes, LAT activation, membrane condensation and the formation of stable signaling complexes at TCR activation sites. However, obtaining a clearer picture of this remains a challenge for the future.
CD4 and CD8
The TCR coreceptors CD4 and CD8 facilitate T cell activation in two ways: they bind to invariant regions on MHC proteins, which stabilizes interactions with antigen presenting cells, and they associate with Lck, which couples the TCR to downstream signaling pathways. Despite having similar functions, the composition and structure of CD4 and CD8 are quite distinct. CD4 is a monomer with an extracellular domain composed of four immunoglobulin-like domains, whereas CD8 is present on most T cells as a disulfide-linked heterodimer of CD8α and CD8β chains, each containing one extracellular immunoglobulin-like domain Citation[70]. Nevertheless, at the cytoplasmic domains both proteins contain palmitoylation sites.
CD4 is palmitoylated at two cysteines located at the junction of the cytoplasmic and transmembrane domains, but this modification does not affect transport, cell surface expression or association with Lck Citation[71]. Since CD4 was observed to be detergent-insoluble, further studies on palmitoylation have focused primarily on a role in lipid raft targeting, and the involvement of this in T cell signaling and HIV entry. An influence of CD4 palmitoylation on T cell signaling has been suggested in studies using Jurkat T cells transfected with either wildtype or palmitoylation-deficient CD4. Reduced levels of tyrosine phosphorylation induced by CD4 crosslinking were observed for the mutant protein, although differences with wildtype CD4 were not very pronounced Citation[72]. In primary murine T cells, palmitoylation of CD4 was shown to be required for the recruitment of TCR and PKCθ to the immunological synapse Citation[73], but the significance of this for T cell signaling was not further established. Thus, at present there is only scarce knowledge on the effect of CD4 palmitoylation on T cell signaling.
Moreover, the importance of palmitoylation for DRM localization of CD4 is not completely clear since contradictory reports on this exist. Two studies demonstrated that the absence of CD4 palmitoylation, either through deletion of palmitoylation sites or by growing cells in the presence of the inhibitor bromo-palmitate, led to a reduction in detergent insolubility Citation[72], Citation[74]. However, a complete absence of detergent insolubility was observed only when both palmitoylation and Lck association did not occur. In contrast, other studies failed to detect any role for palmitoylation in the detergent insolubility of CD4 Citation[75], Citation[76]. Instead, a novel raft-targeting signal that consists of the positively charged residues RHRRR was identified Citation[75]. Mutations in this sequence located adjacent to the palmitoylation sites, abolished DRM localization without interfering with palmitoylation. This suggests that the RHRRR sequence is a dominant signal for raft targeting of CD4, and that although a contribution of palmitoylation cannot be excluded, it does not appear to be essential. In the absence of a role in raft localization, it is not clear how CD4 palmitoylation contributes to T cell signaling.
Conflicting reports also exist regarding the importance of lipid rafts in HIV entry, nonetheless, it has been consistently shown that CD4 palmitoylation is not required for this Citation[74–76]. Raft localization of CD4 was also suggested to contribute to the downmodulation of CD4 by HIV Nef, but here again palmitoylation of CD4 does not appear to play a role Citation[77]. Thus, clear consequences of CD4 palmitoylation have remained elusive so far.
In the case of CD8, palmitoylation was first demonstrated for the mouse CD8αβ heterodimer and occurs here on a single cysteine in the cytoplasmic domain of the CD8β chain Citation[78]. The deletion of this palmitoylation site reduced detergent insolubility of CD8αβ. Absence of CD8β palmitoylation also resulted in a reduction of Lck activation, Ca2+ induction and recruitment of the TCR to DRM following T cell stimulation Citation[79]. Thus, for mouse CD8αβ, palmitoylation appears to contribute to its function in T cell signaling. Interestingly, palmitoylation and detergent insolubility do not occur for the mouse CD8αα homodimer Citation[78], which is expressed on a small subset of T cells as well as on NK and dendritic cells. Despite the fact that CD8α associates with Lck, the CD8αα homodimer is not an efficient TCR coreceptor Citation[80], and this difference in function between CD8αα and CD8αβ has now been attributed to the absence of raft localization of CD8αα.
The role of CD8 palmitoylation is less straightforward when results on human CD8 are considered. Similar to the situation in the mouse, human CD8αβ localizes to DRM, whereas CD8αα does not Citation[81]. Although this points towards a conserved function of these localizations, the involvement of palmitoylation is strikingly different between human and mouse CD8. Human CD8β contains not one, but two palmitoylation sites, but more surprisingly, CD8α is palmitoylated as well. Thus, the human CD8αα homodimer is not detergent insoluble despite being palmitoylated. Also unexpectedly, the CD8αβ heterodimer localizes to DRM in the absence of palmitoylation: deletion of palmitoylation sites in both CD8β and CD8α does not prevent detergent insolubility of CD8αβ. Instead, a cluster of positively charged amino acids functions as a raft-targeting signal for CD8β expressed on its own. However, this motif is not needed for CD8αβ heterodimers, which solely require the pairing of CD8α and CD8β extracellular domains for detergent insolubility. Hence, human CD8αβ has an intrinsic ability to localize to DRM, but palmitoylation does not appear to play a role in this. A role for palmitoylation in protein transport was also not detected, since non-palmitoylated CD8αα and CD8αβ are efficiently expressed at the cell surface Citation[81]. Whether palmitoylation is important for the coreceptor function of human CD8αβ, has not yet been assessed.
Overall, it can be concluded that, whereas there is some evidence that palmitoylation contributes to CD4 and CD8 function, the mechanism for this is not understood. Furthermore, it is obvious that a simple relationship between palmitoylation and DRM localization does not exist. This is also clear from previous data on the Transferrin Receptor that similarly, is not detergent insoluble despite being palmitoylated Citation[6].
The adaptors LAT and PAG/Cbp
The adaptor LAT is primarily expressed in T cells and is essential for the development and activation of these cells Citation[82]. LAT is a transmembrane protein with an extracellular region of only a few amino acids and a large cytoplasmic domain. Tyrosines in the cytoplasmic domain provide docking sites, when phosphorylated, for Grb2, PLC-γ1, SLP-76 and PI3K. At the junction of the transmembrane and cytoplasmic domain, Cys26 and Cys29 are palmitoylated with Cys26 being the most prominent site Citation[83]. The use of mutant proteins with deletions at these sites has shown that palmitoylation of LAT is essential for optimal recruitment and/or phosphorylation of Grb2, PLC-γ?, Vav and SLP-76 Citation[84], Citation[85]. In the absence of LAT palmitoylation, downstream events such as Ca2+ fluxes, Erk activation and the induction of transcription factors AP-1 and NFAT cannot occur.
The involvement of palmitoylation in LAT function has been attributed to a role in lipid raft targeting. In agreement with this, a large proportion of LAT localizes to detergent insoluble membranes in a palmitoylation-dependent manner Citation[69], Citation[83–85]. Furthermore, poly-unsaturated fatty acids (PUFAs) displace both Lck and LAT from DRM, but negative effects on T cell activation could be prevented by targeting LAT to these domains in a PUFA resistant manner Citation[86]. Palmitoylation of LAT was further demonstrated to be important for the formation of TCR signaling assemblies: plasma membrane fractions isolated on anti-CD3 beads (also described with respect to Lck above) contained TCRζ??, Grb2 and PLCγ? only when LAT palmitoylation sites were intact Citation[69]. On the other hand, the diffusion patterns of palmitoylation-deficient and wildtype LAT clusters were similar with respect to TCR activation-induced CD2 signaling clusters Citation[48], arguing against a role for palmitoylation in the movement to and trapping at sites of T cell activation. The significance of LAT raft localization has also been challenged by the forced localization of LAT to non-raft domains, which does not affect its function Citation[87]. A chimera composed of the cytoplasmic region of LAT and the transmembrane and extracellular domains of the adaptor LAX is excluded from DRM, but can reconstitute T cell activation in LAT-deficient T cells. Moreover, this LAX/LAT chimera can rescue T cell development in LAT-deficient mice, and permits the generation of fully functional peripheral T cells. Thus, in the context of LAX/LAT, palmitoylation is not essential for LAT function. Based on the difference in detergent insolubility between LAT and LAX/LAT, these data also argue against a role for rafts in LAT function, although it remains possible that these proteins target to the same membrane subdomains. LAT palmitoylation may serve functions distinct from raft targeting. For instance, it has been demonstrated that non-palmitoylated LAT mislocalizes to some extent to the Golgi region and has a shorter half-life than the wildtype protein, indicating that palmitoylation ensures proper plasma membrane localization and protein stability Citation[88]. Other data are consistent with such a role as well. Peptides composed of the LAT extracellular and transmembrane regions only, do not associate with membranes in the absence of palmitoylation Citation[89]. Lower protein levels compared to the wildtype protein were also shown for palmitoylation-deficient LAT in transfected Jurkat cells Citation[69] and for LAT/GFP fusion proteins Citation[48]. Therefore, defects of T cell activation in the absence of LAT palmitoylation may at least to some extent arise from inefficient expression of LAT at the plasma membrane.
Interestingly, differences in LAT palmitoylation between activated and anergic T cells have been reported. The induction of T cell anergy was shown to coincide with a reduction in LAT palmitoylation and a decrease of LAT levels at the immunological synapse Citation[90]. This suggests that dynamic palmitoylation of LAT occurs and that the regulation of LAT palmitoylation contributes to T cell anergy. A pool of non-palmitoylated LAT exists in T cells, and the size of this may be controlled by the activation state of these cells. The palmitoylation of Fyn was not affected in anergic T cells, which indicates that perhaps the activity of specific palmitoyl transferases are regulated in T cells.
The adaptor Cbp/PAG shares with LAT some degree of sequence homology, has a comparable domain organization and contains two palmitoylation sites at similar positions as LAT Citation[62], Citation[63]. Cbp/PAG is ubiquitously expressed but given its almost exclusive detergent insolubility, an important role in T cell signaling has been postulated. The main function of Cbp/PAG was proposed to be the recruitment of Csk to membranes, resulting in the inactivation of Src kinases. Consistent with a role in T cells, Cbp/PAG is constitutively phosphorylated in resting T cells and dephosphorylated upon T cell activation. Furthermore, overexpression of Cbp/PAG inhibits T cell activation to some extent, which was not observed with Cbp/PAG mutants unable to associate with Csk Citation[63], Citation[91]. Palmitoylation of Cbp/PAG has not been studied extensively, but one recent study reported that palmitoylation-deficient Cbp/PAG is not detergent insoluble, and does not inhibit T cell activation when overexpressed Citation[92]. Whereas these studies point towards a role for Cbp/PAG and its palmitoylation in controlling the activity of Csk in T cells, the generation of Cbp-/- knockout mice has raised strong doubts about this. In contrast to Csk-/- mice that are embryonically lethal, Cbp -/- mice are viable and healthy Citation[93]. Moreover, T cell development and function was not compromised in Cbp-/- mice either Citation[94]. In this light, the roles of Cbp palmitoylation and raft localization also remain unresolved.
Pre-TCR signaling
αβT cells undergo a highly regulated developmental program during maturation in the thymus, which ensures that only those cells that are able to recognize MHC proteins but do not respond to self-peptides, will leave the thymus and make up the T cell repertoire in the blood. One off the first hallmarks of T cell development is the completion of somatic gene recombination to generate a specific TCRβ chain. This TCRβ chain then pairs with a surrogate TCRα chain, pTα, and forms the pre-TCR receptor Citation[95]. Signaling from the preTCR leads to several rounds of cell division, followed by the rearrangement of the TCRα gene, and the subsequent expression of the TCRαβ receptor. Lck and LAT, amongst other proteins, are essential for pre-TCR signaling, but intriguingly, a ligand does not appear to be involved Citation[96]. Instead, it has been hypothesized that constitutive targeting of the pre-TCR to lipid rafts initiates signaling Citation[97]. Raft localization of the pre-TCR has been suggested to depend on pTα palmitoylation. This hypothesis is based on the observations that in contrast to TCRαβ, the preTCR colocalizes with Lck in detergent insoluble fractions and in co-capping experiments. Moreover, the cytoplasmic domain of pTα, but not that of TCRα or TCRβ, is modified by palmitoylation. Furthermore, in an artificial model system using crosslinking of CD3 with cell surface calnexin, raft localization and palmitoylation of calnexin were shown to be required for T cell development Citation[98]. Nevertheless, a convincing role for palmitoylation of pTα has not been demonstrated, as a mutation at the pTα palmitoylation site did not compromise T cell development Citation[99]. In contrast, the deletion of a PPSRK sequence located close to the palmitoylation site did have some effect, but whether this motif is involved in raft localization has not been tested. Again, the role of pTα palmitoylation is not yet clear, but does not appear to correlate with lipid raft localization.
Table I. Summary of the roles of palmitoylation related to raft localization for the proteins discussed in this review.
Summary and conclusions
The role of acylation, in particular palmitoylation, in the function of T cell signaling proteins has been studied primarily in the context of lipid rafts over the past 10 years (summarized in ). Palmitoylation indeed has clear effects on detergent insolubility and function of Lck, Fyn and LAT, but the role of DRM localization is not yet understood. Palmitoylation also affects functions of CD4 and CD8, but here it does not appear to be essential for detergent insolubility. To obtain further insights into palmitoylation, it seems appropriate and timely to analyze this modification for T cell proteins from non-raft perspectives as well. Many areas have remained largely unexplored. For instance, it is not known whether palmitate cycling of T cell proteins is regulated, and/or whether this is influenced by T cell activation. Further, little is known about the effects of palmitoylation on subcellular localizations, in specific on the trafficking of proteins between different membrane compartments, or the endocytosis of transmembrane proteins. Additionally, effects of palmitoylation on lateral mobility of proteins in cell membranes can be further explored, as well as a possible interplay between palmitoylation and other protein modifications such as phosphorylation and ubiquitination. Lastly, the identification and regulation of DHHC proteins in T cells may yield important insights. Thus, a less raft-biased approach to palmitoylation may generate novel knowledge on this modification in general and its role in T cell signaling specifically. Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1999; 1451: 1–16
- Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J Biol Chem 2001; 276: 39501–39504
- Bhatnagar RS, Futterer K, Waksman G, Gordon JI. The structure of myristoyl-CoA:protein N-myristoyltransferase. Biochim Biophys Acta 1999; 1441: 162–172
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol 1997; 7: 14–20
- Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem 2004; 73: 559–587
- Magee AI, Courtneidge SA. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J 1985; 4: 1137–1144
- Schmidt MF. The transfer of myristic and other fatty acids on lipid and viral protein acceptors in cultured cells infected with Semliki Forest and influenza virus. EMBO J 1984; 3: 2295–2300
- Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. Biotechniques 2004; 36: 276–285
- Hang HC, Geutjes EJ, Grotenbreg G, Pollington AM, Bijlmakers MJ, Ploegh HL. Chemical probes for the rapid detection of fatty-acylated proteins in mammalian cells. J Am Chem Soc 2007; 129: 2744–2745
- Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol 2003; 13: 32–42
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem 2002; 277: 41268–41273
- Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 2002; 159: 23–28
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron 2004; 44: 987–996
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res 2006; 47: 1118–1127
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 2005; 307: 1746–1752
- Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE :re 2006; 2006: 14
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol 1998; 16: 523–544
- Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol 1999; 17: 467–522
- Harder T, Engelhardt KR. Membrane domains in lymphocytes – from lipid rafts to protein scaffolds. Traffic 2004; 5: 265–275
- Kabouridis PS. Lipid rafts in T cell receptor signalling. Mol Membr Biol 2006; 23: 49–57
- He HT, Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking point on the involvement of lipid rafts in T-cell activation. EMBO Rep 2008; 9: 525–530
- Horejsi V. Lipid rafts and their roles in T-cell activation. Microbes Infect 2005; 7: 310–316
- Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol 2000; 12: 23–34
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Brown D. Structure and function of membrane rafts. Int J Med Microbiol 2002; 291: 433–437
- Chamberlain LH. Detergents as tools for the purification and classification of lipid rafts. FEBS Lett 2004; 559: 1–5
- London E, Brown DA. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta 2000; 1508: 182–195
- Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol 1992; 149: 3535–3541
- Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 1991; 254: 1016–1019
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol 1993; 5: 349–354
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol 1999; 147: 447–461
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity 1998; 8: 723–732
- Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J 1998; 17: 5334–5348
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell 1994; 76: 411–413
- Abraham N, Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol 1990; 10: 5197–5206
- Paige LA, Nadler MJ, Harrison ML, Cassady JM, Geahlen RL. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem 1993; 268: 8669–8674
- Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal to myristate for membrane binding of p56lck. Biochem Biophys Res Commun 1995; 207: 868–876
- Yasuda K, Kosugi A, Hayashi F, Saitoh S, Nagafuku M, Mori Y, Ogata M, Hamaoka T. Serine 6 of Lck tyrosine kinase: a critical site for Lck myristoylation, membrane localization, and function in T lymphocytes. J Immunol 2000; 165: 3226–3231
- Bijlmakers MJ, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4. J Cell Biol 1997; 137: 1029–1040
- Yurchak LK, Sefton BM. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol 1995; 15: 6914–6922
- Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci 1997; 110: 673–679
- Nadler MJ, Harrison ML, Ashendel CL, Cassady JM, Geahlen RL. Treatment of T cells with 2-hydroxymyristic acid inhibits the myristoylation and alters the stability of p56lck. Biochemistry 1993; 32: 9250–9255
- Kosugi A, Hayashi F, Liddicoat DR, Yasuda K, Saitoh S, Hamaoka T. A pivotal role of cysteine 3 of Lck tyrosine kinase for localization to glycolipid-enriched microdomains and T cell activation. Immunol Lett 2001; 76: 133–138
- Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J 1997; 16: 4983–4998
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol 1994; 126: 353–363
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 2004; 23: 7990–8000
- Hawash IY, Hu XE, Adal A, Cassady JM, Geahlen RL, Harrison ML. The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling. Biochim Biophys Acta 2002; 1589: 140–150
- Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA 2006; 103: 18992–18997
- Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature 1992; 357: 161–164
- Lovatt M, Filby A, Parravicini V, Werlen G, Palmer E, Zamoyska R. Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol Cell Biol 2006; 26: 8655–8665
- Filby A, Seddon B, Kleczkowska J, Salmond R, Tomlinson P, Smida M, Lindquist JA, Schraven B, Zamoyska R. Fyn regulates the duration of TCR engagement needed for commitment to effector function. J Immunol 2007; 179: 4635–4644
- Davidson D, Schraven B, Veillette A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol Cell Biol 2007; 27: 1960–1973
- van't Hof W, Resh MD. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J Cell Biol 1999; 145: 377–389
- Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol 1993; 13: 6385–6392
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell 1997; 8: 1159–1173
- van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol 1997; 136: 1023–1035
- Bijlmakers MJ, Marsh M. Trafficking of an acylated cytosolic protein: newly synthesized p56(lck) travels to the plasma membrane via the exocytic pathway. J Cell Biol 1999; 145: 457–468
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem 1994; 269: 16701–16705
- Liang X, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J Biol Chem 2001; 276: 30987–30994
- Timson Gauen LK, Linder ME, Shaw AS. Multiple features of the p59fyn src homology 4 domain define a motif for immune-receptor tyrosine-based activation motif (ITAM) binding and for plasma membrane localization. J Cell Biol 1996; 133: 1007–1015
- Filipp D, Zhang J, Leung BL, Shaw A, Levin SD, Veillette A, Julius M. Regulation of Fyn through translocation of activated Lck into lipid rafts. J Exp Med 2003; 197: 1221–1227
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 2000; 404: 999–1003
- Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med 2000; 191: 1591–1604
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science 2002; 295: 1539–1542
- Tavano R, Gri G, Molon B, Marinari B, Rudd CE, Tuosto L, Viola A. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J Immunol 2004; 173: 5392–5397
- Ike H, Kosugi A, Kato A, Iino R, Hirano H, Fujiwara T, Ritchie K, Kusumi A. Mechanism of Lck recruitment to the T-cell receptor cluster as studied by single-molecule-fluorescence video imaging. Chemphyschem 2003; 4: 620–626
- Gaus K, Chklovskaia E, Fazekas de St Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol 2005; 171: 121–131
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 2005; 121: 937–950
- Harder T, Kuhn M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J Cell Biol 2000; 151: 199–208
- Leahy DJ. A structural view of CD4 and CD8. Faseb J 1995; 9: 17–25
- Crise B, Rose JK. Identification of palmitoylation sites on CD4, the human immunodeficiency virus receptor. J Biol Chem 1992; 267: 13593–13597
- Fragoso R, Ren D, Zhang X, Su MW, Burakoff SJ, Jin YJ. Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J Immunol 2003; 170: 913–921
- Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol 2004; 172: 5887–5892
- Percherancier Y, Lagane B, Planchenault T, Staropoli I, Altmeyer R, Virelizier JL, Arenzana-Seisdedos F, Hoessli DC, Bachelerie F. HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J Biol Chem 2003; 278: 3153–3161
- Popik W, Alce TM. CD4 receptor localized to non-raft membrane microdomains supports HIV-1 entry. Identification of a novel raft localization marker in CD4. J Biol Chem 2004; 279: 704–712
- Del Real G, Jimenez-Baranda S, Lacalle RA, Mira E, Lucas P, Gomez-Mouton C, Carrera AC, Martinez AC, Manes S. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J Exp Med 2002; 196: 293–301
- Sol-Foulon N, Esnault C, Percherancier Y, Porrot F, Metais-Cunha P, Bachelerie F, Schwartz O. The effects of HIV-1 Nef on CD4 surface expression and viral infectivity in lymphoid cells are independent of rafts. J Biol Chem 2004; 279: 31398–31408
- Arcaro A, Gregoire C, Boucheron N, Stotz S, Palmer E, Malissen B, Luescher IF. Essential role of CD8 palmitoylation in CD8 coreceptor function. J Immunol 2000; 165: 2068–2076
- Arcaro A, Gregoire C, Bakker TR, Baldi L, Jordan M, Goffin L, Boucheron N, Wurm F, van der Merwe PA, Malissen B, Luescher IF. CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med 2001; 194: 1485–1495
- Gangadharan D, Cheroutre H. The CD8 isoform CD8alphaalpha is not a functional homologue of the TCR co-receptor CD8alphabeta. Curr Opin Immunol 2004; 16: 264–270
- Pang DJ, Hayday AC, Bijlmakers MJ. CD8 Raft localization is induced by its assembly into CD8alpha beta heterodimers, Not CD8alpha alpha homodimers. J Biol Chem 2007; 282: 13884–13894
- Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE :R 2000; 2000: E1
- Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 1998; 9: 239–246
- Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol 1999; 11: 943–950
- Lin J, Weiss A, Finco TS. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J Biol Chem 1999; 274: 28861–28864
- Zeyda M, Staffler G, Horejsi V, Waldhausl W, Stulnig TM. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J Biol Chem 2002; 277: 28418–28423
- Zhu M, Shen S, Liu Y, Granillo O, Zhang W. Cutting edge: localization of linker for activation of T cells to lipid rafts is not essential in T cell activation and development. J Immunol 2005; 174: 31–35
- Tanimura N, Saitoh S, Kawano S, Kosugi A, Miyake K. Palmitoylation of LAT contributes to its subcellular localization and stability. Biochem Biophys Res Commun 2006; 341: 1177–1183
- Shogomori H, Hammond AT, Ostermeyer-Fay AG, Barr DJ, Feigenson GW, London E, Brown DA. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem 2005; 280: 18931–18942
- Hundt M, Tabata H, Jeon MS, Hayashi K, Tanaka Y, Krishna R, De Giorgio L, Liu YC, Fukata M, Altman A. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity 2006; 24: 513–522
- Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol 2003; 23: 2017–2028
- Posevitz-Fejfar A, Smida M, Kliche S, Hartig R, Schraven B, Lindquist JA. A displaced PAG enhances proximal signaling and SDF-1-induced T cell migration. Eur J Immunol 2008; 38: 250–259
- Xu S, Huo J, Tan JE, Lam KP. Cbp deficiency alters Csk localization in lipid rafts but does not affect T-cell development. Mol Cell Biol 2005; 25: 8486–8495
- Dobenecker MW, Schmedt C, Okada M, Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol Cell Biol 2005; 25: 10533–10542
- von Boehmer H. Unique features of the pre-T-cell receptor alpha-chain: not just a surrogate. Nat Rev Immunol 2005; 5: 571–577
- Yamasaki S, Saito T. Molecular basis for pre-TCR-mediated autonomous signaling. Trends Immunol 2007; 28: 39–43
- Saint-Ruf C, Panigada M, Azogui O, Debey P, von Boehmer H, Grassi F. Different initiation of pre-TCR and gammadeltaTCR signalling. Nature 2000; 406: 524–527
- Ferrera D, Panigada M, Porcellini S, Grassi F. Recombinase-deficient T cell development by selective accumulation of CD3 into lipid rafts. Eur J Immunol 2008; 38: 1148–1156
- Aifantis I, Borowski C, Gounari F, Lacorazza HD, Nikolich-Zugich J, von Boehmer H. A critical role for the cytoplasmic tail of pTalpha in T lymphocyte development. Nat Immunol 2002; 3: 483–488