Abstract
The efficacy and success of many cellular processes is dependent on a tight orchestration of proteins trafficking to and from their site(s) of action in a time-controlled fashion. Recently, a dynamic cycle of palmitoylation/de-palmitoylation has been shown to regulate shuttling of several proteins, including the small GTPases H-Ras and N-Ras, and the GABA-synthesizing enzyme GAD65, between the Golgi compartment and either the plasma membrane or synaptic vesicle membranes. These proteins are peripheral membrane proteins that in the depalmitoylated state cycle rapidly on and off the cytosolic face of ER/Golgi membranes. Palmitoylation of one or more cysteines, by a Golgi localized palmitoyl transferase (PAT) results in trapping in Golgi membranes, and sorting to a vesicular pathway in route to the plasma membrane or synaptic vesicles. A depalmitoylation step by an acyl protein thioesterase (APT) releases the protein from membranes in the periphery of the cell resulting in retrograde trafficking back to Golgi membranes by a non-vesicular pathway. The proteins can then enter a new cycle of palmitoylation and depalmitoylation. This inter-compartmental trafficking is orders of magnitude faster than vesicular trafficking. Recent advances in identifying a large family of PATs, their protein substrates, and single PAT mutants with severe phenotypes, reveal their critical importance in development, synaptic transmission, and regulation of signaling cascades. The emerging knowledge of enzymes involved in adding and removing palmitate is that they provide an intricate regulatory network involved in timing of protein function and transport that responds to intracellular and extracellular signals.
Palmitoylation
Proteins can be modified by a variety of lipid groups including different fatty acids, isoprenyl groups, cholesterol, and a phosphatidyl-inositol glycan. Most lipid modifications are irreversible. However, palmitoylation, the addition of a 16-carbon saturated palmitate group to the sulfhydryl group of a cysteine to form a thioester, is a reversible modification that affects a variety of functions, including protein trafficking, protein sorting, protein clustering, protein stability, and protein aggregation Citation[1], Citation[2].
In many cases, palmitoylation serves to provide a soluble protein or a protein with a weak membrane avidity, with a hydrophobic membrane anchor. However, many transmembrane proteins (TMPs) are palmitoylated and this modification serves a variety of functions including clustering of the protein, binding to specific lipid and/or protein domains in the residence membrane, folding and/or preventing aggregation Citation[1]. In many cases, palmitoylation is a prerequisite for assembly of signaling complexes Citation[3].
Palmitoylated proteins do not share an easily recognizable consensus sequence and palmitoylation can be found in N-terminal, C-terminal, or middle domains of proteins. However, palmitoylation often involves cysteine residues, which are adjacent to a region mediating membrane association for peripheral membrane proteins or spanning the membrane in TMPs. This is consistent with the finding that most protein acyl transferases (PATs) are TMPs themselves and that a proximity of a cysteine to a membrane may facilitate palmitoylation. Palmitoylated peripheral membrane proteins can be divided into two categories, based on whether palmitoylation is the only lipid modification, or one of several. The first category is exemplified by proteins like GAP-43 Citation[4] and PSD-95 Citation[5], which undergo dual palmitoylation on cysteines near the N-terminus and adjacent to positively charged amino acid residues mediating interaction with negatively charged membrane phospholipids and providing sufficient membrane affinity to enable palmitoylation by a membrane bound PAT. Proteins in the second category undergo palmitoylation following an initial irreversible modification with a different lipid. The first lipid modification varies. Some proteins in this group are modified co-translationally by myristic acid at a glycine that becomes N-terminal after removal of the initial N-terminal methionine and sometimes additional amino acids. This is followed by post-translational palmitoylation of one or more nearby cysteines. Non-receptor tyrosine kinases and the alpha subunit of small trimeric GTP-binding proteins (Gα) belong to this subgroup Citation[6], Citation[7]. A second subgroup includes proteins which are palmitoylated close to the C-terminus. The first lipid modification of these proteins involves a post-translational addition of a hydropbobic isoprenoid group, which can be either a 15-carbon farnesyl or a 20-carbon geranyl-geranyl group. This reaction is catalyzed by a cytosolic transferase and results in a stable thioether bond with a cysteine in a C-terminal CAAX sequence. This process is followed by a removal of the -AAX sequence and addition of a methyl group to form a methyl ester with the now C-terminal cysteine Citation[8]. The two reactions are catalyzed by enzymes located in the endoplasmic reticulum (ER) Citation[9]. The isoprenylated protein is then palmitoylated at one or more adjacent cysteines Citation[10]. The monomeric small GTP-binding proteins N-Ras and H-Ras belong to this group. A third type of irreversible posttranslational hydrophobic modification preceding palmitoylation is found in the smaller isoform of the synthesizing enzyme for GABA, glutamic acid decarboxylase. This protein acquires a hydrophobic modification in the N-terminal domain, which is neither myristoylation nor isoprenylation and has remained elusive Citation[11–13]. Like myristoylation and farnesylation, it confers low avidity membrane association that primes the protein for palmitoylation at two adjacent cysteines by a Golgi membrane bound PAT Citation[12], Citation[13].
There are proteins that become stably palmitoylated by forming an amide bond with the α-amino group of an N-terminal cysteine or an ε-amino groups of a lysine. These permanently palmitoylated proteins and the enzyme that catalyze their palmitoylation Citation[3] are beyond the scope of this review, which will focus on reversible palmitoylation and the enzymes involved.
A palmitoylation/depalmitoylation cycle mediating inter-compartmental shuttling of peripheral membrane proteins
Ras proteins
A model of a palmitoylation/depalmitoylation cycle, which rapidly shuttles proteins back and forth between the Golgi compartment membranes and post-Golgi membranes, is shown in . This cycle was first identified for the N-Ras and H-Ras proteins Citation[14], Citation[15]. Ras proteins are small GTPases involved in regulation of several cellular processes with distinct outcomes, including cell differentiation, growth, proliferation, and apoptosis. Ras is a key regulator of the mitogen-activated protein kinase (MAPK) cascade that receives signals from a variety of growth factors and hormones via tyrosine kinase receptors Citation[16]. Mammalian genomes encode three different ras genes, which give rise to H-Ras, N-Ras, and K-Ras4B. These proteins are highly homologous, have identical effector binding domains, but differ in the C-terminal membrane targeting region that anchors them to cellular membranes, and the adjacent hypervariable domains. All Ras proteins are synthesized as hydrophilic soluble molecules, which are post-translationally modified by a farnesyl group at the C-terminus (Step 1 in ) Citation[8]. The farnesylation of N-Ras and H-Ras results in a low affinity membrane association. The proteins cycle on and off the cytosolic face of different membranes, sampling the environment, until they are further modified by palmitoylation of the adjacent cysteine 181 in N-Ras and cysteines 181 and 184 in H-Ras (Step 2 in ). The palmitoylation occurs on Golgi membranes and results in increased membrane affinity of N-Ras and H-Ras, trafficking through the ER/Golgi network, and sorting to the exocytic vesicular pathway to the plasma membrane () Citation[17]. A series of polybasic lysines upstream from the farnesyl group in K-Ras4B target this protein directly to the plasma membrane without the involvement of endomembranes Citation[17]. Rocks et al. Citation[14] and Goodwin et al. Citation[15] showed that N-Ras and H-Ras dynamically cycle between the plasma membrane and the Golgi compartment. They used fluorescence recovery after photobleaching (FRAP) to analyze trafficking of N-Ras and/or H-Ras into the Golgi compartment. After selective photobleaching of the Golgi compartment, fluorescent N-Ras or H-Ras molecules from other parts of the cell rapidly re-populated the Golgi area and restored fluorescence, demonstrating a retrograde flow from the plasma membrane to Golgi membranes (Step 3 in ). The monopalmitoylated N-Ras populated Golgi faster than the dually palmitoylated H-Ras reflecting a longer residence of H-Ras in plasma membranes Citation[14]. The repopulation of the Golgi compartment following photobleaching was monophasic with a half-time for the wild type N-Ras and H-Ras in the Golgi compartment of ∼1 and 6 min, respectively, in canine kidney cells and ∼1 and 0.6 min in COS-7 cells (). The reason for the 10-fold difference in half-time measured for wild type H-Ras in canine kidney cells and COS-7 cells is not known.
Figure 1. Schematic model of cycling of the Ras and GAD65 proteins between cytosol, ER/Golgi, and PM/SVM. Newly synthesized hydrophilic and soluble protein (green) undergoes an irreversible hydrophobic modification in the cytosol (Step 1). The resulting hydrophobic protein (red) reversibly associates with ER and Golgi membranes, establishing an equilibrium between membrane and cytosolic pools. In Golgi membranes, the protein undergoes palmitoylation (Step 2) catalyzed by a palmitoyl-transferase. The palmitoylated protein (blue) is targeted to the TGN, shifting the equilibrium from ER and toward the Golgi. From the TGN, the protein is targeted to cytosolic vesicles and a pathway to either the PM (Ras) or synaptic vesicles (GAD65). A depalmitoylation along this pathway (Step 3) results in a reversal to the non-palmitoylated form, trafficking back to ER/Golgi membranes by a microtubule-independent pathway, and a new cycle of palmitoylation and targeting to a vesicular pathway. Bilayer membranes are shown as single lines for clarity. In reality, the lipid modifications interact only with the cytoplasmic leaflet of the bilayer. PAT, palmitoyl transferase; APT, acyl-protein thioesterase, ER, Endoplasmic reticulum; PM, plasma membrane; SVM synaptic vesicle membrane.
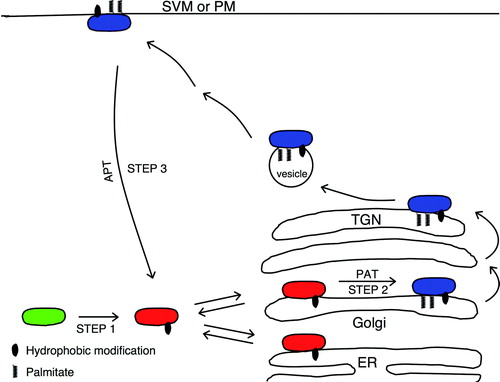
Table I. Half-times of fluorescent protein recovery into the Golgi compartment following photobleaching.
In comparison, the half-time for repopulation of Golgi membranes by the palmitoylation deficient mutants of N-Ras and H-Ras was in the order of a few seconds (), representing the rapid on and off cycling between cytosol and membranes by farnesylated but non-palmitoylated Ras. Further evidence for the retrograde trafficking from the plasma membrane to the Golgi compartment was obtained by analyzing trafficking of Ras proteins coupled to a photoactivatable fluorescent tag. Following activation of the fluorescence on H-Ras and N-Ras in the plasma membrane, fluorescent molecules appeared in the Golgi compartment Citation[14]. The retrograde trafficking of Ras proteins was dependent on both palmitoylation and depalmitoylation Citation[14]. It was already known that activated Ras is found both on the PM and on Golgi membranes Citation[18]. However, prior to the discovery of the cycling of H-Ras and N-Ras, activated protein in the Golgi compartment was thought to represent newly synthesized molecules in route to the plasma membrane. A block in the palmitoylation/depalmitoylation cycle, however, resulted in a loss of activated H-Ras in the Golgi compartment, suggesting that a significant fraction of activated H-Ras in Golgi originates at the plasma membrane. Activation of H-Ras occurred first at the plasma membrane followed by a delay of 10–15 min for activated protein to appear in Golgi membranes. In contrast, N-Ras activation at the Golgi did not show a delay, consistent with a more rapid trafficking from the PM Citation[14]. The identification of the acylation/deacylation cycle revealed a novel layer of functional diversity in Ras signaling and showed that the number of palmitic acids in a molecule influence its distribution between the Golgi compartment and PM.
GAD65
A palmitoylation/depalmitoylation cycle is not limited to distribution of proteins between the Golgi compartment and plasma membranes. Thus, the GABA-synthesizing enzyme GAD65 undergoes a palmitoylation/depalmitoylation cycle that controls its distribution between the Golgi compartment and synaptic vesicle membranes () Citation[19]. GAD65 is the smaller isoform of the GABA-synthesizing enzyme glutamic acid decarboxylase. In brain, it is mainly present as an inactive apoform that is transiently activated by influx of the coenzyme pyridoxal 5′-phosphate Citation[20]. The highly homologous larger isoform, GAD67, a product of a separate non-allelic gene, is constitutively active and synthesizes >90% of basal levels of GABA in the brain. Ablation of the GAD67 gene in mice results in a neonatal lethal phenotype Citation[21], while GAD65−/ − mice develop normally but are prone to epilepsy, anxiety, and visual problems. Numerous studies of GAD65−/ − mice have revealed defects in handling of environmental stimuli, including stress [19 and references therein]. The strategic location of GAD65 in the membrane of synaptic vesicles that secrete its product GABA Citation[11], Citation[22] is believed to be critical for rapid filling of vesicles to sustain repeated firing of GABA-ergic neurons in periods of acute increase in inhibitory neurotransmission.
GAD65 and GAD67 differ mainly in the N-terminal region, which harbors the membrane anchoring signals for GAD65. Similar to the Ras proteins, GAD65 is synthesized as a soluble hydrophilic molecule. The first step of hydrophobic modifications takes place in the cytosol and involves an irreversible addition of a heretofore undefined hydrophobic group to a signal residing in amino acids 24–31 (Step 1 in ) Citation[13]. The result is a hydrophobic form that has a weak membrane affinity and cycles on and off Golgi membranes, establishing an equilibrium between membrane and cytosolic pools similar to farnesylated but non-palmitoylated Ras Citation[19]. However, while non-palmitoylated Ras seems to target indiscriminately to different intracellular membranes and only acquires a specific attachment to Golgi membranes when it is palmitoylated Citation[14], Citation[15], the hydrophobic non-palmitoylated form of GAD65 specifically targets to ER/Golgi membranes by virtue of its N-terminal targeting signal Citation[23], Citation[24]. The second step of post-translational modifications of GAD65 involves palmitoylation of cysteines 30 and 45 catalyzed by a PAT in Golgi membranes (Step 2 in ) Citation[13], Citation[19] and results in a membrane-anchored protein, which is only released from membranes by detergent Citation[11], Citation[12]. From the TGN, the protein is sorted into cytosolic vesicles that initiate a pathway to synaptic vesicle membranes (SVM) in presynaptic termini in neurons. In neurons, the cytosolic vesicles may intersect with the Rab5a pathway described as critical for axon-specific targeting of GAD65 to presynaptic clusters Citation[25].
Recently, FRAP analyses after photobleaching of GAD65-GFP in the Golgi compartment of primary neurons, COS-7 and canine kidney cells revealed that two pools of GAD65 repopulate Golgi membranes following photobleaching () Citation[19]. In both neuronal and non-neuronal cells there was a rapidly recovering pool corresponding to hydrophobic non-palmitoylated GAD65 with a half-time of recovery of less than a second in neurons and 2–3 sec in COS-7 and MDCK cells, and a slower pool which replenishes the Golgi compartment with a half-time of 2–3 minutes (). The fast pool was shown to be independent of palmitoylation and its rate of recovery was similar to the rates of cytosol to Golgi membrane trafficking of a palmitoylation mutant of GAD65 Citation[19] and of farnesylated but non-palmitoylated N-Ras and H-Ras proteins Citation[15]. As for N-Ras and H-Ras, this rate of recovery is orders of magnitude faster than vesicular transport of transmembrane proteins from ER to Golgi Citation[26], Citation[27], which amounts to tens of minutes. It is similar to the recovery kinetics of several peripheral proteins that exchange continually between Golgi membranes and cytoplasmic pools in a non-vesicular fashion Citation[27]. The fast pool represents the non-palmitoylated form of GAD65, i.e. a protein, which only has the first hydrophobic modification and continually cycles on and off ER/Golgi membranes (). The recovery by the slow pool of GAD65-GFP was, however, palmitoylation-dependent. The half-time of recovery of this pool into the Golgi compartment was intermediary between the half-time of recovery of singly palmitoylated N-Ras and dually palmitoylated H-Ras in Golgi membranes following photobleaching Citation[14] reflecting perhaps the longer time it takes for doubly palmitoylated proteins to traffic from the periphery. The rate of recovery of the slow pool of GAD65-GFP is orders of magnitude faster than de novo synthesis of GAD65 which has a half-life of 20–30 h in pancreatic beta cells Citation[11]. It is similar to the rate of depalmitoylation by the cytosolic mammalian depalmitoylation enzyme acyl-protein thioesterase 1 (APT1) Citation[28–30]. Thus, it was concluded that the slow pool represents palmitoylated GAD65, which undergoes depalmitoylation, release from a vesicular pathway and retrograde trafficking to the Golgi compartment (Step 3 in ) where it can undergo a new cycle of de-palmitoylation and re-palmitoylation.
Palmitoylation of N-Ras and H-Ras appears to be more efficient than palmitoylation of GAD65. Thus, the rapid pool of Golgi replenishment by farnesylated but non-palmitoylated N-Ras and H-Ras is only observed in FRAP analyses of palmitoylation deficient mutants but not for the wild type proteins Citation[14], Citation[15]. In contrast, in FRAP analyses of GAD65 the size of the rapid pool of the non-palmitoylated pool is similar or larger than that of the palmitoylated pool suggesting that is is a significant fraction of the protein at any given time Citation[19].
There is evidence to suggest that the de/reacylation cycle shown to shuttle N-Ras, H-Ras and GAD65 between compartments is not limited to those proteins. Thus, four different palmitoylated N-terminal targeting sequences of plasma membrane proteins corresponding to the neuronal growth cone protein GAP43 (amino acids 1–20), a subunit of a small trimeric GTP binding protein, Giα1 (amino acids 1–32), the endothelial nitrogen oxide synthetase eNOS (amino acids 1–55), and an artificial myristoylated and palmitoylated sequence of 33 amino acids, were coupled to YFP and analyzed by FRAP for repopulation of the Golgi compartment following photobleaching in the presence and absence of 2-bromo-palmitate (2BP), an inhibitor of palmitoylation, in canine kidney cells Citation[14]. All four proteins revealed kinetics and 2BP sensitivity consistent with acylation-dependent cycling between Golgi and plasma membranes with kinetics similar to that found for N-Ras, H-Ras, and GAD65 (). Furthermore, recent evidence suggests that trafficking between the Golgi compartment and PM by an acylation/deacylation cycle may be a common property of several subunits of heterotrimeric G proteins Citation[31].
The results suggest that reversible palmitoylation provides a versatile mechanism for dynamic distribution of proteins between the ER/Golgi and post-Golgi membranes. Whether these proteins associate with a chaperone during retroactive transport in the cytosol between PM/SVM and the Golgi compartment, as suggested for cytosolic Ras proteins Citation[32], remains to be shown. What are the functional consequences of compartmentalized signaling of Ras? Rocks et al. Citation[14] showed that an inhibition of palmitoylation prevented activated H-Ras from reaching the Golgi compartment. The different outputs from Ras action are dependent on the specific spatial organization of a given Ras molecule in a particular membrane compartment and the timing of its localization in that compartment Citation[33]. It has been proposed that different membrane compartments, such as the PM and Golgi, provide unique sets of GTPase activating proteins, guanine nucleotide-exchange factors, and downstream effector pathways Citation[34]. The ability of the cell to distribute N-Ras, H-Ras and K-Ras4B differently between Golgi and PM is likely a critical part of the ability of Ras to control so many distinct cellular processes. For GAD65, the cycle may control how much of the protein is available for rapid synthesis of GABA in SV and how much is deposited in the Golgi compartment. The question remains whether the active holoenzyme is localized in both the Golgi compartment and in SVM or whether it is primarily restricted to the latter compartment. It will be of interest to study: (i) The kinetics of trafficking of GAD65 into presynaptic clusters following specific photobleaching of this compartment, and (ii) how the kinetics of repopulation of the Golgi compartment and presynaptic clusters, respectively, are affected by the functional state of GABA-ergic neurons.
Local acylation cycles
For most palmitoylated proteins, the palmitate turns over multiple times during the lifetime of the protein. The half-life of palmitate has typically been estimated based on pulse-chase labeling experiments with [3H]-labeled palmitate and shown to be much faster than the half-life of the protein. Recent studies Citation[14], however, suggest that the pulse-chase experiments underestimate the turnover time of palmitate by 15- to 20-fold possibly because they do not account for released palmitate, which is stored as palmitoyl-CoA and used for reacylation Citation[35], Citation[36].
The palmitoylation/depalmitoylation cycle that shuttles the Ras and GAD65 proteins between the Golgi and membranes in the periphery of the cell, is a mechanism for rapid and dynamic inter-compartmental distribution of peripheral membrane proteins. But local palmitoylation/depalmitoylation cycles may take place to mediate dynamic palmitoylation within a specific membrane compartment. For instance, while palmitoylation of newly synthesized TMPs may take place in the Golgi compartment, the dynamic and rapid acylation cycles of these proteins in their target membrane, which is often the plasma membrane, may involve local PATs and APTs. This would avoid a time-consuming retroactive trafficking by a vesicular pathway to the Golgi for repalmitoylation. This may be particularly relevant in neurons where rapid cycles of palmitoylation are involved in control of several functions at the synapse and the distance from dendrites or from axon termini to the soma is large. It is also possible that local palmitoylation cycles play a role in dynamic palmitoylation of some peripheral membrane proteins, such as the postsynaptic density protein PSD-95, that functions as a scaffolding protein at the postsynapse Citation[36]. Thus, an initial palmitoylation of a newly synthesized PSD-95 in ER/Golgi membranes mediating vesicular trafficking to post-Golgi membranes may be followed by a local acylation/deacylation cycle in post-synaptic membranes. The dual localization of some PATs in Golgi as well as either SVM or PM ( and text below) suggests that palmitoylation of a specific protein substrate can take place in either location. Furthermore, the presence of an APT in presynaptic clusters (see below) is consistent with a local cycle of palmitoylation/depalmitoylation taking place at the presynapse. It is also possible that Golgi structures described in dendrites Citation[37] may serve as stations for inter-compartmental shuttling of proteins between postsynaptic and Golgi membranes locally in dendrites.
Table II. Intracellular localization of mammalian DHHC family of palmitoyl transferases.
Mechanisms of palmitoylation and depalmitoylation
The DHHC family of palmitoyl transferases
Because of the critical role and dynamic nature of palmitoylation in cellular processes, it was long expected to be controlled by PAT and APT enzymes catalyzing the addition and removal of protein palmitate. It was only recently, however, that enzymes mediating transfer of palmitate from palmitoyl-CoA to a protein substrate were identified in Saccharomyces cerevisae and subsequently in mammals. In yeast, Erf2p and Erf4p were shown to form a complex that localizes to the ER and is a PAT for yeast Ras2p Citation[38]. Deletion of either Erf2 or Erf4 caused a reduction in palmitoylation of Ras2p and its relocalization from the plasma membrane to internal membranes Citation[39]. The Akr1 protein in yeast was identified as a PAT for yeast casein kinase 2 Citation[40]. These proteins were found to share a common 50 residue zinc finger-like sequence, containing a cysteine-rich domain (CRD) with a DHHC motif, which mediates the PAT activity. In mammalian cells, a Golgi apparatus-specific protein with the DHHC zinc finger domain (GODZ) was shown to palmitoylate the γ2 subunit of the GABA-A receptor Citation[41]. The yeast and Drosophila genomes contain 7 and 20 DHHC family proteins each Citation[42], Citation[43]. A search in the mouse and human genome, identified 23 genes encoding for proteins containing this motif. Four of the encoded proteins, DHHC-2, -3, -7 and -15, were shown to mediate palmitoylation of PSD-95 in mammalian cells Citation[44]. In a separate in vitro study, the Huntingtin-interacting protein HIP14 was shown to be a palmitoyl transferase (DHHC-17) which mediates palmitoylation of huntingtin as well as several synaptic proteins in vitroCitation[45]. A complex of DHHC-9 and the Golgi complex protein of 16 kDa (GCP16) was shown to add palmitate to mammalian Ras and be the functional ortholog of Erf2p and Erf4p in yeast Citation[46]. All DHHC proteins except the yeast protein Yn1155W are integral membrane proteins with four or more transmembrane domains. Beyond the zinc finger-like region, containing the CRD with most of the DHHC motif, the enzymes share little homology. The DHHC motif is typically located between two transmembrane domains and faces the cytosol, a feature that may provide critical proximity to the catalytic site for proteins adhering transiently to the membrane and becoming palmitoylated Citation[47] as predicted by the membrane trapping model of palmitoylation Citation[48]. This model was originally proposed to explain the two-step membrane anchoring mechanisms of mammalian Ras proteins Citation[8] and heterotrimeric G-proteins Citation[49]. In this model, the low membrane affinity of the myristoyl and prenyl lipids enables proteins to cycle on and off intracellular membranes. The addition of a palmitate by a specific PAT results in increased membrane affinity and determines in which compartment the protein becomes firmly membrane anchored. It may then be sorted to a vesicle-mediated pathway for trafficking to other compartments while depalmitoylation will return the protein to the state where it samples different membranes. The N-Ras and H-Ras proteins are examples of proteins that cycle indiscriminately on and off different intracellular membranes until an ER/Golgi-localized PAT traps them in membranes. In this case, it is the subcellular localization of the PAT that determines their localization in ER/Golgi following palmitoylation. However, GAD65 is an example of a protein that exclusively cycles on and off ER/Golgi membranes in the non-palmitoylated state due to its own targeting sequence. In this case, the only requirement is that a PAT for the substrate protein resides in the Golgi compartment. However, localization of the same PAT in additional compartments will not compromise the Golgi-specific targeting of the substrate protein.
Acyl protein thioesterases
Compared to the large number of PATs in the mammalian genome, and based on the idea that the multiplicity of regulatory functions mediated by palmitoylation/depalmitoylation cycles is reminiscent of the phosphorylation/dephosporylation cycles mediated by kinases and phosphatases, one would expect to find numerous thioesterases involved in depalmitoylation of proteins. However, only three thioesterases have been identified in mammals and of those, only two are known to depalmitoylate protein substrates. Protein palmitoylthioesterase 1 (PPT1) Citation[50] is involved in depalmitoylation of proteins undergoing degradation in lysosomes Citation[51]. However, it is also present in synaptic vesicles in neurons and appears to be critical for trafficking of several presynaptic proteins including VAMP2, SNAP25, syntaxin 1, SYTI, and GAD65 Citation[52]. Mutations in PPT1 result in a devastating neurodegenerative disorder, infantile neuronal ceroid lipofuscinosis Citation[52], Citation[53]. Protein palmitoylthioesterase 2 (PPT2) shares 18% homology with PPT1 and is localized to lysosomes where it hydrolyzes long-chain fatty acyl CoAs Citation[54]. PPT2 and PPT1 activities are not redundant. Thus, PPT2 does not hydrolyze the fatty acid thioesters that accumulate in PPT1-deficient cells and ablation of the PPT1 or PPT2 gene in the mouse results in distinct neuronal ceroid lipofuscinosis phenotypes Citation[55]. Currently, there are no known protein substrates of PPT2 Citation[54]. A third thioesterase, acylprotein thioesterase-1 (APT1) is a cytosolic protein that has been shown to remove palmitate from Giα, H-Ras, and endothelial nitric oxide synthase and may function in the depalmitoylation of numerous proteins Citation[28], Citation[29]. The kinetics of this enzyme are consistent with a role in the depalmitoylation-repalmitoylation cycle for N-Ras, H-Ras, and GAD65 Citation[28–30], Citation[56]. The identification of additional APTs is expected and awaited.
Methods and approaches for identifying the protein substrate(s) of DHHC enzymes
Three different approaches have provided important knowledge on the identity of protein substrates for a specific PT or the candidate PAT for a specific protein substrate. The method of Fukata et al. Citation[44], Citation[57], Citation[58] involves co-transfecting a cDNA for a candidate protein together with a cDNA for a PAT into heterologous cells and analyses of enhancement of palmitoylation by individual enzymes. The cells are biosynthetically labelled with [3H] palmitic acid followed by separation of total cellular proteins by SDS-PAGE and fluorography to detect the [3H]-labeled proteins. Further analyses of a candidate PAT include establishing whether it expresses in the same tissues as the substrate, and whether knock-down of the enzyme by siRNA results in a decreased palmitoylation of the protein susbstrate. This approach was used to identify DHHC-2, 3, 7, 15 as candidate PATs for PSD-95 Citation[44], DHHC-21 as the most likely PAT for eNOS and Lck Citation[58], Citation[59], HIP14 (DHHC-17), DHHC-3, and DHHC-7 for SNAP-25, and DHHC-9 and 18 for H-Ras Citation[58]. DHHC-3 (GODZ) and DHHC-7 for Gαs, GAP-43, and the GABAA receptor γ2 subunit Citation[58], Citation[60], confirming earlier results for DHHC-3 and the GABAA receptor γ2 subunit Citation[41].
Roth et al. Citation[42], developed a different approach to study palmitoylated proteins that circumvents the lengthy autoradiographic exposure required for detection of [3H]-palmitate labeled proteins. This method was applied to the study of the palmitoylproteome and a study of PAT/protein substrate pairs in yeast. The method uses an acyl-biotinyl exchange chemistry Citation[61] substituting biotin for palmitoyl modifications in proteins. The first step involves blockade of free thiols in proteins with N-ethyl maleimide (NEM). Next, palmitoylated thioester linkages are cleaved with hydroxylamine. Then, sulfhydryl groups exposed by the hydroxylamine treatment are biotinylated using a thiol-specific biotinylation reagent. Finally, biotinylated proteins are affinity purified by streptavidin-agarose and identified by mass spectrometry or SDS-PAGE and immunoblotting. An obligatory control involves a sample where the hydroxylamine treatment step is omitted. Proteins unique to the treated sample represent purified palmitoyl proteins. This approach identified 12 of 15 known palmitoylated proteins in yeast and identified 35 new ones. Using yeast strains in which the genes for DHHC PATs were deleted individually or in combination, the study then identified multiple DHHC PAT substrate protein pairs and also showed that the DHHC family of enzymes accounts for most of the protein palmitoylation events in S. cerevisiae. However, in rare cases palmitoylation may involve: (i) PATs that are not members of the DHHC family and/or, (ii) spontaneous palmitoylation with palmitoyl coenzyme A as recently demonstrated for the trafficking factor Bet3 in yeast Citation[62]. Interestingly, none of the individual DHHC PAT mutants in yeast are lethal. However, strain inviabilities are encountered with cumulative mutations of DHHC-enzymes. Many yeast DHHC PATs were shown to have multiple protein substrates while others displayed substrate specificity Citation[42].
A recent and promising approach should make it possible to identify any protein which is a substrate of a specific DHHC PAT Citation[63]. This method was used to identify a protein substrate of the DHHC-2 PAT, an enzyme which has been implicated in human colon and other cancers (). In this approach, the DHHC protein of investigation is expressed in HeLa cells. One half of the culture is subjected to siRNA-mediated knock-down of the DHHC enzyme, while the other half is mock treated. Next, free thiols are blocked using methyl methane thiosulfonate. Then, palmitoylated thioester linkages are cleaved with hydroxylamine. Sulfhydryl groups exposed by the hydroxylamine treatment are biotinylated using a thiol specific biotinylation reagent containing a heavy isotope for the siRNA-treated sample and light isotope for the mock sample. Next, the samples are incubated with trypsin followed by isolation of biotinylated peptides by streptavidin-agarose affinity chromatography. The peptides are separated and subjected to mass spectrometric analyses for identification. Each of the isolated peptides contains a cysteine, which was palmitoylated in the original protein and now is attached to either a heavy or a light biotin group yielding two peptides that can be separated by liquid column chromatography. For a palmitoylated protein, which is not a substrate of the DHHC enzyme, the two biotinylated peptides with a heavy and light isotope from the treated and mock cultures, respectively, are present in similar quantities, i.e. in a ratio of approximately one. In contrast, the two biotinylated peptides from a palmitoylated protein, which is a substrate of the DHHC enzyme under investigation, differ in quantity such that the treated culture has less of that peptide. Using this method, a peptide derived from the CKAP4/p63 protein was identified as a substrate for DHHC-2. CKAP4/p63 is a palmitoylated protein that has been suggested to link ER with microtubules. Apart from the ER, it localizes to endomembranes and PM where it interacts as a receptor for several extracellular ligands. Knock-down of DHHC-2 resulted in an ER-restricted localization of CKAP/p63 and in a collapsed ER which is concentrated into a narrow disc on the nucleus. DHHC-2 has also been implicated in the palmitoylation of the tetraspanins CD9 and CD151 Citation[64] but those proteins were not identified in the screen for DHHC-2 substrates by the above method.
Table III. Mutations in DHHC proteins associated with pathology.
Subcellular localization of DHHC family PAT proteins
Proteins of the DHHC family are typically TMPs. A study of the subcellular localization of all members of the yeast and human DHHC family of proteins was carried out in yeast and HEK 293 cells, respectively Citation[65]. Of the seven DHHC proteins in yeast, three were localized in ER, two in Golgi, one in PM and one in the yeast vacuole. In HEK 293 cells, eight human DHHC proteins were restricted to the ER, six to Golgi, four localized to both ER and Golgi, and three were reported in the plasma membrane Citation[65]. In Drosophila melanogaster, fourteen DHHC enzymes are localized in the ER, six in Golgi and one in PM Citation[43]. The number of DHHC proteins reported in the PM is, however, increasing (). Thus, while Ohno et al. Citation[65] reported DHHC-2, 3, 7, and 8 in Golgi or ER/Golgi membranes in HEK 293 cells, Fernandez-Hernando et al. Citation[59] confirmed the Golgi compartment as a primary localization of those proteins but also detected them in the PM. In addition, DHHC-21 is detected in both PM and Golgi membranes Citation[59], Citation[65]. While HIP14 (DHHC-17) localizes to the Golgi compartment and cytosolic vesicles in non-neuronal cells Citation[45], Citation[66], it is also found in synaptic vesicles in neurons Citation[66]. DHHC-8 is found in the Golgi compartment, vesicles in the dendritic shaft and in postsynaptic membranes Citation[67]. Thus, while a large number of DHHC PAT enzymes is found in the ER and/or Golgi compartment, a subset is also found in the plasma membrane and/or at neuronal synapses, suggesting a role in palmitoylation in both those locations ().
Functional consequences of DHHC enzyme mutation
Single DHHC PAT mutants in yeast do not show an abnormal phenotype, whereas yeast strains with mutations in multiple DHHC proteins reveal defects in function and even lethality Citation[42]. Mutations of single genes encoding DHHC enzymes in Drosophila melanogaster, including hip14 (DHHC-17) and approximated, however, were recently shown to result in severe abnormalities in function and/or morphology and even lethality.
HIP14 (DHHC-17) was originally identified as a PAT for the Huntingtin protein Htt in mammalian cells Citation[45], Citation[68], a protein that is mutated to include expanded repeats in Huntington's disease. It was shown that such repeats decrease the palmitoylation of Htt and enhance its aggregation and localization to inclusion bodies Citation[68]. The location of HIP14 in SVM in mammalian neurons and its reactivity toward several neuronal membrane proteins including GAD65, PSD95, SNAP25, GAP43, synaptophysin 1 and the cysteine string protein (CSP) in vitro suggests a role in local palmitoylation at synapses Citation[45], Citation[66].
Two independent studies screening for mutations that affect neurotransmitter release in Drosophila melanogaster identified a total of four mutants with severe defects in synaptic transmission resulting in lethality Citation[66], Citation[69]. For each of those, the mutation was located to the gene encoding the Drosophila equivalent of hip14 (DHHC-17). In the fly, the HIP14 protein is localized to the Golgi compartment in non-neuronal cells, while in neurons it is primarily found in SVM Citation[66], Citation[69]. The mutants are morphologically normal but have defects in synaptic transmission due to abnormal SV exocytosis. Analyses of the subcellular localization of several candidate substrates for HIP14, including PSD-95, synaptotagmin 1, neuronal synaptobrevin, CSP and SNAP25 revealed aberrant localization of SNAP25 and CSP but not of the other candidates. Because SNAP25 is considered to be redundant in Drosophila melanogaster, due to the function of another SNARE, SNAP24, CSP was indicated as more likely to contribute to the phenotype Citation[69]. Consistent with this hypothesis, expression as a transgene of CSP targeted to presynapses partially compensated for the defect in synaptic transmission Citation[69]. The results showed that HIP14 plays a critical role in synaptic function by mediating the palmitoylation and proper targeting of specific presynaptic proteins and that the cause of the phenotype includes but is not limited to mislocalization of CSP, a chaperone protein that may be particularly important for neuronal function Citation[70]. As detailed above, HIP14 has been implicated as a PAT for numerous synaptic proteins Citation[45]. It is possible that a defect in the palmitoylation of additional candidate substrate proteins, which were not assessed in these studies, including Htt and GAD, may contribute to the hip14 mutant phenotype.
Recently, another mutation in Drosophila melanogaster, causing severe developmental abnormalities was identified as the gene approximated, which is a member of the DHHC PAT protein family and in the same subgroup as human DHHC-9, 14, 18 and yeast Erf2 Citation[71]. The approximated protein is localized in the PM. It was shown to act by controlling the normal subcellular localization and activity of Dachs, an atypical myosin and negative regulator of Ft signaling which is required for a variety of developmental functions. Since Dachs itself is not palmitoylated, it was speculated that the activity of approximated may be to palmitoylate a binding partner or regulator of Dachs.
The studies of the single DHHC-enzyme family mutants in Drosophila melanogaster are the first reports of a single DHHC PAT in a model organism resulting in severe abnormalities and death. The difference in severity of single DHHC enzyme mutants in yeast and flies is likely to reflect the critical role of individual DHHC proteins in neuronal and/or other specialized cellular function in a multi-tissue organism. Palmitoylation appears to be particularly relevant for structure and function at the neuronal synapse. In addition to protein sorting, palmitoylation plays a role in modulating synapse morphology, neurite growth, axon pathfinding, neurotransmitter release and neurotransmitter receptor function Citation[36], Citation[72]. Local palmitoylation cycles are likely to be intricately involved in synaptic transmission. For instance, the post-synaptic scaffolding protein PSD-95 regulates synaptic strength via activity-dependent palmitate cycling in hippocampal neurons Citation[73].
In humans and mice, several diseases, including cancer, schizophrenia, X-linked mental retardation, and Huntington disease Citation[67], Citation[68], Citation[74–78] have been linked to mutations in the DHHC-gene family ().
Concluding remarks
Since the first demonstration of palmitate turnover on a protein Citation[10] and the later evidence that the turnover of palmitate in the Gαs subunit of a small trimeric GTP binding protein is dependent on signals from its upstream ß-adrenergic receptor Citation[79], dynamic palmitoylation has emerged as a critical mechanism for regulation of a variety of cellular functions. The discovery of palmitoylation/depalmitoylation cycles that control continuous inter-compartment cycling of several proteins and the characterization of the enzymes involved, suggests a novel regulatory network. Many aspects of palmitoylation remain unclear. Is there a larger family of APTs? How selective versus redundant are the members of the DHHC-family of PATs? Are there PATs involved in dynamic palmitoylation that do not belong to the DHHC family? How many proteins traffic continuously between Golgi and post-Golgi membranes by a palmitoylation/depalmitoylation cycle, which enzymes are involved, and how are they regulated? What is the contribution of local versus inter-compartmental palmitoylation-depalmitoylation cycles to the function of palmitoylated proteins? How are different PATs and APTs regulated? There is recent evidence to suggest that PAT activity can be regulated by extracellular growth factors. Thus, binding of FGF2 to the FGF receptor was shown to result in increased palmitoylation of NCAM 140 and 180, a shift of these proteins to lipid rafts, and a stimulation of neurite outgrowth Citation[80]. This mechanism was shown to be critically dependent on palmitoylation of NCAM 140 and 180 and DHHC-7 activity was shown to be directly enhanced by FGF2. This study suggests that an extracellular growth factor can regulate neuronal morphogenesis via activation of a DHHC PAT activity, which then modulates signaling via an adhesion molecule. Thus, a link between an external growth factor and PAT activity has been established, suggesting that palmitoylation cycles add a new dimension to the network of responses to extracellular signaling modules.
Acknowledgements
Steinunn Baekkeskov and Jamil Kanaani were supported by the Nora Eccles Treadwell Foundation and by NIH.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Greaves J, Chamberlain LH. Palmitoylation-dependent protein sorting. J Cell Biol 2007; 176: 249–254
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 2007; 8: 74–84
- Resh, MD. 2006. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. re14.
- Skene JH, Virág I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol 1989; 108: 613–624
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron 1999; 22: 497–509
- Degtyarev MY, Spiegel AM, Jones TL. The G protein alpha s subunit incorporates [3H]palmitic acid and mutation of cysteine-3 prevents this modification. Biochemistry 1993; 32: 8057–8061
- Linder ME, Middleton P, Hepler JR, Taussig R, Gilman AG, Mumby SM. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci USA 1993; 90: 3675–3679
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitylation is required in addition to the CAAX motif to localize p21ras to the plasma membrance. Cell 1990; 63: 133–139
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of Ras: the CAAX motif targets proteins to the ER and Golgi. Cell 1999; 98: 69–80
- Magee AI, Gutierrez L, McKay IA, Marshall CJ, Hall A. Dynamic fatty acylation of p21N-Ras. EMBO J. 1987; 6: 3353–3357
- Christgau S, Schierbeck H, Aanstoot HJ, Aagaard L, Begley K, Kofod H, Hejnaes K, Baekkeskov S. Pancreatic β-cells express two autoantigenic forms of glutamic acid decarboxylase, a 65kDa hydrophilic form and a 64kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem 1991; 266: 21257–21264
- Christgau S, Aanstoot HJ, Schierbeck H, Begley K, Tullin S, Hejnaes H, Baekkeskov S. Membrane anchoring of the autoantigen GAD65 to microvesicles in pancreatic ß-cells by palmitoylation in the N-terminal domain. J Cell Biol 1992; 118: 309–320
- Shi Y, Veit B, Baekkeskov S. Amino acid residues 24-31 but not palmitoylation of cysteines 30 and 45 are required for membrane anchoring of glutamic acid decarboxylase, GAD65. J Cell Biol 1994; 124: 927–934
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PIH. An acylation cycle regulates localization and activity of palmitoylated ras isoforms. Science 2005; 307: 1746–1752
- Goodwin JS, Drake KR, Rogers C, Wright L, Lippincott-Schwartz J, Philips MR, Kenworthy AK. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol 2005; 170: 261–272
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol 2008; 9: 517–531
- Apolloni A, Prior IA, Lindsay M, Parton RG, Hancock JF. H-Ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol Cell Biol 2000; 20: 2475–2487
- Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL 2nd, Cox AD, Philips MR. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol 2002; 4: 343–350
- Kanaani J, Patterson G, Schaufele F, Lippincott-Schwartz J, Baekkeskov S. A palmitoylation cycle dynamically regulates partitioning of the GABA-synthesizing enzyme GAD65 between ER-Golgi and post-Golgi membranes. J Cell Sci 2008; 121: 437–449
- Battaglioli GH, Liu H, Martin DL. Kinetic differences between the isoforms of glutamate decarboxylase: Implications for the regulation of GABA synthesis. J Neurochem 2003; 86: 879–887
- Condie BG, Bain G, Gottlieb DI, Capecchi MR. Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proc Natl Acad Sci USA 1997; 94: 11451–11455
- Reetz A, Solimena M, Matteoli M, Folli F, Takei K, DeCamilli P. GABA and pancreatic ß-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic like microvesicles suggests their role in GABA storage and secretion. EMBO J 1991; 10: 1275–1284
- Kanaani J, El-Husseini AE, Aguilera-Moreno A, Diacovo MJ, Bredt DS, Baekkeskov S. A combination of three distinct trafficking signals mediates axonal targeting and presynaptic clustering of GAD65. J Cell Biol 2002; 158: 1229–1238
- Solimena M, Dirkx R, Radzynski M, Mundigl O, De Camilli P. A signal located within amino acids 1-27 of GAD65 is required for its targeting to the Golgi complex region. J Cell Biol 1994; 126: 331–341
- Kanaani J, Diacovo MJ, El-Husseini AE, Bredt D, Baekkeskov S. Palmitoylation controls trafficking of GAD65 from Golgi membranes to axon-specific endosomes and a Rab5a-dependent pathway to presynaptic clusters. J Cell Sci 2004; 117: 2001–2013
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analyses of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol 1998; 143: 1485–1503
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol 2001; 155: 557–570
- Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21RAS. J Biol Chem 1998; 273: 15830–15837
- Duncan JA, Gilman AG. Characterization of Saccharomyces cerevisiae acyl-protein thioesterase 1, the enzyme responsible for G protein alpha subunit deacylation in vivo. J Biol Chem 2002; 277: 31740–31752
- Yeh DC, Duncan JA, Yamashita S, Michel T. Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca2 + -calmodulin. J Biol Chem 1999; 274: 33148–33154
- Chisari M, Saini DK, Kalyanaraman V, Gautam N. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem 2007; 282: 24092–24098
- Meder D, Simons K. Cell biology. Ras on the roundabout. Science. 2005; 307(5716)1731–1733
- Quatela SE, Philips MR. Ras signaling on the Golgi. Curr Opin Cell Biol 2006; 18(2)162–167
- Rocks O, Peyker A, Bastiaens PI. Spatio-temporal segregation of Ras signals: one ship, three anchors, many harbors. Curr Opin Cell Biol 2006; 18: 351–357
- Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol Ther 2003; 97: 1–33
- Huang K, El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr Opin Neurobiol 2005; 15: 527–535
- Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 2005; 48(5)757–771
- Lobo, S, Greentree, WK, Linder, ME, Deschenes, RJ.. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem, 277:41268–41273.
- Zhao L, Lobo S, Dong X, Ault AD, Deschenes RJ. Erf4p and Erf2p form an endoplasmic reticulum-associated complex involved in the plasma membrane localization of yeast Ras proteins. J Biol Chem 2002; 277: 49352–49359
- Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 2002; 159: 23–28
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoè-Pognetto M, Lüscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci 2004; 24: 5881–5891
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR III, Davis NG. Global analysis of protein palmitoylation in yeast. Cell 2006; 125: 1003–1013
- Bannan BA, Van Etten J, Kohler JA, Tsoi Y, Hansen NM, Sigmon S, Fowler E, Buff H, Williams TS, Ault JG, Glaser RL, Korey CA. The Drosophila protein palmitoylome: characterizing palmitoyl-thioesterases and DHHC palmitoyl-transferases. Fly 2008; 2: 198–214
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron 2004; 44: 987–996
- Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, Mullard A, Haigh B, Guathier-Campbell C, Gutekunst C, Hayden MR, El-Husseini A. Huntingtin-Interacting Protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 2004; 44: 977–986
- Swarthout JT, Lobo S, Farh L, Croke MR, Greentree WK, Deschenes RJ, Linder ME. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem 2005; 280: 31141–31148
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res 2006; 47: 1118–1127
- Shahinian S, Silvius JR. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membrances. Biochemistry 1995; 34: 3813–3822
- Michaelsons D, Aheran I, Bergo M, Young S, Phillips M. Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell 2002; 13: 3294–3302
- Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem 1993; 268: 22566–22574
- Hellsten E, Vesa J, Olkkonen VM, Jalanko A, Peltonen L. Human palmitoyl protein thioesterase: evidence for lysosomal targeting of the enzyme and disturbed cellular routing in infantile neuronal ceroid lipofuscinosis. EMBO J 1996; 15: 5240–5245
- Kim SJ, Zhang Z, Sarkar C, Tsai PC, Lee YC, Dye L, Mukherjee AB. Palmitoyl protein thioesterase-1 deficiency impairs synaptic vesicle recycling at nerve terminals, contributing to neuropathology in humans and mice. J Clin Invest 2008; 118: 3075–3086
- Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature 1995; 376: 584–587
- Soyombo AA, Hofmann SL. Molecular cloning and expression of palmitoyl-protein thioesterase 2 (PPT2), a homolog of lysosomal palmitoyl-protein thioesterase with a distinct substrate specificity. J Biol Chem 1997; 272: 27456–27463
- Gupta P, Soyombo AA, Atashband A, Wisniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci USA 2001; 98: 13566–13571
- Veit M, Schmidt MFG. Enzymatic depalmitoylation of viral glycoproteins with acyl-protein thioesterase 1 in vitro. Virology 2001; 288: 89–95
- Fukata Y, Iwanaga T, Fukata M. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods 2006; 40: 177–182
- Tsutsumi R, Fukata Y, Fukata M. Discovery of protein-palmitoylating enzymes. Pflugers Arch – Eur J Physiol 2008; 456: 1199–1206
- Fernández-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol 2006; 174: 369–377
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Lüscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci 2006; 26: 12758–12768
- Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. Biotechniques 2004; 36: 276–285
- Kümmel D, Heinemann U, Veit M. Unique self-palmitoylation activity of the transport protein particle component Bet3: a mechanism required for protein stability. Proc Natl Acad Sci USA 2006; 103: 12701–12706
- Zhang J, Planey SL, Ceballos C, Stevens SM, Jr, Keay SK, Zacharias DA. Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Mol Cell Proteomics 2008; 7: 1378–1388
- Sharma C, Yang XH, Hemler ME. DHHC2 affects palmitoylation, stability, and functions of tetraspanins CD9 and CD151. Mol Biol Cell 2008; 19: 3415–3425
- Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta 2006; 1761: 474–483
- Stowers RS, Isacoff EY. Drosophila huntingtin-interacting protein 14 is a presynaptic protein required for photoreceptor synaptic transmission and expression of the palmitoylated proteins synaptosome-associated protein 25 and cysteine string protein. Neuroscience 2007; 27: 12874–12883
- Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet 2004; 36: 725–731
- Yanai A, Huang K, Kang R, Singaraja RR, Arstikaitis P, Gan L, Orban PC, Mullard A, Cowan CM, Raymond LA, Drisdel RC, Green WN, Ravikumar B, Rubinsztein DC, El-Husseini A, Hayden MR. Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function. Nat Neurosci 2006; 9: 824–831
- Ohyama T, Verstreken P, Ly CV, Rosenmund T, Rajan A, Tien AC, Haueter C, Schulze KL, Bellen HJ. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J Cell Biol 2007; 179: 1481–1496
- Greaves J, Salaun C, Fukata Y, Fukata M, Chamberlain LH. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J Biol Chem 2008; 283: 25014–25026
- Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol 2008; 18: 1390–1395
- El-Husseini Ael-D, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci 2002; 3: 791–802
- El-Husseini Ael-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 2002; 108: 849–863
- Oyama T, Miyoshi Y, Koyama K, Nakagawa H, Yamori T, Ito T, Matsuda H, Arakawa H, Nakamura Y. Isolation of a novel gene on 8p21.3-22 whose expression is reduced significantly in human colorectal cancers with liver metastasis. Genes Chromosomes Cancer. 2000; 29: 9–15
- Raymond FL, Tarpey PS, Edkins S, Tofts C, O'Meara S, Teague J, Butler A, Stevens C, Barthorpe S, Buck G, Cole J, Dicks E, Gray K, Halliday K, Hills K, Hinton J, Jones D, Menzies A, Perry J, Raine K, Shepherd R, Small A, Varian J, Widaa S, Mallya U, Moon J, Luo Y, Shaw M, Boyle J, Kerr B, Turner G, Quarrell O, Cole T, Easton DF, Wooster R, Bobrow M, Schwartz CE, Gecz J, Stratton MR, Futreal PA. Mutations in ZDHHC9, which encodes a palmitoyltransferase of N-RAS and H-RAS, cause X-linked mental retardation associated with a Marfanoid habitus. Am J Hum Genet 2007; 80: 982–987
- Mansilla F, Birkenkamp-Demtroder K, Kruhøffer M, Sørensen FB, Andersen CL, Laiho P, Aaltonen LA, Verspaget HW, Orntoft TF. Differential expression of DHHC9 in microsatellite stable and instable human colorectal cancer subgroups. Br J Cancer 2007; 96: 1896–1903
- Yamamoto Y, Chochi Y, Matsuyama H, Eguchi S, Kawauchi S, Furuya T, Oga A, Kang JJ, Naito K, Sasaki K. Gain of 5p15.33 is associated with progression of bladder cancer. Oncology 2007; 72: 132–138
- Mansouri MR, Marklund L, Gustavsson P, Davey E, Carlsson B, Larsson C, White I, Gustavson KH, Dahl N. Loss of ZDHHC15 expression in a woman with a balanced translocation t(X;15)(q13.3;cen) and severe mental retardation. Eur J Hum Genet 2005; 13: 970–977
- Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gs alpha. Cell 1994; 77: 1063–1070
- Ponimaskin E, Dityateva G, Ruonala MO, Fukata M, Fukata Y, Kobe F, Wouters FS, Delling M, Bredt DS, Schachner M, Dityatev A. Fibroblast growth factor-regulated palmitoylation of the neural cell adhesion molecule determines neuronal morphogenesis. J Neurosci 2008; 28: 8897–8907