Abstract
To investigate the effect of fluorescent probe on the properties of membranes, we studied model membranes composed of 1,2- dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1-palmitoyl 2-oleoyl-sn-glycero-3-phosphocholine (POPC) in the presence and absence of fluorescent probe. The morphology of giant unilamellar vesicles (GUVs) has been observed as a function of temperature and composition by fluorescence microscopy using NBD-DOPE or C6-NBD-PC as the probe. The phase behavior of model membranes containing no fluorescent probe was investigated by 2H-NMR spectroscopy. We found that the bright phase observed on GUVs was the fluid phase enriched in POPC and the dark phase was the gel phase enriched in DPPC. NBD-DOPE and C6-NBD-PC preferentially participated in the fluid-phase domains when GUVs were in the gel + fluid phase coexistence. Inclusion of both fluorescent probes (1 mol%) lowered the transition temperature of POPC/DPPC membranes. In addition, C6-NBD-PC exhibited a stronger effect than NBD-DOPE, which was considered to be associated with the structures of fluorescent molecules.
Introduction
Fluorescence microscopy is a powerful tool for studying membranes organization (Bagatolli, Citation2006; Veatch & Keller, Citation2005). There are many fluorescence studies on model membranes composed of binary or ternary mixtures (Loura et al., Citation2009; Veatch & Keller, Citation2005). These measurements involve the use of fluorescent probes. However, the effect of fluorescent probe on the properties of membranes is less studied. It is reported that fluorescent probes can potentially perturb membrane structure and dynamics (Cadenhead et al., Citation1977; Lesslauer et al., Citation1972). The phase transition temperature of model membranes is shifted by fluorescent probes, depending on the type and concentration of the probe (Klausner and Wolf, Citation1980; Loura et al., Citation2008; Veatch et al., Citation2007). In addition, the transitions become broader. It is thus important to have a better understanding of the probe-induced effect in order to properly interpret the results of fluorescence experiments.
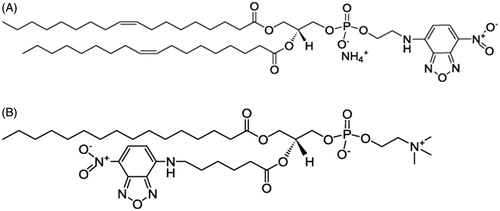
Phospholipids labeled with the 7-nitro-2-1,3-benzoxadiazol-4-yl (NBD) fluorophore have been used as fluorescence probes in the study of model membranes (Loura et al., Citation2009; Mazères et al., Citation1996; Mukherjee et al., Citation2004). The NBD moiety is either attached to the headgroup or the acyl chain of phospholipids. It is of interest how fluorophore location affects the membranes properties, in particular the phase behavior and phase partition preference. We therefore chose head- and acyl chain-labeled phospholipids to study. shows the structure of a headgroup-labeled probe NBD-DOPE, which has been used to observe membrane domains successfully (Baumgart et al., Citation2007; Loura et al., Citation2009). It is also reported that NBD-DOPE preferentially partitions into the disordered phase. C6-NBD-PC, shown in , is an acyl chain-labeled probe with the NBD moiety attached to the sn-2 chain.
In this work, we studied model membranes composed of POPC and DPPC in the presence and absence of fluorescence probe (NBD-DOPE or C6-NBD-PC). The morphology and phase behavior of model membranes were investigated by fluorescence microscopy and 2H-NMR spectroscopy, respectively. The results of fluorescence study were then compared with those obtained from 2H-NMR in the absence of fluorescence probe. The effect of fluorescent probe on membrane properties will be discussed.
Materials and methods
POPC, DPPC, POPC-d31, DPPC-d31 were purchased from Avanti Polar Lipids (Alabaster, AL). 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DOPE) and 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (C6-NBD-PC or 16:0-06:0 NBD PC) were also purchased from Avanti Polar Lipids (Alabaster, AL). Sucrose and glucose were purchased from Merck (Darmstadt, Germany).
GUVs of 30:70, 50:50, 70:30 POPC/DPPC containing 1mol% NBD-DOPE (or C6-NBD-PC) were prepared using electroformation (Angelova et al., Citation1992). Lipids and the fluorescent probe were dissolved in chloroform and mixed in the appropriate quantities. The lipid/chloroform solution was deposited onto ITO (Indium Tin Oxide) coated glass and then lyophilized. Lipid film was hydrated with sucrose solution (0.2M) at 50 °C in the chamber for 40 min, then subject to an AC field (sinusoidal wave function with a frequency of 10 Hz and amplitude of 3 V) for another 40 min. After the vesicle formation, the AC field was turned off and the glucose solution (0.2 M) was added to the chamber. The temperature of the chamber was slowly cooled down to 13 °C at the rate of 0.3 °C/min. After 30 min of waiting, the temperature was raised to the desired value in the rate of 1 °C/min. GUVs were allowed to equilibrate for 30 min before the images were acquired.
GUVs were observed under inverted fluorescence microscope (Nikon TE2000-U). The excitation/emission wavelengths for NBD-DOPE were 460/535 nm, respectively. The excitation/emission wavelengths for C6-NBD-PC probe were 460/534 nm, respectively. The bright-field and fluorescence images of GUVs were acquired by a CCD camera (Evolution VF Digital Camera Kit). The size of GUVs ranged from 10–30 μm in diameter.
Multi-lamellar vesicles of 50:50 POPC-d31/DPPC and 50:50 POPC/DPPC-d31 were prepared for the 2H NMR measurements. Lipids were mixed in the appropriate quantities, dissolved in benzene/methanol 4:1 (v/v), and then lyophilized. Samples were hydrated using a pH 7.4 buffer prepared in deuterium-depleted water containing 50 mM HEPES, 150 mM NaCl, and 4 mM EDTA followed by freeze-thaw-vortex cycling five times between liquid nitrogen temperature and 50 °C. 2H NMR experiments were performed on a Varian NMR spectrometer (Infinityplus 300) at 46.06 MHz or a locally built spectrometer at 46.8 MHz using the quadrupolar echo technique (Davis et al., Citation1976). The samples were heated from −7–51 °C. At each temperature, the sample was allowed to equilibrate for 30 min before a measurement.
Results and discussion
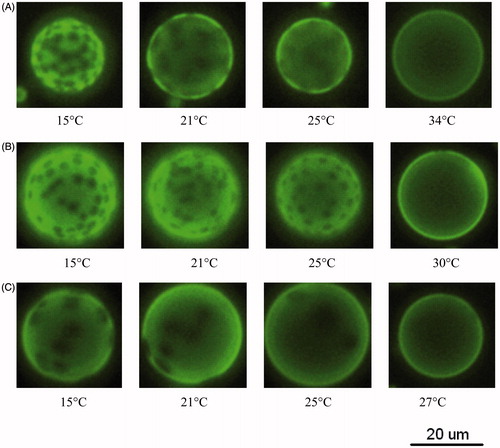
The bright-field and fluorescence images were taken for the GUVs of (POPC/DPPC) + 1mol% NBD-DOPE. shows the fluorescence images of POPC/DPPC GUVs as a function of temperature. Bright and dark phases are observed in the GUVs of 30:70, 50:50 or 70:30 POPC/DPPC over certain temperature ranges, suggesting that POPC and DPPC do not mix well. NBD-DOPE is known to preferentially participates in a disordered region (Baumgart et al., Citation2007; Loura et al., Citation2009), thus the bright phase is supposed to be more disordered than the dark phase. As shown in , the area fraction of bright phase for 50:50 POPC/DPPC increased as the temperature increased from 15–30 °C. Concurrently, the area fraction of dark phase, which are circular domains, decreased with increasing temperature. A uniform fluorescence was observed at 30 °C, indicating that POPC and DPPC were well mixed at this temperature. Therefore, the miscibility temperature, Tmis, for the GUV of 50:50 POPC/DPPC was 30 ± 1 °C. Tmiss for the GUVs of 30:70 POPC/DPPC and 70:30 POPC/DPPC were 34 ± 1 °C and 27 ± 1 °C, respectively, as shown in .
Figure 2. Fluorescence images as a function of temperature for GUVs of (A) 30:70 POPC/DPPC; (B) 50:50 POPC/DPPC; (C) 70:30 POPC/DPPC. Each sample contains 1 mol% NBD-DOPE.
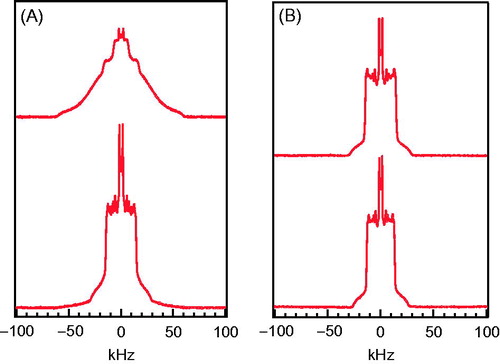
To further study the bright and dark phases observed in , a 2H NMR experiment were performed. The sn-1 chain of each lipid was deuterated in turn. displays 2H NMR spectra of 50:50 POPC/DPPC-d31 and 50:50 POPC-d31/DPPC at 17 °C. The spectrum of 50:50 POPC/DPPC-d31 was a superposition of characteristic gel and characteristic fluid (or liquid crystalline) spectra, with the gel component dominant. Therefore, DPPC molecules were predominantly in the gel phase with a small population in the fluid phase. The spectrum of 50:50 POPC-d31/DPPC was also a superposition of characteristic gel and characteristic fluid spectra, however with the fluid component dominant. Therefore, the majority of POPC molecules were in the fluid phase with a small portion in the gel phase. The above results show that the membrane is in the gel + fluid phase coexistence at 17 °C. The gel phase was rich in DPPC and the fluid phase was rich in POPC. In , 2H NMR spectra of 50:50 POPC/DPPC-d31 and 50:50 POPC-d31/DPPC display characteristic fluid spectra at 33 °C, showing that the membranes are in the fluid phase. In short, 50:50 POPC/DPPC was in gel + fluid phase coexistence at 17 °C. As the temperature increased, the proportion of the fluid-phase domains increased. At 33 °C, the membranes had fully transformed into the fluid phase.
We next compared the results of and . The observation of bright and dark phases in matched with the gel + fluid phase coexistence discussed in . In addition, the proportions of the bright and fluid phases both increased with temperature, suggesting that NBD-DOPE preferentially participates in the fluid-phase domains when GUVs are in the gel + fluid coexistence. Therefore, the bright and dark phases observed in the GUV of 50:50 POPC-d31/DPPC () corresponded to the POPC-rich fluid phase and DPPC-rich gel phase, respectively.
The bright-field and fluorescence images were taken for the GUVs of (POPC/DPPC) + 1 mol% C6-NBD-PC. shows the fluorescence images of POPC/DPPC GUVs as a function of temperature. Bright and dark phases were observed in the GUVs of 70:30, 50:50 or 30:70 POPC/DPPC over certain temperature ranges, suggesting that POPC and DPPC did not mix well. Like NBD-DOPE, C6-NBD-PC prefer disordered region rather than ordered region (Loura et al., Citation2008), thus the bright phase is supposed to be more disordered than the dark phase. As shown in , the area fraction of bright phase for 50:50 POPC/DPPC increased as the temperature increased from 15–28 °C. Concurrently, the area fraction of dark phase, which are circular domains, decreased with increasing temperature. A uniform fluorescence was observed at 28 °C, indicating that POPC and DPPC were well mixed at this temperature. Therefore, the miscibility temperature, Tmis, for the GUV of 50:50 POPC/DPPC was 28 ± 1 °C. Tmiss for the GUVs of 30:70 POPC/DPPC and 70:30 POPC/DPPC were 30 ± 1 °C and 27 ± 1 °C, respectively, as shown in .
Figure 4. Fluorescence image as a function of temperature for GUVs of (A) 30:70 POPC/DPPC; (B) 50:50 POPC/DPPC; (C) 70:30 POPC/DPPC. Each sample contains 1 mol% C6-NBD-PC.
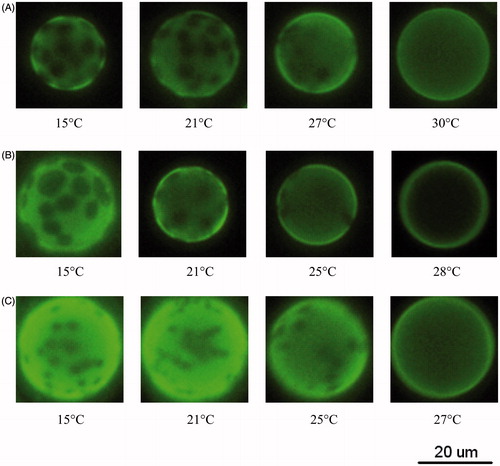
We next compared the results of and . The bright and dark phases in matched with the gel + fluid phase coexistence discussed in . In addition, the proportions of the bright and fluid phases both increased with temperature, suggesting that C6-NBD-PC preferentially participates in the fluid-phase domains when GUVs are in the gel + fluid coexistence. Therefore, the bright and dark phases observed in the GUV of 50:50 POPC-d31/DPPC () corresponded to the POPC-rich fluid phase and DPPC-rich gel phase, respectively.
The miscibility temperatures Tmis,1 obtained from and Tmis,2 obtained from for GUVs of various compositions are summarized in . The temperature Tend, where 50:50 POPC/DPPC membranes had fully transformed into the fluid phase as seen in the spectra of , was also included. We compared Tmis,1 and Tend for 50:50 POPC/DPPC bilayers. The former was measured in the presence of 1 mol% NBD-DOPE. This suggests that the addition of NBD-DOPE decreased the (gel + fluid)-to-fluid transition temperature of 50:50 POPC/DPPC by 5 °C. We next compared Tmis,2 and Tend for 50:50 POPC/DPPC bilayers. The former was measured in the presence of 1 mol% C6-NBD-PC. This showed that the addition of C6-NBD-PC also decreased the transition temperature of 50:50 POPC/DPPC, however by 7 °C. Therefore the presence of fluorescent probe NBD-DOPE or C6-NBD-PC lowered the (gel + fluid)-to-fluid transition temperature, and C6-NBD-PC had a stronger effect. As shown in , the NBD group was attached to the sn-2 chain of C6-NBD-PC, whereas in NBD-DOPE, NBD was attached to the headgroup. Our results suggest that fluorescent probe with NBD moiety at the acyl chain disturbs the membrane structure more strongly than that at the headgroup. Similar results were also found in 30:70 POPC/DPPC, given that the transition temperature is 40 °C for the probe-free membrane (Curatolo et al., Citation1985).
Table 1. A comparison for Tmis,1 of NBD-DOPE-containing GUVs, Tmis,2 of NBD-DOPE-containing GUVs, and Tend measured without the fluorescent probe. Considering that the transition temperature was reduced by 2 °C due to the lipid chain deuteration, the transition temperature for non-deuteraturated lipid bilayers should be shifted by +2 °C.
While 2H NMR can probe structures down to submicron scale, fluorescence microscopy probes to micron scale. When temperature approaches the gel + fluid/fluid transition, small gel domains (dark phase) of submicron size may not be detectable by fluorescence microscopy. Hence the measured miscibility temperature might be lower than the one measured by 2H NMR, despite the presence of the fluorescent probe. It was reported by an AFM study that micron-size gel domains were observed 2 °C below the gel + fluid/fluid transition (Bernchou et al., Citation2009; Lin, Citation2011; Xie et al., Citation2002). This implies that if submicron gel domains exist, it probably happens within 1–2 °C below the transition temperature. In this case, miscibility temperature measured by these two techniques should be quite close if not perturbed by the other probe. It is thus plausible that the discrepancy in transition temperature observed by fluorescence and 2H NMR, shown in , was mainly caused by the NBD probe.
The concentration of C6-NBD-PC and other NBD-labeled phospholipids as fluorescent probes typically range from 0.1 ∼ 1 mol% (Baumgart et al., Citation2007; Mukherjee et al., Citation2004), or higher in some cases (Loura et al., Citation2008; Mazères et al., Citation1996). The effect of 1mol% of C6-NBD-PC or NBD-DOPE has been discussed in . Here we briefly discuss the effect of low fluorophore concentration 0.1 mol%. Loura et al. (Citation2008) found that the addition of C6-NBD-PC lowers the main transition temperature of DPPC and the change in transition temperature was positively correlated to C6-NBD-PC concentration as it increased from 0–4 mol%. Assuming that the change in transition temperature of POPC/DPPC was also positively correlated to C6-NBD-PC concentration, the decrease in transition temperature for 0.1mol% C6-NBD-PC should be smaller than that for 1 mol% C6-NBD-PC. In this case, milder perturbation of POPC/DPPC bilayers was expected.
Conclusions
Bright and dark phases were observed in GUVs of POPC/DPPC in wide temperature and composition ranges, suggesting that POPC and DPPC do not mix well. As the temperature reached the miscibility temperature, e.g. 30 °C for 50:50 POPC/DPPC, two types of lipids mixed well, showing uniform fluorescence. The NMR results suggest that the bright phase is the fluid phase enriched in POPC and the dark phase is the gel phase enriched in DPPC. In addition, we observed that fluorescent probe NBD-DOPE and C6-NBD-PC preferentially participate in the fluid-phase domains when GUVs are in the gel + fluid phase coexistence.
A further comparison of fluorescence and NMR results showed that the two phases (gel + fluid) to one phase (fluid) transition temperature observed in membranes containing NBD-DOPE was 5 °C lower than that containing no NBD-DOPE. On the other hand, the transition temperature observed in membranes containing C6-NBD-PC was 7 °C lower than that containing no NBD-DOPE. The addition of fluorescent probe NBD-DOPE or C6-NBD-PC decreased the transition temperature of 5:5 POPC/DPPC bilayers, and C6-NBD-PC had a stronger effect. This suggests that fluorescent probe with NBD at the acyl chain disturbs the membrane structure more strongly than that at the headgroup.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This work was supported by research grants from the Ministry of Science and Technology of Taiwan [Grant Nos. NSC 97-2112-M-008 -004 -MY2].
References
- Angelova MI, Soleau S, Meleard P, Faucon JF, Bothorel P. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Progr Colloid Polym Sci 89:127–131.
- Bagatolli L. 2006. To see or not to see: lateral organization of biological membranes and fluorescence microscopy. Biochim Biophys Acta 1758:1541–1556.
- Baumgart T, Hunt G, Farkas ER, Webb WW, Feigenson GW. 2007. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim Biophys Acta 1768:2182–2194
- Bernchou U, Ipsen JH, Simonsen AC. 2009. Growth of solid domains in model membranes: quantitative image analysis reveals a strong correlation between domain shape and spatial position. J Phys Chem B 113:7170–7177.
- Cadenhead DA, Kellner BMJ, Jacobson K, Papahadjopoulos D. 1977. Fluorescent probes in model membranes I: anthroyl fatty acid derivatives in monolayers and liposomes of dipalmitoylphosphatidylcholine. Biochemistry 16:5386–5392.
- Curatolo W, Sears B, Neuringer LJ. 1985. A calorimetry and deuterium NMR study of mixed model membranes of 1-palmitoyl-2-oleylphosphatidylcholine and saturated phosphatidylcholines. Biochim Biophys Acta 817:261–270.
- Davis JH, Jeffrey KR, Bloom M, Valic MI, Higgs TP. 1976. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem Phys Lett 42:390–394.
- Klausner RD, Wolf DE. 1980. Selectivity of fluorescent lipid analogues for lipid domains. Biochemistry 19:6199–6203.
- Lesslauer W, Cain JE, Blasie JK. 1972. X-ray diffraction studies of lecithin bimolecular leaflets with incorporated fluorescent probes. Proc Natl Acad Sci USA 69:1499–1503.
- Lin C-Y. 2011. The effects of composition and thermal history on the properties of supported lipid bilayers [Master thesis]. Jung-Li, Taiwan: National Central University.
- Loura LMS, de Almeida RFM, Silva LC, Prieto M. 2009. FRET analysis of domain formation and properties in complex membrane systems. Biochim Biophys Acta 1788:209–224.
- Loura LMS, Fernandes F, Fenandes AC, Ramalho JP. 2008. Effects of fluorescent probe NBD-PC on the structure, dynamics and phase transition of DPPC. A molecular dynamics and differential scanning calorimetry study. Biochim Biophys Acta 1778:491–501.
- Mazères S, Schram V, Tocanne JF, Lopez A. 1996. 7-nitrobenz-2-oxa-1, 3-diazole-4-yl-labeled phospholipids in lipid membranes: differences in fluorescence behaviour. Biophys J 71:327–335.
- Mukherjee S, Raghuraman H, Dasgupta S, Chattopadhyay A. 2004. Organization and dynamics of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: a fluorescence approach. Chem Phys Lipids 127:91–101.
- Veatch SL, Keller SL. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta 1746:172–185.
- Veatch SL, Leung SS, Hancock RE, Thewalt JL. 2007. Fluorescent probes alter miscibility phase boundaries in ternary vesicles. J Phys Chem B 111:502–504.
- Xie AF, Yamada R, Gewirth AA, Granick S. 2002. Materials science of the gel to fluid phase transition in a supported phospholipid bilayer. Phys Rev Lett 89:246103.