Abstract
Modification of proteins with the lipid palmitate, a process called palmitoylation, is important for the normal function of neuronal cells. However, most attention has focused on how palmitoylation regulates the targeting and trafficking of neurotransmitter receptors and non-enzymatic scaffold proteins. In this review we discuss recent studies that suggest that palmitoylation also plays additional roles in neurons by controlling the localization, interactions and perhaps even the activity of protein kinases that play key roles in physiological neuronal regulation and in neuropathological processes.
Introduction
Proteins can undergo a variety of post-translational modifications that regulate their subcellular localization and function. One such modification is covalent lipid attachment, which increases a protein’s hydrophobicity and hence its association with intracellular and/or plasma membranes. Protein-lipid attachment thus has the potential to dramatically affect a protein’s localization, trafficking and interactions.
Several protein-lipid attachment mechanisms are known for intracellular proteins, including S-acylation, myristoylation, farnesylation and geranylgeranylation. The first of these, S-acylation, refers to the addition of a fatty acid to protein cysteine residues via a thioester bond. When the conjugated fatty acid is the C16 lipid palmitate then this modification is referred to as S-palmitoylation, or simply palmitoylation. For simplicity, the term palmitoylation will be used in this review, although we note that the precise identity of the conjugated lipid has not been determined for many S-acylated proteins.
Palmitoylation occurs even in simple, unicellular organisms such as yeast and is likely important in all eukaryotic cells (Roth et al., Citation2006). However, control of protein localization and/or function by palmitoylation might be predicted to be especially critical in polarized, morphologically complex cells such as neurons. Consistent with this notion, studies of human patients and genetically modified mice have revealed that loss of function of several individual palmitoyl acyltransferases (PATs, the enzymes that catalyze palmitoylation) causes predominantly neuropathological defects (Li et al., Citation2010; Mansouri et al., Citation2005; Mukai et al., Citation2008; Raymond et al. Citation2007).
These findings raise the question of which PAT substrate(s) account for these phenotypes and, more broadly, which cellular processes in neurons are palmitoylation-dependent. Results from palmitoyl-proteomic studies suggest that these are not simple questions because a large number of neuronal proteins are palmitoylated. These palmitoyl-proteins include numerous neurotransmitter receptors, as well as scaffolding and adhesion proteins (Kang et al., Citation2008; Wan et al., Citation2013). In particular, many key pre- and post-synaptic proteins at glutamatergic synapses, the major excitatory synaptic connections in the brain, are palmitoylated. Postsynaptic palmitoyl-proteins include multiple AMPA- and NMDA-type glutamate receptor subunits and several of their scaffolding/auxiliary proteins, while presynaptic palmitoyl-proteins include multiple components of the neurotransmitter release machinery (Craven et al., Citation1999; Fukata & Fukata, Citation2010; Hayashi et al., Citation2005, Citation2009; Kang et al., Citation2008; Oku et al., Citation2013; Prescott et al., Citation2009; Thomas et al., Citation2012,Citation2013). Moreover, palmitoylation-dependent regulation is not just a feature of glutamatergic synapses – specific receptors for the inhibitory neurotransmitter GABA are also palmitoylated, along with gephyrin, a key scaffolding protein at inhibitory synapses (Dejanovic et al., Citation2014; Fang et al., Citation2006; Keller et al., Citation2004). Key roles for palmitoylation in the targeting and trafficking of many of these receptors and non-enzymatic proteins have been described (reviewed by Prescott et al., Citation2009; Fukata & Fukata, Citation2010; Thomas & Huganir, Citation2013). Recently, however, several studies suggest that palmitoylation can more directly regulate neuronal enzymatic signaling, because a select group of protein kinases are modified by palmitoylation. Here, we focus on recent developments in our understanding of how palmitoylation regulates these kinases. Importantly, these recent findings not only provide new insights into neuronal regulation but also reveal broader roles for palmitoylation at both the cellular and molecular levels.
Palmitoylation-dependent signaling by Src-family kinases (SFKs)
The possibility that palmitoylation might be critical for signaling by specific kinases was first raised when several members of the Src family of non-receptor tyrosine kinases (SFKs) were identified as palmitoyl-proteins. In particular, the SFKs Fyn, Lck, Yes, Fgr and Hck were all found to be palmitoylated in non-neuronal cells, although interestingly Src itself was not (Alland et al., Citation1994; Koegl et al., Citation1994; Paige et al., Citation1993; Shenoy-Scaria et al., Citation1994). A series of key papers revealed that palmitoylation is critical for signaling by both Fyn and Lck SFKs in specific cells of the immune system. In particular, point mutation of Fyn or Lck palmitoylation sites, or pharmacological inhibition of palmitoylation with the compound 2-Bromopalmitate markedly impairs signaling from activated T cell or B cell receptors (Kabouridis et al., Citation1997; van’t Hof & Resh, Citation1999; Webb et al., Citation2000). Interestingly, both Fyn and Lck are constitutively modified with the lipid myristate, but myristoylation alone cannot support stable membrane association, so palmitoylation is a critical second lipid modification that is essential for Fyn/Lck membrane targeting and downstream signaling. We highlight several excellent reviews e.g. (Bijlmakers, Citation2009; Resh, Citation2006; Yount et al., Citation2013) for those interested in further details of palmitoylation-dependent SFK signaling in the immune system and in other non-neuronal cells.
Synaptic regulation by the palmitoylated SFK Fyn
In addition to their importance in the immune system and other non-neuronal cells, several palmitoylated SFKs are also expressed in neurons, where they are known to play key roles. In particular, the importance of Fyn for neuronal signaling has long been appreciated because Fyn knockout mice show impairments in hippocampal long-term potentiation (LTP, a long-lasting increase in the strength of transmission at specific synapses that is the best-known cellular correlate of learning and memory). In contrast, genetic deletion of Src or Yes, two other SFKs that are expressed in the hippocampus, does not affect LTP (Grant et al., Citation1992; Kojima et al., Citation1997). The importance of palmitoylation for Fyn membrane targeting and signaling in non-neuronal cells strongly suggests that neuronal Fyn signaling likewise requires its palmitoylation-dependent membrane association.
Consistent with the notion that Fyn is active at synaptic membranes, neuronal Fyn substrates include several transmembrane or membrane-associated proteins that are key components of excitatory synapses. One of the best-known neuronal Fyn substrates is the GluN2B subunit of the NMDA-type glutamate receptor. Fyn phosphorylates multiple tyrosine residues in the GluN2B C-terminal tail, one of which (Tyr1472) lies in a motif that binds clathrin adaptor proteins and controls GluN2B internalization. Tyr1472 phosphorylation by Fyn has been proposed to mask this motif, thus stabilizing GluN2B at the neuronal cell surface (Prybylowski et al., Citation2005; Trepanier et al., Citation2012). Interestingly, the synaptic scaffolding proteins PSD-95 and PSD-93 are also Fyn binding partners and substrates (Nada et al., Citation2003; Trepanier et al., Citation2012). PSD-95/-93 also bind the C-terminal tails of the GluN2A/GluN2B subunits and can thus promote Fyn’s ability to regulate NMDA receptors (Tezuka et al., Citation1999; Trepanier et al. Citation2012). Like Fyn, GluN2A/2B and PSD-95/-93 are palmitoyl-proteins (El-Husseini et al., Citation2002; Hayashi et al., Citation2009; Kang et al., Citation2008), raising the possibility that palmitoylation targets Fyn and its substrates to similar membrane microdomains and thereby facilitates their phospho-dependent regulation.
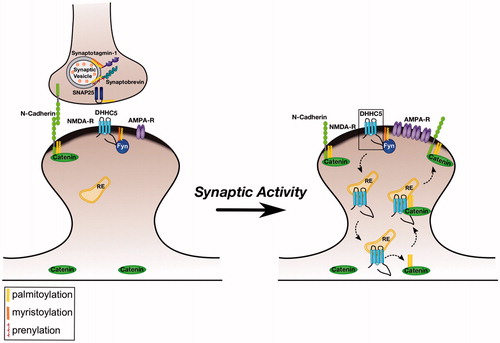
Another aspect of palmitoylation-dependent signaling involving Fyn arises from the recent identification of the PAT DHHC5 as a neuronal Fyn substrate. Fyn phosphorylates DHHC5 at a specific residue (Tyr533) which, like the Tyr1472 site on GluN2B, forms part of a clathrin-dependent internalization motif (Brigidi et al., Citation2015). Again, phosphorylation by Fyn appears to mask this motif, because Tyr533 phosphorylation promotes DHHC5 plasma membrane localization in non-neuronal cells and increases DHHC5 stability on the plasma membrane of neuronal dendritic spines (Brigidi et al., Citation2015). In an elegant series of experiments, Bamji and colleagues further showed that DHHC5 stability in spines involves a tri-partite complex of Fyn, DHHC5 and PSD-95 (Brigidi et al., Citation2015). Interestingly, this complex can be rapidly regulated by changes in neuronal activity, with important consequences for synaptic transmission and plasticity. In particular, enhancing neuronal activity leads to rapid dephosphorylation of DHHC5-Tyr533, which triggers rapid DHHC5 internalization. This acute activity-dependent relocalization is necessary for DHHC5 to subsequently palmitoylate the adhesion protein delta-catenin (Brigidi et al., Citation2014,Citation2015), which then traffics back into spines with DHHC5. Importantly, previous work from the same group revealed that DHHC5-dependent delta-catenin palmitoylation stabilizes synaptic cadherin-catenin complexes and also plays a key role in activity-dependent increases in the number of synaptic AMPA-type glutamate receptors (Brigidi et al., Citation2014). Thus, Fyn is not only palmitoylated but is also a key regulator of neuronal palmitoylation events that control excitatory synaptic transmission ().
Figure 1. Regulation of excitatory synapses by Palmitoylated Fyn. Left panel: Several key pre- and post-synaptic proteins are palmitoylated (marked with yellow lipid). These palmitoyl-proteins include the tyrosine kinase Fyn, which is likely localized to the postsynaptic plasma membrane by dual lipid modification (myristate plus palmitate). Under normal conditions, Fyn phosphorylates NMDA receptors and the PAT DHHC5, preventing their internalization. Right panel: Elevated synaptic activity leads to dephosphorylation of DHHC5 at the Fyn site, triggering DHHC5 internalization to recycling endosomes (RE). Here, DHHC5 palmitoylates delta-catenin and the two proteins are trafficked back to the spine membrane, where delta-catenin associates with N-cadherin to increase the synaptic pool of AMPA-type glutamate receptors. Note that several other synaptic palmitoyl-proteins, such as PSD-95, are omitted from the schematic for clarity. Also not pictured is the synaptodendritic PAT DHHC2, which palmitoylates several dendritic/postsynaptic proteins, and may also palmitoylate Fyn.
Further questions regarding neuronal roles of palmitoyl-Fyn
Although Fyn plays key roles in the regulation of excitatory synapses, additional Fyn substrates in different subcellular locations have been reported. For example, Fyn also likely phosphorylates GABA receptors, which localize to inhibitory synapses, and the sodium channel NaV1.2 subunit, which preferentially localizes to axons (Ahn et al., Citation2007; Jurd et al., Citation2010). In addition, Fyn is implicated in the correct formation of both dendrites and axons during early neurodevelopment, prior to the appearance of mature synapses (Meriane et al., Citation2004; Sasaki et al., Citation2002). It will be interesting to determine whether palmitoylation is also critical for Fyn activity towards these additional substrates, and how Fyn targeting to different membrane locations within the neuron is controlled.
The importance of Fyn, and likely its palmitoylation, in postsynaptic regulation raises the additional question of how Fyn palmitoylation is itself regulated. The binding of Fyn to DHHC5 might initially suggest that this PAT palmitoylates Fyn in neurons, but several findings suggest this may not be the case. First, Fyn palmitoylation was not decreased by DHHC5 knockdown in cultured cortical neurons, nor was it decreased in neuronal stem cells from a gene trap mouse line that expresses very low levels of DHHC5 (Li et al., Citation2012; Thomas et al., Citation2012). Although other PATs may compensate for the absence of DHHC5 in these situations, it is of note that Fyn palmitoylation in non-neuronal cells is mediated by DHHC21 (Mill et al., Citation2009). This PAT has not been characterized in the nervous system but is likely expressed in hippocampal neurons and other neuronal cell types (Cajigas et al., Citation2012; Doyle et al., Citation2008). It is thus possible that DHHC21 indeed palmitoylates Fyn and perhaps other neuronal proteins. Another possible Fyn PAT is DHHC2, which can palmitoylate the related SFK Lck in non-neuronal cells (Zeidman et al., Citation2011) and also palmitoylates other key synaptic proteins (Noritake et al., Citation2009; Woolfrey et al., Citation2015).
Closer examination of structural differences between these candidate Fyn PATs provides additional interesting, though not definitive, insights into which of them might palmitoylate Fyn in neurons. For example, the assignment of DHHC21 as a Fyn PAT is in part based on Fyn’s abnormal localization in hair follicles of depilated (dep) mice, a mouse line in which a single amino acid in the C-terminal non-catalytic region of DHHC21 is missing (Mill et al., Citation2009). It is tempting to speculate that this region of DHHC21 (which is poorly conserved in other PATs) is the key factor that accounts for its regulation of Fyn in dep hair follicles. However, even if this is the case, whether the same mechanism is important for Fyn palmitoylation in neurons remains to be established. An additional factor arguing against the palmitoylation of Fyn by DHHC5 is that the C-terminus of this PAT forms a PSD-95/discs large/ZO-1 (PDZ) domain-binding motif that is essential for DHHC5 to interact with and palmitoylate certain substrates (Thomas et al., Citation2012). Fyn lacks a PDZ domain and so should not be recognized by DHHC5 in this way. However, not all DHHC5 substrates contain PDZ domains (Brigidi et al., Citation2014; Howie et al., Citation2014) and so DHHC5 cannot be ruled out as a potential Fyn PAT. The identity of the Fyn PAT(s) may thus differ depending on neuron type and/or subcellular location but is an interesting area for future study. Another intriguing question is whether Fyn palmitoylation (and hence its localization and/or kinase activity) is itself regulated by synaptic activity or other stimuli.
Regulation of neuronal cytoskeletal dynamics by palmitoylated kinases
Normal brain function requires not only tight regulation of the number and function of synaptic neurotransmitter receptors, but also precise control of neuronal structure and morphology. This is particularly so for dendritic spines, the actin-rich protrusions that are the site of most excitatory synapses (Hotulainen & Hoogenraad, Citation2010). Just as receptor number and function can be regulated in a synapse-specific manner, so can the morphology of individual spines (Harvey & Svoboda, Citation2007; Matsuzaki et al., Citation2004). Importantly, dynamic changes in the size of individual dendritic spines are frequently observed following LTP and are thus closely associated with higher brain functions such as learning and memory (Bosch & Hayashi, Citation2012; Fifkova & Van Harreveld, Citation1977; Murakoshi & Yasuda, Citation2012). Links between dendritic spine structure and cognitive function are strengthened by findings that spine morphology and/or density is altered in an array of neuropathological conditions, ranging from autism-spectrum disorders to Alzheimer’s Disease (Fiala et al. Citation2002, Penzes et al. Citation2011).
Rapid changes in spine shape and size largely reflect dynamic modulation of the spine’s underlying actin cytoskeleton (Hotulainen and Hoogenraad Citation2010), suggesting that neurons possess mechanisms to spatially control spine actin dynamics. However, many actin regulatory proteins are small and thought to be freely diffusible, so how such spatially precise actin regulation might be achieved was unclear. George et al. recently hypothesized that modification of actin regulatory proteins by palmitoylation might facilitate precise control of spine morphology. Using bioinformatic tools (Obenauer et al. Citation2003, Oku et al. Citation2013) they searched for predicted palmitoyl-motifs in actin regulators and identified such a motif close to the N-terminus of LIM Kinase-1 (LIMK1). LIMK1 regulates actin dynamics by phosphorylating and inactivating the actin severing/disassembly protein cofilin, and LIMK1 knockout mice display specific alterations in dendritic spine morphology (Meng et al. Citation2002). This raised the possibility that palmitoylation of LIMK1 might be particularly important for the control of spine actin dynamics. Consistent with this notion, LIMK1’s N-terminal palmitoyl-motif was found to be necessary and, strikingly, sufficient for its enrichment in dendritic spines (George et al. Citation2015). The authors then used a shRNA knockdown/rescue approach to replace endogenous LIMK1 in hippocampal neurons with shRNA-resistant wild type or palmitoyl-mutant forms of LIMK1. They found that LIMK1 ‘knockdown’ impairs normal actin turnover in dendritic spines and also attenuates the enlargement of individual spines that normally occurs in response to elevated neuronal activity (George et al. Citation2015). The deficits in actin turnover and activity-dependent spine enlargement could be ‘rescued’ by wild type LIMK1 but not by a LIMK1 palmitoyl-mutant (George et al. Citation2015).
These findings strongly suggested that palmitoylation is critical for LIMK1 dependent regulation of dendritic spines. However, it was initially surprising that the LIMK1 palmitoyl-mutant failed to rescue these phenotypes, despite being detectable in spines, only less enriched than wtLIMK1. The authors first addressed whether the LIMK1 palmitoyl-site mutant is simply misfolded and inactive. However, arguing against this possibility, palmitoyl-site mutation did not affect either LIMK1 activation by the upstream kinase p21-activated kinase-3 (PAK3), or the ability of activated LIMK1 to phosphorylate its downstream substrate cofilin in vitro (George et al. Citation2015). In marked contrast, though, mutation of LIMK1’s palmitoylation sites completely prevented LIMK1 phosphorylation at the activatory site, T508, in neurons. The authors suggested that this lack of T508 phosphorylation was because palmitoyl-mutant LIMK1 cannot localize to the spine membrane, where its upstream kinase PAK is located (George et al. Citation2015). Thus, while exerting a direct effect only on LIMK1 localization, palmitoylation also ensures that only LIMK1 localized to the spine membrane is active in neurons (). The authors further suggested that palmitoylation-dependent control of LIMK1 activity might create a ‘shell-to-core’ gradient of actin polymerizing activity at the sub-spine level. This is because palmitoyl-LIMK1 might be predicted to specifically phosphorylate and inactivate cofilin in juxtamembrane regions, thus favoring actin polymerization. In contrast, cofilin in the spine core would remain unphosphorylated and active to ensure that actin filaments are maintained at the appropriate length. However, experimental evidence is needed to test this hypothesis.
Figure 2. Control of spine morphology by palmitoylated Cdc42 and LIMK1. Left panel: Palmitoyl-LIMK1 on the dendritic spine membrane is phosphorylated and activated via a pathway that involves membrane-bound upstream activators such as Rac, Cdc42 (which is also palmitoylated) and PAK. In contrast, any non-palmitoylated LIMK1 in the spine ‘core’ remains inactive. This palmitoylation-dependent control of LIMK1 localization and activity may facilitate juxtamembrane phosphorylation and inactivation of cofilin, thus allowing spine-specific, and perhaps even sub-spine, control of actin dynamics. Right panel: Elevated synaptic activity triggers Rac/Cdc42/PAK activation and recruitment of additional cofilin to spines (Bosch et al., Citation2014; Murakoshi et al., Citation2011). Both these events are required for activity-dependent spine enlargement, which also depends on palmitoyl-LIMK1 (George et al., Citation2015). Control of sub-spine actin polymerization by this signaling pathway may provide the force required for the enlargement of specific spines that is frequently observed during LTP.
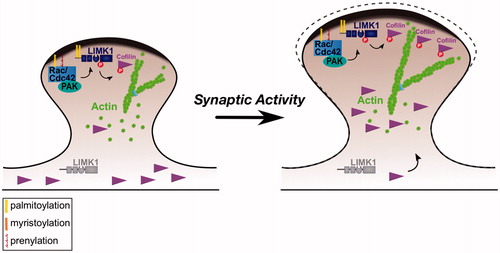
These findings not only provide new insights into the spatial control of actin dynamics at the single spine and perhaps even the sub-spine level, but also highlight intriguing areas for future study. For example, the ‘upstream’ proteins that regulate PAK/LIMK1 activity in spines are not known, but it is striking that palmitoylated forms of both Rac and Cdc42, small GTPases that are well known regulators of LIMK1 signaling, are detected in neurons (Kang et al., Citation2008; Wirth et al., Citation2013). Moreover, a palmitoylated form of Cdc42 is dominantly expressed in hippocampus (compared to the prenylated form, which is dominant in most other tissues), is preferentially targeted to dendritic spines, and is important for the control of spine morphology (Kang et al., Citation2008; Wirth et al., Citation2013). These findings raise the possibility that palmitoylation controls the spine localization and activity of multiple elements of the Rac/Cdc42-PAK-LIMK1 signaling module ().
Roles for palmitoyl-kinases in earlier stages of dendritic development
Interestingly, palmitoylated forms of LIMK1 and cdc42 are also detectable in cultured neurons prior to the formation of the majority of dendritic spines (George et al., Citation2015; Kang et al., Citation2008). This finding suggests that palmitoylation-dependent Rac/Cdc42-PAK-LIMK1 signals may also control earlier stages of development of the neuronal cytoskeleton, for example dendritic branching and/or extension. Indeed, another palmitoylated kinase, Calcium/calmodulin-dependent protein kinase-1 gamma (CaMKI-gamma, also known as CLICK-III) may regulate dendritogenesis via the Rac/Cdc42-PAK-LIMK1 pathway (Takemoto-Kimura et al., Citation2007). CaMKI-gamma is part of a family of CaMKs that play key roles in activity-dependent dendritic arborization (Redmond et al., Citation2002; Wayman et al., Citation2006). Of the neuronal CaMKs, CAMKI-gamma undergoes two sequential lipid modifications, prenylation followed by palmitoylation, which together recruit CAMKI-gamma to dendritic lipid rafts (Takemoto-Kimura et al., Citation2007). CAMKI-gamma knockdown impairs dendritic outgrowth, an effect that is rescued by wild type, but not palmitoyl-deficient, CAMKI-gamma (Takemoto-Kimura et al., Citation2007), suggesting that palmitoylation of CAMKI-gamma is critical for its dendritogenic role. Interestingly, the impaired dendritic growth caused by CAMKI-gamma knockdown was also rescued by constitutively active Rac, suggesting that raft-localized CAMKI-gamma controls dendritogenesis via Rac (and hence potentially PAK/LIMK1). Taken together these studies on Rac/Cdc42, CAMKI-gamma and LIMK1 suggest that palmitoylation-dependent kinase signaling is critical for multiple stages in the development and regulation of the dendritic cytoskeleton. These findings also raise the possibility that other processes that require precise control of neuronal cytoskeletal dynamics (e.g., rapid, cue-directed steering of axonal growth cones [Gomez & Letourneau, Citation2014]) may be palmitoylation-dependent.
Synaptic regulation by palmitoylated Protein A Kinase anchoring proteins (AKAPs)
Thus far we have focused our discussion exclusively on neuronal kinases that are directly palmitoylated. However, recent studies reveal that even signaling by non-palmitoylated kinases can still be palmitoylation-dependent. A good example of this situation is AKAP79/150, a palmitoyl-protein that is important for synaptic regulation by Protein Kinase A (PKA) (Keith et al., Citation2012; Sanderson & Dell’Acqua, Citation2011). AKAP79/150 targeting to synapses is palmitoylation-independent under basal conditions, but palmitoylation is required for rapid AKAP79/150 synaptic recruitment following elevated neuronal activity (Keith et al., Citation2012). This rapid recruitment of AKAP79/150 likely involves its trafficking via dendritic endosomes, where AKAP79/150 is palmitoylated by the endosomal PAT DHHC2 (Woolfrey et al., Citation2015). Consistent with this model, AKAP79/150 palmitoyl-site mutation, or RNAi-mediated knockdown of DHHC2, prevent activity-dependent increases in both dendritic spine size and synaptic localization of AMPA-type glutamate receptors (Keith et al., Citation2012; Woolfrey et al., Citation2015). It will be of interest to determine whether these palmitoylation-dependent effects of AKAP79/150 on synapses solely depend on targeting of PKA activity, or also involve other signaling molecules to which AKAP79/150 binds (Sanderson & Dell’Acqua, Citation2011). A related question is whether the recently described role of AKAP79/150-PKA signaling in the regulation of specific pools of AMPA-type glutamate receptors also requires AKAP79/150 palmitoylation (Sanderson et al., Citation2016). More broadly, these findings raise the important possibility that spatial regulation of signaling by other kinases may be controlled by palmitoylation of their specific binding partners.
New roles for palmitoyl-kinases in the peripheral nervous system (PNS)
Palmitoylation-dependent kinase signaling is thus critical for multiple aspects of Central Nervous System (CNS) function, but recent findings also reveal the importance of palmitoyl-kinases in peripheral neurons. The axons of PNS neurons often project long distances to innervate their target tissues, presenting a great challenge for the relay of both anterograde signals (from soma to axons) and also retrograde signals (from axons to soma). Axonal retrograde signals are critical to stimulate transcriptional programs that control regenerative responses to peripheral nerve injury (Abe & Cavalli, Citation2008; Rishal & Fainzilber, Citation2014). Several key mediators of retrograde injury signaling have recently been identified, of which Dual Leucine-zipper Kinase (DLK) has received considerable attention. DLK is an evolutionarily conserved upstream activator (a MAP3K) of the Mitogen-activated Protein Kinases (MAPKs) c-Jun N-terminal Kinase (JNK) and p38 MAPK (Tedeschi & Bradke, Citation2013). In mammalian peripheral neurons, DLK-dependent JNK activation is heavily implicated in retrograde signals that result in phosphorylation and activation of the c-Jun transcription factor (Ghosh et al., Citation2011; Shin et al., Citation2012). However, it had been unknown how DLK, a predicted soluble kinase, conveys these long-distance signals. Recently, DLK was found to be palmitoylated at a conserved site adjacent to its kinase domain (Holland et al., Citation2016). ShRNA knockdown/rescue experiments, in which endogenous DLK in sensory neurons was replaced with a palmitoyl-site mutant, revealed that palmitoylation at this site is critical for DLK-dependent retrograde injury signaling (Holland et al., Citation2016) ().
Figure 3. Axonal retograde signaling by palmitoylated DLK-JNK pathway kinases. Palmitoylation is essential for DLK’s ability to convey retrograde injury signals following distal axonal injury. At the cellular level, palmitoylation targets DLK to trafficking vesicles where DLK forms complexes with its direct substrate MKK4. These complexes also contain the scaffold protein JIP3, which binds dynein/dynactin retrograde motor proteins, and may also involve the downstream palmitoyl-kinase JNK3, which is heavily implicated in axonal retrograde signaling. In addition to this role in DLK localization, palmitoylation is necessary for DLK’s ability to activate the JNK pathway. This additional ‘security feature’ ensures that depalmitoylated DLK does not inappropriately phosphorylate cytosolic MKKs.
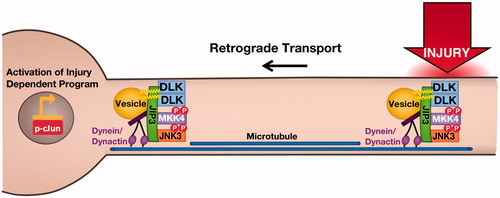
At the cellular level, palmitoylation targets DLK to motile trafficking vesicles, some of which move retrogradely and may thus convey axonal retrograde signals. In addition to this clear effect on trafficking, though, palmitoylation also impacts DLK at the molecular level. In particular, palmitoyl-site mutation prevents DLK’s ability to form multiprotein complexes that contain the scaffold protein JNK-interacting Protein-3 (JIP3) and the downstream kinases MAP Kinase Kinase-4 (MKK4) and MKK7 (Holland et al., Citation2016). Both MKK4 and JIP3 can co-traffic with wild type (i.e., palmitoylated) DLK in axons, suggesting that palmitoylation of DLK may be critical for the assembly and/or stability of axonal complexes that contain multiple JNK pathway signaling components. Finally, palmitoylation is also critical for DLK’s ability to phosphorylate its direct substrates MKK4 and MKK7, both in vitro and in transfected cells (Holland et al., Citation2016). These findings strongly suggest that palmitoylation plays an additional role to control DLK-dependent signaling and raise the exciting possibility that palmitoylation directly controls DLK’s kinase activity.
Is there any precedent for this unexpected potential role of palmitoylation? The answer would appear to be yes, because another palmitoyl-kinase, G protein-coupled Receptor Kinase-6 (GRK6) was also reported to be markedly more active, even in vitro, when palmitoylated (Babu et al., Citation2004; Loudon & Benovic, Citation1997). It will be of considerable interest to determine whether roles of GRK6 in regulation of receptors for the neurotransmitter dopamine are palmitoylation-dependent (Gainetdinov et al., Citation2003). However, it is important to note that the in vitro assays that revealed elevated activity of the palmitoylated forms of GRK6 and DLK both used kinases that had been isolated from mammalian cells. Elevated DLK/GRK6 activity may thus have been a secondary effect of a palmitoylation-dependent modification in cells (e.g., activatory phosphorylation by an additional membrane-associated kinase), which persists following isolation of the kinase in vitro. Testing the hypothesis that palmitoylation instead acts autonomously to alter DLK and/or GRK6 conformation and hence kinase activity will be challenging and may require high-resolution structures of the palmitoylated and non-palmitoylated forms of these kinases. Nonetheless, regardless of which mechanism is correct, an emerging consensus is that palmitoylation restricts the activity of a given palmitoyl-kinase to a specific subcellular location. Thus, because palmitoylation targets DLK to vesicles, only vesicle-bound DLK is likely active in neurons. Similarly, the activity of LIMK1 is restricted to dendritic spines, whereas active GRK6 is enriched on membranes that contain its G-protein coupled receptor substrates. It will be of considerable interest to address whether neurons or other cells use ‘multi-functional’ palmitoylation of this type to regulate other kinases and/or other families of signaling enzymes.
Additional roles for palmitoylation in DLK-JNK pathway signaling
The importance of palmitoylation in DLK-JNK signaling is reinforced by a report that another member of this pathway, the neurally-enriched JNK3, is also palmitoylated (Yang et al., Citation2012). In peripheral neurons JNK3 plays key roles in retrograde responses to axonal injury and was previously reported to colocalize with JIP3 on axonal vesicles (Cavalli et al., Citation2005; Keramaris et al., Citation2005). It is thus tempting to speculate that palmitoylation accounts for this vesicular targeting of JNK3 and that these JNK3-JIP3-positive vesicles are similar, or perhaps identical, to the recently reported DLK-JIP3 positive vesicles (Holland et al., Citation2016). If so, then palmitoylation would play a key role in modifying multiple DLK-JNK pathway components to ensure the specificity and transport of neuronal injury signals. It is important to note, though, that in CNS neurons, the palmitoylated form of JNK3 was reported to regulate secretory trafficking (Yang et al., Citation2013) and axonal development (Yang et al., Citation2012). These different roles of JNK3 may reflect CNS-PNS differences, but multiple functions for JNK3 palmitoylation should perhaps be expected, given that this kinase can play both physiological and pathological roles in neurons (Waetzig & Herdegen, Citation2003).
Future questions: Additional palmitoyl-kinases, additional roles in neuronal development and function?
Recent studies have thus revealed several novel roles for palmitoyl-kinases, but these roles have largely been elucidated on a case-by-case basis using low throughput approaches. Excitingly, though, high throughput studies have identified several other kinases that are likely palmitoylated, yet for which the roles of their palmitoylation in neurons remain unexplored. For example, members of the Casein Kinase 1 gamma (CK1 gamma) subfamily have been identified in palmitoyl-proteomic studies from several mammalian tissues (Blanc et al., Citation2015; Sanders et al., Citation2015). CK1 is highly evolutionarily conserved and roles of palmitoylation in subcellular trafficking and localization have been defined for the yeast CK1 ortholog (e.g., Babu et al., Citation2004). In contrast, much less is known about roles of CK1 palmitoylation in multicellular organisms. Interestingly, though, the Drosophila CK1 gamma ortholog, Gilgamesh, is preferentially expressed in neurons of the mushroom bodies (MBs), regions of the fly brain that are critical for olfactory-dependent learning (Tan et al., Citation2010). Moreover, forced expression of Gilgamesh in adult MBs rescues learning deficits in gilgamesh mutants, suggesting that this CK1 gamma ortholog actively participates in signaling events within MB neurons that underlie memory formation (Tan et al., Citation2010). CK1 gamma is also highly expressed in several mammalian CNS and PNS neuronal populations (Cajigas et al., Citation2012; Lerch et al., Citation2012), suggesting that this kinase, and perhaps its palmitoylation, may also be critical for neuronal function in higher organisms.
A second group of kinases identified in palmitoyl-proteomic studies is the Eph (ephrin receptor) family of receptor tyrosine kinases (Kang et al., Citation2008). Signaling via Ephs and their ephrin ligands regulates a diverse array of processes, including axon guidance, synaptogenesis and the stability and plasticity of mature synapses (Kania & Klein, Citation2016; Klein, Citation2009; McClelland et al., Citation2010). It will be interesting to determine the extent to which the diverse roles of ephrin/Eph signaling in neuronal development and function are palmitoylation-dependent.
Two further kinases that are reported to be palmitoylated are the brain-specific kinases (BRSKs)-1 and -2 (Kang et al., Citation2008; Rodriguez-Asiain et al., Citation2011), also known as SAD kinases A and B (Barnes et al., Citation2007, Citation2008; Kishi et al., Citation2005; Shelly et al., Citation2007). BRSK-1/-2 play important roles in neuronal polarization, the correct specification of a neuron’s axon and dendrites, which is critical for the appropriate transmission and integration of neural information. The process of neuronal polarization likely requires differential activity and/or distribution of intracellular signaling proteins in response to asymmetrically distributed cues and would thus appear to be a prime candidate to be subject to palmitoylation-dependent regulation. Experiments using a recently developed Fluorescence Resonance Energy Transfer (FRET) kinase activity reporter suggest that BRSK activity is indeed spatially regulated, and is elevated in axons compared to other regions of developing hippocampal neurons (Sample et al., Citation2015). In addition, experiments from non-neuronal cells revealed that BRSK1 associates with lipid rafts and, interestingly, the raft-associated BRSK1 has higher kinase activity than ‘non-raft’ BRSK1 (Rodriguez-Asiain et al., Citation2011). It will thus be of interest to determine whether axonal BRSK activity is due to the palmitoylated form of BRSK-1 and/or BRSK-2 and whether palmitoylation is critical for BRSK1/2-dependent control of neuronal polarization. The finding that the BRSK1/2 ‘upstream’ activator Liver Kinase B1 (LKB1) is farnesylated (Collins et al., Citation2000) raises the possibility that signaling via multiple lipid-modified kinases is critical for axon polarization. This scenario, which would be reminiscent of the Rac/Cdc42/PAK/LIMK1 module, would suggest that lipid modification of multiple signaling proteins in a common pathway represents a frequently used mechanism to ensure spatially precise neuronal signaling.
How many palmitoyl-kinases are there?
The human genome codes for > 500 protein kinases (Manning et al., Citation2002). So far around 20 of these kinases are reported to be palmitoylated, but several lines of evidence suggest that additional palmitoyl-kinases likely exist. First, palmitoyl-proteomic databases list numerous other kinases that have been identified as palmitoyl-proteins in single reports (Blanc et al., Citation2015; Sanders et al., Citation2015). Cognizant that a number of these ‘hits’ may turn out to be false positives, we thus mainly focused our review on kinases that were identified in multiple palmitoyl-proteomic studies, or those for which targeted studies have been performed. However, palmitoylation is not a constitutive modification, so palmitoylation of a particular kinase may occur only in a specific cell type, at a particular developmental stage, or in response to a specific stimulus. Because palmitoyl-proteomic studies have been performed from a diverse range of cell types and tissues it appears likely that some of these singleton ‘hits’ will indeed turn out to be bona fide palmitoyl-kinases. A related point is that palmitoylation appears to play particularly important roles in neurons, consistent with the morphological complexity of these cells and their consequent need for precise subcellular control of protein targeting. However, only two palmitoyl-proteomic studies have thus far been carried out using neuronal tissue (Kang et al., Citation2008; Wan et al., Citation2013), and none as yet from peripheral neurons. These studies reported an impressive number of palmitoyl-proteins, but did not identify several palmitoylated neuronal proteins, including kinases, that have been identified in targeted studies, e.g., (George et al., Citation2015; Holland et al., Citation2016; Levy et al., Citation2011; Yang et al., Citation2012). This raises the strong possibility that additional studies from different neuronal preparations may identify additional palmitoyl-kinases.
How is palmitoylation of neuronal kinases regulated?
How palmitoylation is regulated at the cellular level is a key outstanding question for those interested in this regulatory mechanism. Remarkably little is known about this issue even for well-studied palmitoyl-proteins, so the paucity of information regarding regulation of neuronal palmitoyl-kinases, many of which were only recently identified, is unsurprising. Indeed, current knowledge is largely limited to observations that palmitate turnover on LIMK1 in hippocampal neurons is reasonably slow (half-time approximately 6 h [George et al., Citation2015]) while palmitate turnover of DLK in cultured sensory neurons is much more rapid (half-time less than 1 h) (Holland et al., Citation2016). Specific stimuli that increase (or decrease) LIMK1 or DLK palmitoylation have not been described. However, an intriguing recent report of rapid palmitoylation of the SFK Lck in response to Fas ligand engagement suggests that palmitoylation of specific kinases may indeed be acutely regulated in response to extracellular stimuli (Akimzhanov & Boehning, Citation2015).
Perhaps unsurprisingly, even less is known regarding the identity and/or regulation of thioesterase(s) whose activity accounts for palmitate turnover on palmitoyl-kinases. Nonetheless, given the strong links between several palmitoyl-kinases and disease conditions, the identity and regulation of palmitoyl-kinase PATs and thioesterases and the evaluation of these enzymes as potential therapeutic targets are all key areas for future study.
Conclusions
Although initially limited to a few isolated reports, their growing number suggests that palmitoyl-kinases represent a newly defined enzyme family. Several palmitoyl-kinases have been recently described in neurons and so we have focused on this cell type. However, precise subcellular regulation is important in all cells, so findings from neurons may enhance our knowledge of how a wide variety of cell types ensure spatially precise kinase signaling. It will be of great interest to see how these insights shape our understanding of intracellular signaling in health and disease as this field continues to evolve.
Acknowledgements
We thank Drs Sabrina Holland and Francesca DeSimone for comments on the manuscript. This study was supported by grants from NIH (R21NS087414 and R01NS094402) and Shriners Hospitals for Children (85600 and 86610) to G.M.T.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abe N, Cavalli V. 2008. Nerve injury signaling. Curr Opin Neurobiol 18:276–283
- Ahn M, Beacham D, Westenbroek RE, Scheuer T, Catterall WA. 2007. Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J Neurosci 27:11533–11542
- Akimzhanov AM, Boehning D. 2015. Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci USA 112:11876–11880
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. 1994. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem 269:16701–16705
- Babu P, Deschenes RJ, Robinson LC. 2004. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J Biol Chem 279:27138–27147
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN. et al. 2007. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129:549–563
- Barnes AP, Solecki D, Polleux F. 2008. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol 18:44–52
- Bijlmakers MJ. 2009. Protein acylation and localization in T cell signaling (Review). Mol Membr Biol 26:93–103
- Blanc M, David F, Abrami L, Migliozzi D, Armand F, Burgi J, Van Der Goot FG. 2015. SwissPalm: Protein Palmitoylation database. F1000Research 4:261
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. 2014. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82:444–459
- Bosch M, Hayashi Y. 2012. Structural plasticity of dendritic spines. Curr Opin Neurobiol 22:383–388
- Brigidi GS, Santyr B, Shimell J, Jovellar B, Bamji SX. 2015. Activity-regulated trafficking of the palmitoyl-acyl transferase DHHC5. Nature Commun 6:8200
- Brigidi GS, Sun Y, Beccano-Kelly D, Pitman K, Mobasser M, Borgland SL. et al. 2014. Palmitoylation of delta-catenin by DHHC5 mediates activity-induced synapse plasticity. Nature Neurosci 17:522–532
- Cajigas IJ, Tushev G, Will TJ, Tom Dieck S, Fuerst N, Schuman EM. 2012. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 74:453–466
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. 2005. Sunday Driver links axonal transport to damage signaling. J Cell Biol 168:775–787
- Collins SP, Reoma JL, Gamm DM, Uhler MD. 2000. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J 345(Pt 3):673–680
- Craven SE, El-Husseini AE, Bredt DS. 1999. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron 22:497–509
- Dejanovic B, Semtner M, Ebert S, Lamkemeyer T, Neuser F, Luscher B. et al. 2014. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS Biol 12:e1001908
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G. et al. 2008. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135:749–762
- El-Husseini AD, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O. et al. 2002. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108:849–863
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. 2006. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci 26:12758–12768
- Fiala JC, Spacek J, Harris KM. 2002. Dendritic spine pathology: Cause or consequence of neurological disorders? Brain Res 39:29–54
- Fifkova E, Van Harreveld A. 1977. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol 6:211–230
- Fukata Y, Fukata M. 2010. Protein palmitoylation in neuronal development and synaptic plasticity. Nature reviews. Neuroscience 11:161–175
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD. et al. 2003. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38:291–303
- George J, Soares C, Montersino A, Beique JC, Thomas GM. 2015. Palmitoylation of LIM Kinase-1 ensures spine-specific actin polymerization and morphological plasticity. eLife 4
- Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. 2011. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol 194:751–764
- Gomez TM, Letourneau PC. 2014. Actin dynamics in growth cone motility and navigation. J Neurochem 129:221–234
- Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. 1992. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258:1903–1910
- Harvey CD, Svoboda K. 2007. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450:1195–1200
- Hayashi T, Rumbaugh G, Huganir RL. 2005. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron 47:709–723
- Hayashi T, Thomas GM, Huganir RL. 2009. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron 64:213–226
- Holland SM, Collura KM, Ketschek A, Noma K, Ferguson TA, Jin Y. et al. 2016. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci USA 113:763–768
- Hotulainen P, Hoogenraad CC. 2010. Actin in dendritic spines: Connecting dynamics to function. J Cell Biol 189:619–629
- Howie J, Reilly L, Fraser NJ, Vlachaki Walker JM, Wypijewski KJ, Ashford ML. et al. 2014. Substrate recognition by the cell surface palmitoyl transferase DHHC5. Proc Natl Acad Sci USA 111:17534–17539
- Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. 2010. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Mol Cell Neurosci 44:129–134
- Kabouridis PS, Magee AI, Ley SC. 1997. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J 16:4983–4998
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO. et al. 2008. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 456:904–909
- Kania A, Klein R. 2016. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nature reviews. Molecular Cell Biol 17:240–256
- Keith DJ, Sanderson JL, Gibson ES, Woolfrey KM, Robertson HR, Olszewski K. et al. 2012. Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms. J Neurosci 32:7119–7136
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. 2004. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci 24:5881–5891
- Keramaris E, Vanderluit JL, Bahadori M, Mousavi K, Davis RJ, Flavell R. et al. 2005. c-Jun N-terminal kinase 3 deficiency protects neurons from axotomy-induced death in vivo through mechanisms independent of c-Jun phosphorylation. J Biol Chem 280:1132 –1141
- Kishi M, Pan YA, Crump JG, Sanes JR. 2005. Mammalian SAD kinases are required for neuronal polarization. Science 307:929–932
- Klein R. 2009. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nature Neurosci 12:15–20
- Koegl M, Zlatkine P, Ley SC, Courtneidge SA, Magee AI. 1994. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem J 303(Pt 3):749–753
- Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. 1997. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci USA 94:4761–4765
- Lerch JK, Kuo F, Motti D, Morris R, Bixby JL, Lemmon VP. 2012. Isoform diversity and regulation in peripheral and central neurons revealed through RNA-Seq. PLoS One 7:e30417
- Levy AD, Devignot V, Fukata Y, Fukata M, Sobel A, Chauvin S. 2011. Subcellular Golgi localization of Stathmin family proteins is promoted by a specific set of DHHC palmitoyl transferases. Mol Biol Cell 22:1930–1942
- Li Y, Hu J, Hofer K, Wong AM, Cooper JD, Birnbaum SG. et al. 2010. DHHC5 interacts with PDZ domain 3 of post-synaptic density-95 (PSD-95) protein and plays a role in learning and memory. J Biol Chem 285:13022–13031
- Li Y, Martin BR, Cravatt BF, Hofmann SL. 2012. DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of neuronal differentiation in cultured cells. J Biol Chem 287:523–530
- Loudon RP, Benovic JL. 1997. Altered activity of palmitoylation-deficient and isoprenylated forms of the G protein-coupled receptor kinase GRK6. J Biol Chem 272:27422–27427
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. 2002. The protein kinase complement of the human genome. Science 298:1912–1934
- Mansouri MR, Marklund L, Gustavsson P, Davey E, Carlsson B, Larsson C. et al. 2005. Loss of ZDHHC15 expression in a woman with a balanced translocation t(X;15)(q13.3;cen) and severe mental retardation. Eur J Human Gen 13:970–977
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. 2004. Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766
- Mcclelland AC, Hruska M, Coenen AJ, Henkemeyer M, Dalva MB. 2010. Trans-synaptic EphB2-ephrin-B3 interaction regulates excitatory synapse density by inhibition of postsynaptic MAPK signaling. Proc Natl Acad Sci USA 107:8830–8835
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M. et al. 2002. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 35:121–133
- Meriane M, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S. et al. 2004. Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J Cell Biol 167:687–698
- Mill P, Lee AW, Fukata Y, Tsutsumi R, Fukata M, Keighren M. et al. 2009. Palmitoylation regulates epidermal homeostasis and hair follicle differentiation. PLoS Genetics 5:e1000748
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB. et al. 2008. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nature Neurosci 11:1302–1310
- Murakoshi H, Wang H, Yasuda R. 2011. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472:100–104
- Murakoshi H, Yasuda R. 2012. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci 35:135–143
- Nada S, Shima T, Yanai H, Husi H, Grant SG, Okada M, Akiyama T. 2003. Identification of PSD-93 as a substrate for the Src family tyrosine kinase Fyn. J Biol Chem 278:47610–47621
- Noritake J, Fukata Y, Iwanaga T, Hosomi N, Tsutsumi R, Matsuda N. et al. 2009. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J Cell Biol 186:147–160
- Obenauer JC, Cantley LC, Yaffe MB. 2003. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31:3635–3641
- Oku S, Takahashi N, Fukata Y, Fukata M. 2013. In silico screening for palmitoyl substrates reveals a role for DHHC1/3/10 (zDHHC1/3/11)-mediated neurochondrin palmitoylation in its targeting to Rab5-positive endosomes. J Biol Chem 288:19816–19829
- Paige LA, Nadler MJ, Harrison ML, Cassady JM, Geahlen RL. 1993. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem 268:8669–8674
- Penzes P, Cahill ME, Jones KA, Vanleeuwen JE, Woolfrey KM. 2011. Dendritic spine pathology in neuropsychiatric disorders. Nature Neurosci 14:285–293
- Prescott GR, Gorleku OA, Greaves J, Chamberlain LH. 2009. Palmitoylation of the synaptic vesicle fusion machinery. J Neurochem 110:1135–1149
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. 2005. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47:845–857
- Raymond FL, Tarpey PS, Edkins S, Tofts C, O’Meara S, Teague J. et al. 2007. Mutations in ZDHHC9, which encodes a palmitoyltransferase of NRAS and HRAS, cause X-linked mental retardation associated with a Marfanoid habitus. Am J Human Genet 80:982–987
- Redmond L, Kashani AH, Ghosh A. 2002. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 34:999–1010
- Resh MD. 2006. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Science’s STKE 2006:re14
- Rishal I, Fainzilber M. 2014. Axon-soma communication in neuronal injury. Nature Rev Neurosci 15:32–42
- Rodriguez-Asiain A, Ruiz-Babot G, Romero W, Cubi R, Erazo T, Biondi RM. et al. 2011. Brain specific kinase-1 BRSK1/SAD-B associates with lipid rafts: Modulation of kinase activity by lipid environment. Biochim Biophys Acta 1811:1124–1135
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN. et al. 2006. Global analysis of protein palmitoylation in yeast. Cell 125:1003–1013
- Sample V, Ramamurthy S, Gorshkov K, Ronnett GV, Zhang J. 2015. Polarized activities of AMPK and BRSK in primary hippocampal neurons. Molec Biol Cell 26:1935–1946
- Sanders SS, Martin DD, Butland SL, Lavallee-Adam M, Calzolari D, Kay C. et al. 2015. Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Computat Biol 11:e1004405
- Sanderson JL, Dell’Acqua ML. 2011. AKAP signaling complexes in regulation of excitatory synaptic plasticity. The Neuroscientist 17:321–336
- Sanderson JL, Gorski JA, Dell’Acqua ML. 2016. NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca(2+)-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron 89:1000–1015
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T. et al. 2002. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35:907–920
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. 2007. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129:565–577
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. 1994. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol 126:353–363
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, Diantonio A. 2012. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74:1015–1022
- Takemoto-Kimura S, Ageta-Ishihara N, Nonaka M, Adachi-Morishima A, Mano T, Okamura M. et al. 2007. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron 54:755–770
- Tan Y, Yu D, Pletting J, Davis RL. 2010. Gilgamesh is required for rutabaga-independent olfactory learning in Drosophila. Neuron 67:810–820
- Tedeschi A, Bradke F. 2013. The DLK signalling pathway – a double-edged sword in neural development and regeneration. EMBO Rep 14:605–614
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. 1999. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci USA 96:435–440
- Thomas GM, Hayashi T, Chiu SL, Chen CM, Huganir RL. 2012. Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking. Neuron 73:482–496
- Thomas GM, Hayashi T, Huganir RL, Linden DJ. 2013. DHHC8-dependent PICK1 palmitoylation is required for induction of cerebellar long-term synaptic depression. J Neurosci 33:15401–15407
- Thomas GM, Huganir RL. 2013. Palmitoylation-dependent regulation of glutamate receptors and their PDZ domain-containing partners. Biochem Soc Transact 41:72–78
- Trepanier CH, Jackson MF, MacDonald JF. 2012. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J 279:12–19
- Van’t Hof W, Resh MD. 1999. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J Cell Biol 145:377–389
- Waetzig V, Herdegen T. 2003. A single c-Jun N-terminal kinase isoform (JNK3-p54) is an effector in both neuronal differentiation and cell death. J Biol Chem 278:567–572
- Wan J, Savas JN, Roth AF, Sanders SS, Singaraja RR, Hayden MR, Yates JR 3rd, Davis NG. 2013. Tracking brain palmitoylation change: Predominance of glial change in a mouse model of Huntington’s disease. Chem Biol 20:1421–1434
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. 2006. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50:897–909
- Webb Y, Hermida-Matsumoto L, Resh MD. 2000. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem 275:261–270
- Wirth A, Chen-Wacker C, Wu YW, Gorinski N, Filippov MA, Pandey G, Ponimaskin E. 2013. Dual lipidation of the brain-specific Cdc42 isoform regulates its functional properties. Biochem J 456:311–322
- Woolfrey KM, Sanderson JL, Dell’Acqua ML. 2015. The palmitoyl acyltransferase DHHC2 regulates recycling endosome exocytosis and synaptic potentiation through palmitoylation of AKAP79/150. J Neurosci 35:442–456
- Yang G, Liu Y, Yang K, Liu R, Zhu S, Coquinco A. et al. 2012. Isoform-specific palmitoylation of JNK regulates axonal development. Cell Death Differentiat 19:553–561
- Yang G, Zhou X, Zhu J, Liu R, Zhang S, Coquinco A. et al. 2013. JNK3 couples the neuronal stress response to inhibition of secretory trafficking. Science Signal 6:ra57
- Yount JS, Zhang MM, Hang HC. 2013. Emerging roles for protein S-palmitoylation in immunity from chemical proteomics. Curr Opin Chem Biol 17:27–33
- Zeidman R, Buckland G, Cebecauer M, Eissmann P, Davis DM, Magee AI. 2011. DHHC2 is a protein S-acyltransferase for Lck. Mol Membr Biol 28:473–486
