Abstract
Ghrelin is a small peptide hormone that requires a unique post-translational modification, serine octanoylation, to bind and activate the GHS-R1a receptor. Initially demonstrated to stimulate hunger and appetite, ghrelin-dependent signaling is implicated in a variety of neurological and physiological processes influencing diseases such as diabetes, obesity, and Prader-Willi syndrome. In addition to its cognate receptor, recent studies have revealed ghrelin interacts with a range of binding partners within the bloodstream. Defining the scope of ghrelin’s interactions within the body, understanding how these interactions work in concert to modulate ghrelin signaling, and developing molecular tools for controlling ghrelin signaling are essential for exploiting ghrelin for therapeutic effect. In this review, we discuss recent findings regarding the biological effects of ghrelin signaling, outline binding partners that control ghrelin trafficking and stability in circulation, and summarize the current landscape of inhibitors targeting ghrelin octanoylation.
Discovery and characterization of ghrelin
Ghrelin is a 28-amino acid peptide hormone discovered in 1999 by Kojima and co-workers in their search to find the endogenous ligand for a growth hormone secretagogue receptor (GHS-R1a) (Kojima et al., Citation1999). Ghrelin was originally found to be expressed by endocrine X/A cells (P/D cells in humans) in the gastric mucosa of the stomach and small intestine (Date et al., Citation2000; Mizutani et al., Citation2009; Rindi et al., Citation2002; Stengel & Tache, Citation2012). In subsequent studies, both ghrelin and GHS-R1a expression have been detected in a range of tissues including the pancreatic alpha cells, the pituitary gland and hypothalamus using a range of techniques including in situ hybridization, immunohistochemistry monitored by optical microscopy, immunostaining coupled with electron microscopy, and RT-PCR (Date et al., Citation2000, Citation2002; Howard et al., Citation1996; Kojima et al., Citation1999; Korbonits et al., Citation2001). A broad study of human tissues identified the presence of ghrelin and GHS-R1a mRNA in multiple tissues, indicating that both ghrelin and the ghrelin receptor are expressed throughout the body (Gnanapavan et al., Citation2002). This widespread expression of ghrelin and its cognate receptor could provide the molecular foundation for the multiple physiological effects attributed to ghrelin signaling.
In the relatively short time since its discovery, ghrelin and associated signaling pathways have been linked to a wide range of physiological processes. These include growth hormone secretion (Kojima et al., Citation1999; Peino et al., Citation2000; Takaya et al., Citation2000), appetite stimulation and adiposity (Kamegai et al., Citation2001; Nakazato et al., Citation2001; Shintani et al., Citation2001; Tschop et al., Citation2000; Wren et al., Citation2001), insulin secretion and glucose homeostasis (Egido et al., Citation2002; Gagnon et al., Citation2015; Heppner et al., Citation2012; Reimer et al., Citation2003; Tong et al., Citation2010; Yada et al., Citation2014), and organismal response to starvation (Goldstein et al., Citation2011; Li et al., Citation2012). In recent work, ghrelin has been associated with a growing number of neurological processes such as memory, stress response, learning, sleep, mood levels and behavior (Andrews et al., Citation2009; Broglio et al., Citation2002, Citation2004; Gahete et al., Citation2011; Lutter et al., Citation2008). Recent studies have also implicated ghrelin as a major factor in neonatal hypothalamus development which influences lifelong metabolic regulation (Steculorum et al., Citation2015). We direct the reader to a recent review of ghrelin’s role in physiology and signaling for a comprehensive discussion of these topics (Muller et al., Citation2015).
Ghrelin expression and processing
Like other peptide hormones, ghrelin is expressed as a larger polypeptide that undergoes a series of processing steps prior to secretion from the cell () (Chen et al., Citation2009; Romero et al., Citation2010; Takahashi et al., Citation2009; Zhu et al., Citation2006). Ghrelin is expressed as a 117-amino acid precursor (preproghrelin), which contains an N-terminal signal peptide targeting preproghrelin for secretion. Cotranslational recognition of the signal peptide leads to preproghrelin trafficking to the endoplasmic reticulum (ER) where the signal peptide is cleaved to yield the 94-amino acid ghrelin precursor proghrelin (Zhu et al., Citation2006). In a processing step unique to ghrelin and essential for ghrelin’s biological activity through the GHS-R1a receptor, proghrelin is then acylated with octanoic acid on a specific serine side chain hydroxyl (Ser-3) near its N-terminus. Ghrelin octanoylation is catalyzed by ghrelin O-acyltransferase (GOAT), one of three members of the membrane bound O-acyltransferase (MBOAT) superfamily of enzymes that modify protein substrates (Buglino & Resh, Citation2012; Doubravska et al., Citation2011; Hofmann, Citation2000; Konitsiotis et al., Citation2015; Matevossian & Resh, Citation2015; Pepinsky et al., Citation1998; Takada et al., Citation2006; Willert et al., Citation2003). Acylated proghrelin is packaged into secretory vesicles and cleaved by a prohormone convertase (PC 1/3, PC2, or furin) to yield the mature 28-amino acid acylated ghrelin (‘ghrelin’), which can then be secreted into the bloodstream (Zhou et al., Citation1999; Zhu et al., Citation2006). In a recent study, Seim and co-workers identified an exon-deleted splice variant of ghrelin in multiple vertebrate species that yields a 13-amino acid ‘minighrelin’ upon expression and maturation (Seim et al., Citation2016). Minighrelin exhibits similar biological activity to full length ghrelin at the cell and organism level, supporting the potential for ghrelin splice variants to play significant roles in ghrelin signaling.
Figure 1. Ghrelin maturation and processing. Following several proteolytic steps and octanoylation by GOAT, ghrelin is secreted into the bloodstream where it can undergo deacylation via esterases in circulation. The dotted arrow (left) reflects the potential for acylation of desacyl ghrelin in bone marrow adipocytes as recently reported (Hopkins et al., Citation2017).
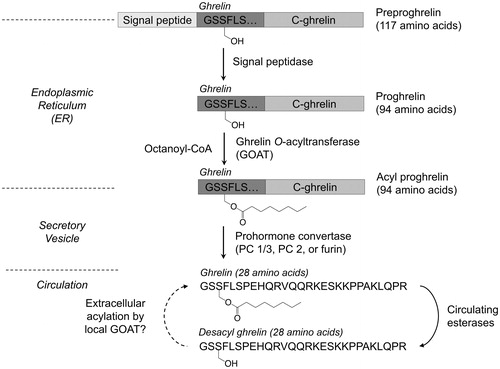
While this processing pathway for ghrelin maturation and acylation has been well established, a recent study suggests a potential new level of regulation within ghrelin signaling (Hopkins et al., Citation2017). Hopkins and co-workers demonstrated that both unacylated and acylated ghrelin promote bone marrow adipogenesis in mice in the presence of GOAT, with these effects absent in GOAT knockout mice. Their study also identifies new cellular localization of GOAT including both large and small lipid-trafficking vesicles as well as the plasma membrane (Hopkins et al., Citation2017). This is the first evidence of GOAT localization beyond the ER (Taylor et al., Citation2013). The observed GOAT localization, fatty acid availability in bone marrow tissue, and the observed effects of desacyl ghrelin treatment on adipogenesis in bone marrow support a model wherein unacylated ghrelin in circulation can be reacylated at the downstream cellular site of ghrelin signaling (Hopkins et al., Citation2017).
Ghrelin interactions within the body
GHS-R1a receptor
Ghrelin’s discovery occurred during the search for the endogenous ligand for the GHS-R1a receptor following its cloning and expression, with this receptor initially linked to growth hormone secretion (Howard et al., Citation1996). Studies of this orphan receptor initially utilized synthetic peptide growth hormone secretagogues to investigate receptor signaling and function (Callaghan & Furness, Citation2014; Howard et al., Citation1996). Ghrelin was identified as the endogenous ligand for the GHS-R1a receptor in 1999 (Kojima et al., Citation1999), at which time the functional necessity of ghrelin octanoylation for receptor binding and activation was realized (). Structure-activity analysis of ghrelin mimetic peptides modified with variable length acyl groups established that the GHS-R1a receptor requires the first five amino acids of ghrelin for recognition and activation equivalent to that observed with full-length ghrelin (Bednarek et al., Citation2000). The first four amino acids of ghrelin bound weakly to the receptor but yielded activation nearly equivalent to ghrelin, with complete receptor activation also requiring a large hydrophobic group at the Ser-3 equivalent position (Bednarek et al., Citation2000). Receptor activation can be achieved using ligands bearing medium to long acyl chains (up to 16 carbons) at the equivalent position to Ser-3, with reduced efficiency observed for acyl chains with less than 7 carbons (Bednarek et al., Citation2000). In addition to its signaling role, GHS-R1a mediates ghrelin uptake and degradation via endosomal processing followed by receptor recycling to the plasma membrane (Camina et al., Citation2004). In light of the potential of ghrelin signaling as a therapeutic target, there have been sustained efforts to develop agonists, antagonists, and inverse agonists targeting the GHS-R1a receptor. Several of these molecules have now progressed to clinical investigations, as discussed in several recent reviews of the topic (Avau et al., Citation2013; Cameron et al., Citation2014; Chollet et al., Citation2009; McGovern et al., Citation2016).
Figure 2. Ghrelin interacts with multiple partners in circulation. Ghrelin and desacyl ghrelin can interact with multiple binding partners in the bloodstream (equilibrium arrows), with esterases converting ghrelin to desacyl ghrelin by serine ester hydrolysis (single arrows). In interaction with lipoproteins (VLDL, HDL, and LDL), desacyl ghrelin shows a preference for binding to HDL (bold).
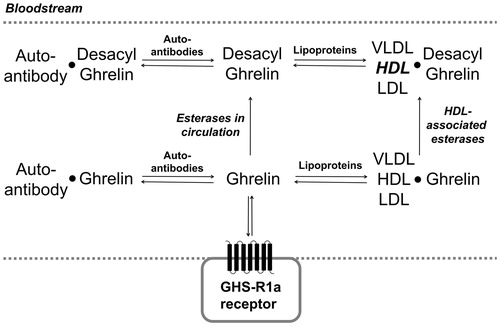
Lipoproteins in circulation
Both ghrelin and desacyl ghrelin lacking the serine octanoyl ester bind to lipoproteins in the blood plasma, with an increased abundance of cholesterol lipoproteins in the bloodstream leading to higher levels of ghrelin supporting the potential for these particles to act as ghrelin transporters (Purnell et al., Citation2003). The two different forms of ghrelin in circulation exhibit distinct binding behaviors to serum lipoproteins, with acylated ghrelin binding VLDL, LDL and HDL equally while desacyl ghrelin exhibits preferential binding to HDL () (De Vriese et al., Citation2007; Holmes et al., Citation2009). The binding of both ghrelin and desacyl ghrelin to HDL suggests these HDL-ghrelin complexes can serve as a ghrelin reservoir, effectively immobilizing circulating ghrelin in the bloodstream (Purnell et al., Citation2003). The interaction of ghrelin with HDL-associated esterases such as paraoxonase 1 (PON1) also accelerates its deacylation and degradation (Beaumont et al., Citation2003). Lipoprotein binding and associated enzymatic deacylation broadens the array of biological interactions regulating ghrelin trafficking, with the impact of these processes on ghrelin signaling remaining largely unexplored.
Ghrelin autoantibodies
Ghrelin-reactive autoantibodies are a recently discovered part of the body’s native immune system with recent evidence that these antibodies can be stored and released upon physiological stimulus and need (Fetissov et al., Citation2017). Ghrelin-reactive IgG was identified in human plasma by Takagi and co-workers, who proposed these autoantibodies stabilize ghrelin in the plasma by reducing serine ester hydrolysis and peptide degradation () (Takagi et al., Citation2013). In addition to protecting ghrelin from enzymatic deacylation, ghrelin autoantibodies are reported to enhance the ability of ghrelin to stimulate appetite (Francois et al., Citation2016a, Citation2016b; Takagi et al., Citation2013). Ghrelin-reactive IgG in obese individuals was found to exhibit higher affinity for ghrelin compared to ghrelin-reactive IgG from lean (non-obese) patient serum, which was proposed to enhance ghrelin trafficking and stability in obese patients (Francois et al., Citation2016a; Takagi et al., Citation2013). In contrast, individuals with anorexia nervosa have lower plasma levels of ghrelin autoantibodies and exhibit lower affinity ghrelin-IgG complexes which could lead to inefficient ghrelin signaling through a loss of effective ghrelin stabilization (Fetissov et al., Citation2017). As with the ghrelin-lipoprotein interactions described above, the impact of autoantibody binding on ghrelin trafficking and stabilization remains an active area of inquiry.
Ghrelin esterases
While in circulation, the octanoyl serine ester required for ghrelin binding and activation of the GHS-R1a receptor has a limited lifetime before removal by enzymatic hydrolysis to yield desacyl ghrelin () (De Vriese et al., Citation2004). Many protein components of human serum and tissues have been demonstrated to exhibit ghrelin deacylation ability, including platelet activating factor (PAF), paraoxanase (PON), carboxypeptidase, butyrylcholinesterase, carboxylesterases, APT1, and alpha 2-macroglobulin (Dantas et al., Citation2011; De Vriese et al., Citation2004, Citation2007; Eubanks et al., Citation2011; Satou et al., Citation2010). Butyrylcholinesterase, carboxylesterases and alpha 2-macroglobulin have been identified as potential ghrelin esterases in rat blood serum (De Vriese et al., Citation2004; Eubanks et al., Citation2011). Ghrelin deacylation was reduced in human and rat serum following the addition of phenylmethylsulfonyl fluoride or water-soluble derivatives thereof that serve as protease and esterase inhibitors (Delhanty et al., Citation2015; De Vriese et al., Citation2004). Subsequent studies have explored additional treatments to stabilize ghrelin acylation in serum, with a recent report using an alkyl fluorophosphonate reagent demonstrating rapid and complete protection of ghrelin from deacylation in biological samples (McGovern-Gooch et al., Citation2016).
Several recent studies have supported butyrylcholinesterase (BChE) as a potential ghrelin esterase within human circulation (Brimijoin et al., Citation2016; Chen et al., Citation2015, Citation2017; Schopfer et al., Citation2015). BChE composes a large proportion of total esterase activity in human serum, and recombinantly expressed BChE can hydrolyze ghrelin to desacyl ghrelin (Schopfer et al., Citation2015; Yao et al., Citation2016). BChE knock-out mice challenged with a high fat diet exhibit larger weight gain compared to control mice, with associated implications for BChE expression involvement in body energy regulation and weight control (Chen et al., Citation2015; Li et al., Citation2008; Schopfer et al., Citation2015). While these studies support the potential for BChE to act as a ghrelin esterase in human circulation, studies of human populations with BChE activity deficiencies such as the Vysya community suggest another esterase can either compensate for loss of BChE-catalyzed ghrelin hydrolysis or serve as the predominant ghrelin esterase (Manoharan et al., Citation2006, Citation2007). Defining the esterase-catalyzed deacylation limb of the ghrelin signaling pathway in circulation remains an important challenge in understanding the regulation of ghrelin-dependent processes at the organismal level.
Ghrelin O-acyltransferase: Catalyzing a unique protein modification required for biological signaling
As described above, ghrelin undergoes a unique posttranslational modification – serine octanoylation – during its maturation process (). While intriguing as a matter of protein biochemistry, this modification carries immense biological and physiological significance as octanoylation of Ser-3 is essential for ghrelin to bind and activate its cognate receptor (Kojima et al., Citation1999; Muller et al., Citation2015). The enzyme that catalyzes this modification, ghrelin O-acyltransferase (GOAT), was reported in 2008 by two research groups (Gutierrez et al., Citation2008; Yang et al., Citation2008a). GOAT is an integral membrane protein found most prominently in the ER (Taylor et al., Citation2013), and is a member of the MBOAT (membrane-bound O-acyltransferase) enzyme superfamily (Hofmann, Citation2000). GOAT is a topologically complex membrane protein containing 11 predicted transmembrane helices and one reentrant loop, with the topology of the C-terminal ‘MBOAT domain’ of GOAT matching closely that determined for fellow MBOAT member Hedgehog acyltransferase (Hhat) (Konitsiotis et al., Citation2015; Matevossian & Resh, Citation2015; Taylor et al., Citation2013). GOAT has proven resistant to purification in active form (Barnett et al., Citation2010; Taylor et al., Citation2013), which has complicated biochemical studies of GOAT-catalyzed ghrelin octanoylation.
GOAT substrate selectivity and potential catalytic domains
The first study of ghrelin recognition by GOAT utilized mutagenesis of the proghrelin precursor and determined that several residues near the N-terminus of proghrelin are crucial for effective octanoylation (Yang et al., Citation2008a, Citation2008b). Several early investigations established the ability of ghrelin mimetic short peptides to serve as GOAT substrates and determined that the N-terminal sequence of ghrelin/proghrelin is essential for recognition by GOAT (Ohgusu et al., Citation2009; Yang et al., Citation2008b). Studies by our group and others have used these synthetic peptide substrates to characterize GOAT substrate selectivity, confirming the importance of the N-terminal sequence of ghrelin and establishing the contribution of each side chain in the first four amino acids of ghrelin for recognition by GOAT (Barnett et al., Citation2010; Darling et al., Citation2013, Citation2015; Taylor et al., Citation2015). There is some tolerance for modification at the site of acylation as the mouse and human isoforms of GOAT accept substrates containing a threonine at the third residue, and bullfrog ghrelin has been confirmed to be octanoylated at a threonine (Darling et al., Citation2015; Kaiya et al., Citation2001, Citation2006, Citation2011; Yang et al., Citation2008b). In addition to its natural activity modifying a hydroxyl group, GOAT can catalyze modification of a substrate containing an amine at the third residue of a ghrelin mimetic peptide, resulting in an octanamide modification (Taylor et al., Citation2015). Regions of ghrelin downstream of the N-terminal ‘GSSF’ sequence may also interact with GOAT, although these downstream interactions appear to play a much smaller role in ghrelin binding to GOAT (Darling et al., Citation2015; Taylor et al., Citation2015). Utilizing the GOAT substrate selectivity preferences defined in these studies, bioinformatics analysis of the human proteome established that it is likely that ghrelin serves as the only substrate for GOAT in humans (Darling et al., Citation2015).
While ghrelin is predominantly modified by octanoic acid (Hosoda et al., Citation2003; Kojima et al., Citation1999), the mouse and human forms of GOAT can accept a range of acyl CoA substrates (Gutierrez et al., Citation2008; Nishi et al., Citation2005; Ohgusu et al., Citation2009; Yoh et al., Citation2011). Acyl donors with long carbon chains do not serve as efficient substrates for GOAT (Ohgusu et al., Citation2009; Yang et al., Citation2008b), unlike other MBOAT family members (Buglino & Resh, Citation2012; Chang & Magee, Citation2009; Hofmann, Citation2000). The preference of GOAT for medium-chain fatty acids is unique among MBOAT family members and provides a potential element for increasing GOAT inhibitor specificity. Inhibition studies using a series of acylated ghrelin mimetic peptides suggest that the acyl chain binding pocket within GOAT is composed of two distinct regions, with GOAT most strongly binding a peptide bearing an octanoyl group (Darling et al., Citation2015).
While the location and nature of the active site and substrate binding sites within GOAT remain undefined, several lines of investigation have provided insights into the regions and residues within GOAT that are essential for catalytic function. Sequence conservation analysis of MBOAT family members suggested conserved and potentially required catalytic residues essential for enzyme function, with subsequent studies of GOAT and Hhat indicating a shared membrane topology within this C-terminal region (Konitsiotis et al., Citation2015; Matevossian & Resh, Citation2015; Taylor et al., Citation2013). Photocrosslinking studies using acylated ghrelin mimetic peptides similarly localize ghrelin binding to the C-terminal half of GOAT (Barnett et al., Citation2010; Taylor et al., Citation2013, Citation2015). At the amino acid level, Asn 307 is highly conserved and His 338 is absolutely conserved within the MBOAT superfamily and mutation of either residue abrogates acylation activity (Gutierrez et al., Citation2008; Hofmann, Citation2000; Taylor et al., Citation2015; Yang et al., Citation2008b). However, the predicted topology of GOAT places these two residues on opposite sides of the ER membrane, suggesting that both cannot be directly involved in catalysis (Taylor et al., Citation2013). A recent study provided evidence that GOAT contains a functionally essential cysteine residue, as N-ethylmaleimide treatment inhibits the human isoform of GOAT (McGovern-Gooch et al., Citation2017). Our understanding of the structural and mechanistic basis for GOAT-catalyzed ghrelin octanoylation has expanded rapidly in the last decade, but many of the aspects of GOAT enzymatic function remain to be determined.
Towards modulating ghrelin signaling for therapeutic effect: Desacyl ghrelin mimics and GOAT inhibitors
Ghrelin signaling has been linked to multiple physiological functions impacting health and disease, including diabetes, obesity, symptoms of Prader-Willi syndrome, psychological stress and anxiety, depression, aging and neuroprotection, taste sensitivity and reward-seeking behavior, sleep regulation and deprivation, cardiac health and function, gastric mobility and acid secretion, and protection against muscle atrophy (Anderwald et al., Citation2003; Andrews et al., Citation2009; Barim et al., Citation2009; Broglio et al., Citation2001; Celi et al., Citation2005; Chuang & Zigman, Citation2010; Cummings et al., Citation2002; Erdmann et al., Citation2005; Falken et al., Citation2010; Heppner et al., Citation2012; Kamegai et al., Citation2001; Kurt et al., Citation2007; Kweh et al., Citation2015; Lutter et al., Citation2008; Mager et al., Citation2006; Moon et al., Citation2009; Murray et al., Citation2005; Muller et al., Citation2015; Poykko et al., Citation2003; Shiiya et al., Citation2002; Spencer et al., Citation2015; Tack et al., Citation2006; Tschop et al., Citation2000; Tong et al., Citation2010; Van der Ploeg et al., Citation2014). Modulating the ghrelin pathway presents a potential therapeutic avenue for treating these disorders and diseases. While significant work has focused on controlling ghrelin signaling using molecules targeting the GHS-R1a receptor (Cameron et al., Citation2014; McGovern et al., Citation2016), recent studies have shifted focus to ghrelin and ghrelin acylation as potential therapeutic targets. We discuss two approaches currently being explored for altering ghrelin signaling – analogs of desacyl ghrelin and GOAT inhibitors.
Analogs of desacyl ghrelin: AZP-531 and CF801
Although initially thought to play no biological role, desacyl ghrelin demonstrates an antagonistic effect with ghrelin in improving insulin sensitivity and decreasing availability of free fatty acids (Barazzoni et al., Citation2007; Benso et al., Citation2012; Broglio et al., Citation2004; Cederberg et al., Citation2012; Delhanty et al., Citation2013, Citation2015; Delhanty & van der Lely, Citation2011; Heppner et al., Citation2014; Hosoda et al., Citation2000; Lear et al., Citation2010; Stevanovic et al., Citation2014; Togliatto et al., Citation2015). Microarray expression studies also support desacyl ghrelin involvement in lipid metabolism and regulation through gene regulation in fat, muscle, and liver cells (Delhanty et al., Citation2010). Investigation of truncated analogs of desacyl ghrelin led to the development of a biologically active linear peptide composed of residues 6–13 of desacyl ghrelin, with circularization of this sequence generating a compound with increased biostability and improved pharmacokinetics named AZP-531 () (Delhanty et al., Citation2013; Julien et al., Citation2012). Mice treated with AZP-531 exhibited increased insulin sensitivity, reduced glucose intolerance, and reduced fat accumulation when challenged with a high-fat diet (Delhanty et al., Citation2013). In a Phase I clinical trial, AZP-531 was well-tolerated and resulted in metabolic improvements. Subjects with impaired glucose tolerance treated with AZP-531 exhibited decreased glucose concentrations, and obese subjected exhibited greater weight loss compared to placebo controls, despite receiving identical meals (Allas et al., Citation2016). In a 2016 press release describing the top-line results of phase II clinical trial investigating AZP-531 as a treatment for patients with Prader-Willi syndrome, Alizè Pharma reported patients treated with AZP-531 reported reduced appetite and showed improved glucose control compared to those in the placebo group.
Figure 3. Desacyl ghrelin mimics. (a) The CF801 inhibitor consists of the first 10 amino acids of ghrelin with an S3A mutation appended to an HIV Tat sequence. (b) The Alizè pharmaceutical AZP-531 is a circularized peptide consisting of ghrelin residues 6–13.
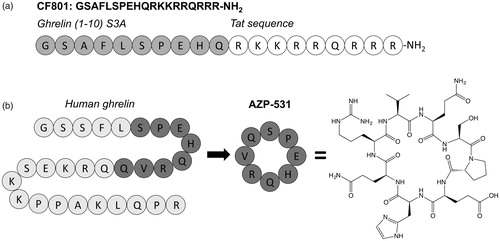
In another approach to develop an agent capable of lowering ghrelin levels in circulation, Wellman and co-workers designed the desacyl ghrelin analog CF801 () (Wellman et al., Citation2015). This analog consists of the first 10 amino acids of ghrelin with an alanine mutation at the octanoylation site and an attached Tat sequence to enable cell permeability. Intriguingly, CF801 does not serve as a GOAT inhibitor. It does not block ghrelin octanoylation in recombinant enzyme-based assays but CF801 treatment decreased acylated ghrelin levels in SG-1 cells (Wellman et al., Citation2015; Zhao et al., Citation2010). Administration of CF801 also decreased weight gain in mice fed a high-fat diet. The biological target of this peptide is not clear, but the beneficial effects observed with both AZP-531 and CF801 treatment provides support for examining desacyl ghrelin analogs for potential therapeutic applications.
GOAT inhibitors
As noted above, ghrelin must be octanoylated in order to bind and active the GHS-R1a receptor. Therefore, blocking GOAT-catalyzed ghrelin octanoylation presents an attractive option for modulating ghrelin signaling. With ghrelin predicted to be the only substrate for GOAT within the human proteome (Darling et al., Citation2015), inhibition of GOAT acylation activity also carries a reduced likelihood of impacting the modification of other proteins leading to undesired off-target side-effects. The lack of structural and mechanistic information about the GOAT active site and substrate binding sites has made rational design of GOAT inhibitors problematic, but several classes of GOAT inhibitors have been described in the scientific and patent literature in recent years. The reported GOAT inhibitors can be sorted into three classes which will be discussed in detail below: acylated ghrelin/product mimetics, bisubstrate analogs, and small molecules.
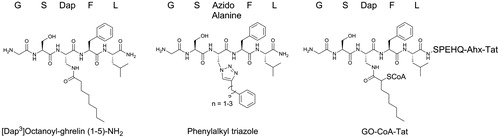
Product-mimetic inhibitors: [Dap3]octanoyl-ghrelin(1–5)-NH2, [Dap3]octanoyl-ghrelin(1–28)-NH2 and triazole-linked acylated ghrelin analogs
Immediately following the initial identification of GOAT as the enzyme responsible for octanoylating ghrelin, Yang and co-workers developed potent GOAT inhibitors by replacing the hydrolytically susceptible ester linkage of octanoylated ghrelin with an amide linkage (Yang et al., Citation2008b). In these molecules, the Ser-3 residue of both full-length ghrelin (amino acids 1–28) and a truncated form of ghrelin (amino acids 1–5) were replaced with octanoylated (S)-2, 3-diaminopropionic acid (Dap) (). In assays measuring radiolabeled [H3]octanoate transfer to His-tagged proghrelin by GOAT in the microsomal fraction, both [Dap3]octanoyl-ghrelin(1-28)-NH2 and [Dap3]octanoyl-ghrelin(1-5)-NH2 effectively inhibited proghrelin octanoylation. Using variations of the shorter [Dap3]octanoyl-ghrelin(1-5)-NH2 inhibitor, Darling and co-workers demonstrated that an 8-carbon acyl group is required for maximum potency against GOAT (Darling et al., Citation2015). While useful for investigating GOAT activity in enzyme-based assays (Darling et al., Citation2013, Citation2015; Yang et al., Citation2008b), these inhibitors have limited therapeutic potential due to two factors: (1) as peptides, these molecule are unlikely to exhibit high cell permeability; and (2) mimics of octanoylated ghrelin are likely to bind to and activate GHS-R1a, thus contravening the rationale for inhibiting ghrelin acylation. The receptor requires only the first four residues of ghrelin and a hydrophobic moiety at position 3 for equivalent receptor agonism to full-length ghrelin (Bednarek et al., Citation2000).
The lipid chain mimicking acyl ghrelin’s octanoyl modification is important for GOAT inhibitor potency (Darling et al., Citation2015; Yang et al., Citation2008b). Inspired by the [Dap3]-ghrelin(1-5)-NH2 inhibitor, Zhao and co-workers replaced the amide linkage to the lipid chain with a bioisosteric 1,2,3-triazole linkage to an phenyl group through a short alkyl chain () (Zhao et al., Citation2015). Triazoles, in addition to being stable against hydrolysis, also allow for the synthesis of a diverse panel of inhibitor candidates using ‘click’ chemistry (Holub & Kirshenbaum, Citation2010). In a microsomal GOAT activity assay, these phenylalkyl triazole peptidomimetics inhibited GOAT activity, with the most potent exhibiting an IC50 of 0.7 μM. Varying the alkyl chain length yielded a similar structure-activity profile to that observed with amide-linked acylated ghrelin mimics (Darling et al., Citation2015).
Bisubstrate analog: GO-CoA-Tat
Barnett and co-workers designed a bisubstrate analog combining chemical aspects of ghrelin and octanoyl-CoA as a potential GOAT inhibitor based on the success of similar approaches in creating inhibitors targeting histone acyl transferase (Barnett et al., Citation2010; Lau et al., Citation2000; Parang et al., Citation2001). The bisubstrate analog, named GO-CoA-Tat, couples the first 10 amino acids of ghrelin to octanoyl CoA via a hydrolytically stable amide linkage (). GO-CoA-Tat also includes an 11-amino acid Tat peptide appended to the C-terminus of the ghrelin sequence to increase cell permeability. Importantly, despite including the first 10 residues of ghrelin and an octanoyl chain GO-CoA-Tat does not activate the GHS-R1a receptor (Barnett et al., Citation2010).
In both microsomal enzyme assays and cell studies, GO-CoA-Tat inhibited production of acylated ghrelin at micromolar or lower concentrations. In animal studies, mice treated with GO-CoA-Tat show decreased levels of serum acyl ghrelin, resistance to weight gain when fed a medium-chain triglyceride-rich high-fat diet and a significant increase of insulin in response to a glucose challenge (Barnett et al., Citation2010). In subsequent studies, GO-CoA-Tat treatment decreased fasting-induced food foraging and hoarding in hamsters and reduced meal frequency in rats (Teubner et al., Citation2013; Teuffel et al., Citation2015). While the in vivo utility and pharmaceutical potential of GO-CoA-Tat is limited by its susceptibility to proteolytic degradation and its high molecular weight (∼3600 Da), studies using GO-CoA-Tat remain the best reported data supporting inhibition of ghrelin octanoylation as a therapeutic avenue for treating conditions impacted by ghrelin signaling.
Small molecule GOAT inhibitors
The first small molecule inhibitors of GOAT were identified by Garner and Janda in 2011 using a screen of small library of lipidated molecules in a microsomal GOAT activity assay () (Garner & Janda, Citation2010, Citation2011). The most potent of these molecules exhibited half-maximal inhibitory concentrations in the single to tens of micromolar concentration range. The presence of medium-chain length alkyl chains in these compounds suggests their inhibitory activity could derive from competing for the octanoyl CoA binding site. While these inhibitors presaged the potential for small molecule GOAT inhibitors, no studies of their effectiveness in cells or animal studies have been reported.
Figure 5. Small molecule GOAT inhibitors. Small molecule GOAT inhibitors currently reported in the scientific and patent literature; molecules in the top row are described in peer-reviewed publications and molecules in the bottom row are reported in patent applications or issued patents.
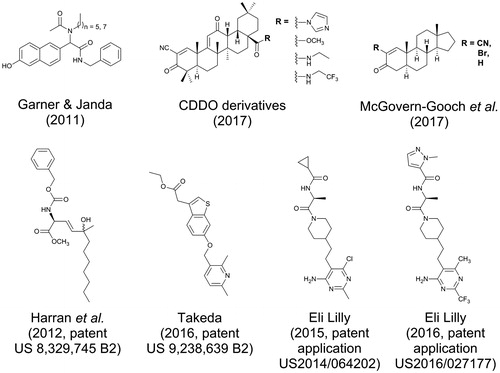
A number of small-molecule GOAT inhibitors have been reported by both academic and industrial research groups in the patent literature. Working from a proposed mechanism for ghrelin acylation including a classical tetrahedral intermediate, Harran and co-workers proposed a series of small molecule transition state analogs mimicking this structure (Harran et al., Citation2012). While originally intending to mimic the side chains of the serine and phenylalanine residues flanking the serine octanoylation site within ghrelin, their most potent inhibitor BK1114 lacks these functionalities (). BK1114 inhibits GOAT activity at micromolar concentrations in a previously reported microsomal assay (Yang et al., Citation2008b).
Industrial researchers have also contributed to the small molecule GOAT inhibitor patent literature. In patents assigned to Takeda Pharmaceuticals, a number of small molecules featuring multiple aromatic rings are reported to potently inhibit GOAT activity using an ELISA-based high throughput assay for detection of acyl ghrelin () (Takakura et al., Citation2013). Two recently published patents assigned to Eli Lilly report substituted piperidyl-ethyl-pyrimidine derived GOAT inhibitors with demonstrated potency in enzyme-, cell-, and animal-base studies. The lead compound in this class was discovered using a high-throughput ELISA-based GOAT activity screening assay, and optimized inhibitors were reported to inhibit GOAT in the mid-nanomolar range in in vitro GOAT activity assays (Galka et al., Citation2016; Martinez-Grau, Citation2014).
In the most recent report of small molecule GOAT inhibitors, a class of molecules derived from synthetic triterpenoids was found to inhibit the human GOAT (hGOAT) ortholog (McGovern-Gooch et al., Citation2017). In screening a NIH-provided library of structurally diverse small molecules, two derivatives of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) were identified as hGOAT inhibitors with IC50 values in the low micromolar range as assessed by a microsomal hGOAT activity assay () (Darling et al., Citation2013). Structure-activity analysis of these inhibitors revealed the α-cyanoenone Michael acceptor moiety in ring A was essential for inhibition. Further studies of these compounds and simpler derivatives containing Michael addition electrophiles support a covalent reversible inhibition mechanism for these molecules, implicating the presence of a functionally essential cysteine residue within hGOAT. Surprisingly, the mouse isoform of GOAT did not demonstrate the same susceptibility to these cysteine-modifying inhibitors, indicating an unexpected but important distinction between these two closely related enzyme orthologs.
These CDDO derivatives have been studied as potential therapeutics for inflammation and oxidative stress in multiple cell signaling pathways including the Nrf2 and NF-κB pathways (Liby & Sporn, Citation2012). In rodent and human studies, treatment with CDDO derivatives was reported to produce side-effects such as weight loss, reduced insulin resistance, and improved glucose tolerance (Camer et al., Citation2015; de Zeeuw et al., Citation2013; Dinh et al., Citation2015; Saha et al., Citation2010). These side-effects would be consistent with altered ghrelin signaling resulting from GOAT inhibition by CDDO derivatives, supporting future studies of the physiological impact of CDDO derivatives and similar molecules on ghrelin signaling.
Conclusions
Ghrelin is a unique peptide hormone impacting multiple physiological processes, playing a central role in energy metabolism while exerting effects on a wide range of systems within the body. Following its production and secretion into the bloodstream, ghrelin can interact with a number of different proteins including lipoproteins, autoantibodies, and ghrelin esterases prior to binding the GHS-R1a receptor. Many of the physiological effects of ghrelin are dependent upon activation of the ghrelin receptor GHS-R1a, which requires that ghrelin be acylated with a medium-chain fatty acid. A growing body of evidence supports a biological role for desacyl ghrelin as well, although less is known about the specific signaling pathways affected by desacyl ghrelin.
The enzyme responsible for ghrelin acylation, ghrelin O-acyltransferase (GOAT), is a member of the MBOAT family of enzymes including fellow protein modifying members PORCN and Hhat. With ghrelin as the unique substrate for GOAT within the human proteome, development of GOAT inhibitors has been identified as an exciting approach to modulating ghrelin-dependent signaling. Ranging from ghrelin-mimetic peptides to bisubstrate inhibitors to small molecules, increasing numbers of reported GOAT inhibitors in recent years justifies a growing enthusiasm in the ghrelin-GOAT system as a potential therapeutic target. Ongoing mechanistic and structural investigation of GOAT-catalyzed ghrelin acylation will further accelerate rational design of small molecule inhibitors for preclinical studies.
Ghrelin signaling is proposed to be connected to an ever-expanding list of diseases and disorders. Modulation of this pathway through GOAT inhibition is a promising strategy in treating these diseases. Defining the scope of ghrelin’s interactions within the body will help to identify possible approaches for controlling ghrelin signaling. Through a combination of these approaches, the ghrelin-GOAT system has great potential as a novel treatment avenue for diabetes, obesity, and other diseases impacted by ghrelin.
Acknowledgements
We thank members of the Hougland research group for discussions and helpful comments.
Disclosure statement
JLH has patent interests in the use of multiple compounds reported herein to target ghrelin signaling and associated health conditions. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Allas S, Delale T, Ngo N, Julien M, Sahakian P, Ritter J, et al. 2016. Safety, tolerability, pharmacokinetics and pharmacodynamics of azp-531, a first-in-class analogue of unacylated ghrelin, in healthy and overweight/obese subjects and subjects with type 2 diabetes. Diabetes Obes Metab 18:868–874.
- Anderwald C, Brabant G, Bernroider E, Horn R, Brehm A, Waldhausl W, Roden M. 2003. Insulin-dependent modulation of plasma ghrelin and leptin concentrations is less pronounced in type 2 diabetic patients. Diabetes 52:1792–1798.
- Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, et al. 2009. Ghrelin promotes and protects nigrostriatal dopamine function via a ucp2-dependent mitochondrial mechanism. J Neurosci 29:14057–14065.
- Avau B, Carbone F, Tack J, Depoortere I. 2013. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil 25:720–732.
- Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, et al. 2007. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab 92:3935–3940.
- Barim AO, Aydin S, Colak R, Dag E, Deniz O, Sahin I. 2009. Ghrelin, paraoxonase and arylesterase levels in depressive patients before and after citalopram treatment. Clin Biochem 42:1076–1081.
- Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, et al. 2010. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 330:1689–1692.
- Beaumont NJ, Skinner VO, Tan TM, Ramesh BS, Byrne DJ, Maccoll GS, et al. 2003. Ghrelin can bind to a species of high density lipoprotein associated with paraoxonase. J Biol Chem 278:8877–8880.
- Bednarek MA, Feighner SD, Pong SS, Mckee KK, Hreniuk DL, Silva MV, et al. 2000. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: Minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43:4370–4376.
- Benso A, St-Pierre DH, Prodam F, Gramaglia E, Granata R, Van Der Lely AJ, et al. 2012. Metabolic effects of overnight continuous infusion of unacylated ghrelin in humans. Eur J Endocrinol 166:911–916.
- Brimijoin S, Chen VP, Pang YP, Geng L, Gao Y. 2016. Physiological roles for butyrylcholinesterase: a BCHE-Ghrelin axis. Chem Biol Interact 259:271–275.
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, et al. 2001. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86:5083–5086.
- Broglio F, Arvat E, Benso A, Papotti M, Muccioli G, Deghenghi R, Ghigo E. 2002. Ghrelin: endocrine and non-endocrine actions. J Pediatr Endocrinol Metab 15(Suppl 5):1219–1227.
- Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, et al. 2004. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab 89:3062–3065.
- Buglino JA, Resh MD. 2012. Palmitoylation of hedgehog proteins. Vitam Horm 88:229–252.
- Callaghan B, Furness JB. 2014. Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol Rev 66:984–1001.
- Camer D, Yu Y, Szabo A, Dinh CH, Wang H, Cheng L, Huang XF. 2015. Bardoxolone methyl prevents insulin resistance and the development of hepatic steatosis in mice fed a high-fat diet. Mol Cell Endocrinol 412:36–43.
- Cameron KO, Bhattacharya SK, Loomis AK. 2014. Small molecule ghrelin receptor inverse agonists and antagonists. J Med Chem 57:8671–8691.
- Camina JP, Carreira MC, El Messari S, Llorens-Cortes C, Smith RG, Casanueva FF. 2004. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology 145:930–940.
- Cederberg H, Koivisto VM, Jokelainen J, Surcel HM, Keinanen-Kiukaanniemi S, Rajala U. 2012. Unacylated ghrelin is associated with changes in insulin sensitivity and lipid profile during an exercise intervention. Clin Endocrinol 76:39–45.
- Celi F, Bini V, Papi F, Santilli E, Ferretti A, Mencacci M, et al. 2005. Circulating acylated and total ghrelin and galanin in children with insulin-treated type 1 diabetes: relationship to insulin therapy, metabolic control and pubertal development. Clin Endocrinol 63:139–145.
- Chang SC, Magee AI. 2009. Acyltransferases for secreted signalling proteins (Review). Mol Membr Biol 26:104–113.
- Chen CY, Fujimiya M, Asakawa A, Chang FY, Cheng JT, Lee SD, Inui A. 2009. At the cutting edge: Ghrelin gene products in food intake and gut motility. Neuroendocrinology 89:9–17.
- Chen VP, Gao Y, Geng L, Brimijoin S. 2017. Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes. 41:1413–1419.
- Chen VP, Gao Y, Geng L, Parks RJ, Pang YP, Brimijoin S. 2015. Plasma butyrylcholinesterase regulates ghrelin to control aggression. Proc Natl Acad Sci USA 112:2251–2256.
- Chollet C, Meyer K, Beck-Sickinger AG. 2009. Ghrelin – a novel generation of anti-obesity drug: design, pharmacomodulation and biological activity of ghrelin analogues. J Pept Sci 15:711–730.
- Chuang JC, Zigman JM. 2010. Ghrelin’s roles in stress, mood, and anxiety regulation. Int J Pept 2010:460549.
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630.
- Dantas VG, Furtado-Alle L, Souza RL, Chautard-Freire-Maia EA. 2011. Obesity and variants of the ghrl (ghrelin) and bche (butyrylcholinesterase) genes. Genet Mol Biol 34:205–207.
- Darling JE, Prybolsky EP, Sieburg M, Hougland JL. 2013. A fluorescent peptide substrate facilitates investigation of ghrelin recognition and acylation by ghrelin O-acyltransferase. Anal Biochem 437:68–76.
- Darling JE, Zhao F, Loftus RJ, Patton LM, Gibbs RA, Hougland JL. 2015. Structure-activity analysis of human ghrelin O-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition. Biochemistry 54:1100–1110.
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. 2000. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261.
- Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. 2002. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 51:124–129.
- De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. 2004. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 145:4997–5005.
- De Vriese C, Hacquebard M, Gregoire F, Carpentier Y, Delporte C. 2007. Ghrelin interacts with human plasma lipoproteins. Endocrinology 148:2355–2362.
- De Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. 2013. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369:2492–2503.
- Delhanty PJ, Huisman M, Baldeon-Rojas LY, Van Den Berge I, Grefhorst A, Abribat T, et al. 2013. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. faseb J 27:1690–1700.
- Delhanty PJ, Huisman M, Julien M, Mouchain K, Brune P, Themmen AP, et al. 2015. The acylated (AG) to unacylated (UAG) ghrelin ratio in esterase inhibitor-treated blood is higher than previously described. Clin Endocrinol 82:142–146.
- Delhanty PJ, Sun Y, Visser JA, Van Kerkwijk A, Huisman M, Van Ijcken WF, et al. 2010. Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted mice. PLoS One 5:e11749.
- Delhanty PJ, Van Der Lely AJ. 2011. Ghrelin and glucose homeostasis. Peptides 32:2309–2318.
- Dinh CH, Szabo A, Camer D, Yu Y, Wang H, Huang XF. 2015. Bardoxolone methyl prevents fat deposition and inflammation in the visceral fat of mice fed a high-fat diet. Chem Biol Interact 229:1–8.
- Doubravska L, Krausova M, Gradl D, Vojtechova M, Tumova L, Lukas J, et al. 2011. Fatty acid modification of wnt1 and wnt3a at serine is prerequisite for lipidation at cysteine and is essential for wnt signalling. Cell Signal 23:837–848.
- Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. 2002. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 146:241–244.
- Erdmann J, Lippl F, Wagenpfeil S, Schusdziarra V. 2005. Differential association of basal and postprandial plasma ghrelin with leptin, insulin, and type 2 diabetes. Diabetes 54:1371–1378.
- Eubanks LM, Stowe GN, De Lamo Marin S, Mayorov AV, Hixon MS, Janda KD. 2011. Identification of alpha2 macroglobulin as a major serum ghrelin esterase. Angew Chem Int Ed Engl 50:10699–10702.
- Falken Y, Hellstrom PM, Sanger GJ, Dewit O, Dukes G, Gryback P, et al. 2010. Actions of prolonged ghrelin infusion on gastrointestinal transit and glucose homeostasis in humans. Neurogastroenterol Motil 22:e192–e200.
- Fetissov SO, Lucas N, Legrand R. 2017. Ghrelin-reactive immunoglobulins in conditions of altered appetite and energy balance. Front Endocrinol 8:10.
- Francois M, Barde S, Legrand R, Lucas N, Azhar S, El Dhaybi M, et al. 2016a. High-fat diet increases ghrelin-expressing cells in stomach, contributing to obesity. Nutrition 32:709–715.
- Francois M, Takagi K, Legrand R, Lucas N, Beutheu S, Bole-Feysot C, et al. 2016b. Increased ghrelin but low ghrelin-reactive immunoglobulins in a rat model of methotrexate chemotherapy-induced anorexia. Front Nutr 3:23.
- Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. 2015. Ghrelin is a novel regulator of glp-1 secretion. Diabetes 64:1513–1521.
- Gahete MD, Cordoba-Chacon J, Kineman RD, Luque RM, Castano JP. 2011. Role of ghrelin system in neuroprotection and cognitive functions: implications in Alzheimer’s disease. Peptides 32:2225–2228.
- Galka CS, Hembre EJ, Honigschmidt NA, Martinez-Grau MA, Plaza GR, Rubio A. 2016. Ghrelin o-acyl transferase inhibitors. US Patent Application PCT/US2016/027177.
- Garner AL, Janda KD. 2010. Cat-elcca: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT). Angew Chem Int Ed Engl 49:9630–9634.
- Garner AL, Janda KD. 2011. A small molecule antagonist of ghrelin O-acyltransferase (GOAT). Chem Commun (Camb) 47:7512–7514.
- Gnanapavan S, Kola B, Bustin SA, Morris DG, Mcgee P, Fairclough P, et al. 2002. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-r, in humans. J Clin Endocrinol Metab 87:2988.
- Goldstein JL, Zhao TJ, Li RL, Sherbet DP, Liang G, Brown MS. 2011. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol 76:121–127.
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. 2008. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105:6320–6325.
- Harran PG, Brown MS, Goldstein JL, Yang J, Zhao TJ. 2012. Small molecule inhibitors of ghrelin O-acyltransferase. US Patent 8329745.
- Heppner KM, Piechowski CL, Muller A, Ottaway N, Sisley S, Smiley DL, et al. 2014. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes 63:122–131.
- Heppner KM, Muller TD, Tong J, Tschop MH. 2012. Ghrelin in the control of energy, lipid, and glucose metabolism. Meth Enzymol 514:249–260.
- Hofmann K. 2000. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci 25:111–112.
- Holmes E, Davies I, Lowe G, Ranganath LR. 2009. Circulating ghrelin exists in both lipoprotein bound and free forms. Ann Clin Biochem 46:514–516.
- Holub JM, Kirshenbaum K. 2010. Tricks with clicks: modification of peptidomimetic oligomers via copper-catalyzed azide-alkyne [3 + 2] cycloaddition. Chem Soc Rev 39:1325–1337.
- Hopkins AL, Nelson TA, Guschina IA, Parsons LC, Lewis CL, Brown RC, et al. 2017. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyltransferase and ghs-r1a activity: evidence for target cell-induced acylation. Sci Rep 7:45541.
- Hosoda H, Kojima M, Matsuo H, Kangawa K. 2000. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279:909–913.
- Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. 2003. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem 278:64–70.
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. 1996. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974–977.
- Julien M, Kay RG, Delhanty PJ, Allas S, Granata R, Barton C, et al. 2012. In vitro and in vivo stability and pharmacokinetic profile of unacylated ghrelin (UAG) analogues. Eur J Pharm Sci 47:625–635.
- Kaiya H, Koizumi Y, Konno N, Yamamoto K, Uchiyama M, Kangawa K, Miyazato M. 2011. Ghrelin receptor in two species of anuran amphibian, bullfrog (Rana catesbeiana), and Japanese tree frog (Hyla japonica). Front Endocrinol 2:31.
- Kaiya H, Kojima M, Hosoda H, Koda A, Yamamoto K, Kitajima Y, et al. 2001. Bullfrog ghrelin is modified by n-Octanoic acid at its third threonine residue. J Biol Chem 276:40441–40448.
- Kaiya H, Sakata I, Yamamoto K, Koda A, Sakai T, Kangawa K, Kikuyama S. 2006. Identification of immunoreactive plasma and stomach ghrelin, and expression of stomach ghrelin mRNA in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol 148:236–244.
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. 2001. Chronic central infusion of ghrelin increases hypothalamic neuropeptide y and agouti-related protein mRNA levels and body weight in rats. Diabetes 50:2438–2443.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660.
- Konitsiotis AD, Jovanovic B, Ciepla P, Spitaler M, Lanyon-Hogg T, Tate EW, Magee AI. 2015. Topological analysis of hedgehog acyltransferase, a multipalmitoylated transmembrane protein. J Biol Chem 290:3293–3307.
- Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, et al. 2001. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 86:881–887.
- Kurt E, Guler O, Serteser M, Cansel N, Ozbulut O, Altinbas K, et al. 2007. The effects of electroconvulsive therapy on ghrelin, leptin and cholesterol levels in patients with mood disorders. Neurosci Lett 426:49–53.
- Kweh FA, Miller JL, Sulsona CR, Wasserfall C, Atkinson M, Shuster JJ, et al. 2015. Hyperghrelinemia in Prader-Willi syndrome begins in early infancy long before the onset of hyperphagia. Am J Med Genet A 167A:69–79.
- Lau OD, Kundu TK, Soccio RE, Ait-Si-Ali S, Khalil EM, Vassilev A, et al. 2000. Hats off: Selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol Cell 5:589–595.
- Lear PV, Iglesias MJ, Feijoo-Bandin S, Rodriguez-Penas D, Mosquera-Leal A, Garcia-Rua V, et al. 2010. Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology 151:3286–3298.
- Li B, Duysen EG, Lockridge O. 2008. The butyrylcholinesterase knockout mouse is obese on a high-fat diet. Chem Biol Interact 175:88–91.
- Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. 2012. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem 287:17942–17950.
- Liby KT, Sporn MB. 2012. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64:972–1003.
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. 2008. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11:752–753.
- Mager U, Lindi V, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, et al; Finnish Diabetes Prevention Study Group. 2006. Association of the leu72met polymorphism of the ghrelin gene with the risk of type 2 diabetes in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention study. Diabet Med 23:685–689.
- Manoharan I, Boopathy R, Darvesh S, Lockridge O. 2007. A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India. Clin Chim Acta 378:128–135.
- Manoharan I, Wieseler S, Layer PG, Lockridge O, Boopathy R. 2006. Naturally occurring mutation leu307pro of human butyrylcholinesterase in the Vysya community of India. Pharmacogenet Genom 16:461–468.
- Martinez-Grau MA. 2014. Substituted piperidyl-ethyl-pyrimidine as ghrelin O-acyltransferase inhibitor. US Patent Application PCT/US2014/064202.
- Matevossian A, Resh MD. 2015. Membrane topology of hedgehog acyltransferase. J Biol Chem 290:2235–2243.
- McGovern-Gooch KR, Mahajani NS, Garagozzo A, Schramm AJ, Hannah LG, Sieburg MA, et al. 2017. Synthetic triterpenoid inhibition of human ghrelin O-acyltransferase: the involvement of a functionally required cysteine provides mechanistic insight into ghrelin acylation. Biochemistry 56:919–931.
- McGovern-Gooch KR, Rodrigues T, Darling JE, Sieburg MA, Abizaid A, Hougland JL. 2016. Ghrelin octanoylation is completely stabilized in biological samples by alkyl fluorophosphonates. Endocrinology 157:4330–4338.
- McGovern KR, Darling JE, Hougland JL. 2016. Progress in small molecule and biologic therapeutics targeting ghrelin signaling. Mini Rev Med Chem 16:465–480.
- Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, et al. 2009. Localization of acyl ghrelin- and des-Acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric ph. Am J Physiol Gastrointest Liver Physiol 297:G974–G980.
- Moon M, Kim HG, Hwang L, Seo JH, Kim S, Hwang S, et al. 2009. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease by blocking microglial activation. Neurotox Res 15:332–347.
- Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. 2015. Ghrelin. Mol Metab 4:437–460.
- Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, et al. 2005. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut 54:1693–1698.
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. 2001. A role for ghrelin in the central regulation of feeding. Nature 409:194–198.
- Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, et al. 2005. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology 146:2255–2264.
- Ohgusu H, Shirouzu K, Nakamura Y, Nakashima Y, Ida T, Sato T, Kojima M. 2009. Ghrelin O-acyltransferase (GOAT) has a preference for n-Hexanoyl-coa over n-Octanoyl-coa as an acyl donor. Biochem Biophys Res Commun 386:153–158.
- Parang K, Till JH, Ablooglu AJ, Kohanski RA, Hubbard SR, Cole PA. 2001. Mechanism-based design of a protein kinase inhibitor. Nat Struct Biol 8:37–41.
- Peino R, Baldelli R, Rodriguez-Garcia J, Rodriguez-Segade S, Kojima M, Kangawa K, et al. 2000. Ghrelin-induced growth hormone secretion in humans. Eur J Endocrinol 143:R11–R14.
- Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, et al. 1998. Identification of a palmitic acid-modified form of human sonic hedgehog. J Biol Chem 273:14037–14045.
- Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O. 2003. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 52:2546–2553.
- Purnell JQ, Weigle DS, Breen P, Cummings DE. 2003. Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab 88:5747–5752.
- Reimer MK, Pacini G, Ahren B. 2003. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 144:916–921.
- Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, et al. 2002. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol 117:511–519.
- Romero A, Kirchner H, Heppner K, Pfluger PT, Tschop MH, Nogueiras R. 2010. GOAT: the master switch for the ghrelin system? Eur J Endocrinol 163:1–8.
- Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. 2010. Antidiabetic effect and mode of action of the triterpenoid, CDDO-Me, in diet-induced type 2 diabetic mice and Lepr(db/db) mice. Diabetes 59:A372–A372.
- Satou M, Nishi Y, Yoh J, Hattori Y, Sugimoto H. 2010. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase i as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology 151:4765–4775.
- Schopfer LM, Lockridge O, Brimijoin S. 2015. Pure human butyrylcholinesterase hydrolyzes octanoyl ghrelin to desacyl ghrelin. Gen Comp Endocrinol 224:61–68.
- Seim I, Jeffery PL, Thomas PB, Walpole CM, Maugham M, Fung JN, et al. 2016. Multi-species sequence comparison reveals conservation of ghrelin gene-derived splice variants encoding a truncated ghrelin peptide. Endocrine 52:609–617.
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. 2002. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87:240–244.
- Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. 2001. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide y/y1 receptor pathway. Diabetes 50:227–232.
- Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. 2015. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol Psychiatry 78:19–27.
- Steculorum SM, Collden G, Coupe B, Croizier S, Lockie S, Andrews ZB, et al. 2015. Neonatal ghrelin programs development of hypothalamic feeding circuits. J Clin Invest 125:846–858.
- Stengel A, Tache Y. 2012. Ghrelin: a pleiotropic hormone secreted from endocrine x/a-like cells of the stomach. Front Neurosci 6:24.
- Stevanovic DM, Grefhorst A, Themmen AP, Popovic V, Holstege J, Haasdijk E, et al. 2014. Unacylated ghrelin suppresses ghrelin-induced neuronal activity in the hypothalamus and brainstem of male rats [corrected]. PLoS One 9:e98180.
- Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, et al. 2006. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 55:327–333.
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, et al. 2006. Monounsaturated fatty acid modification of WNT protein: its role in WNT secretion. Dev Cell 11:791–801.
- Takagi K, Legrand R, Asakawa A, Amitani H, Francois M, Tennoune N, et al. 2013. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat Commun 4:2685.
- Takahashi T, Ida T, Sato T, Nakashima Y, Nakamura Y, Tsuji A, Kojima M. 2009. Production of n-Octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghrelin O-acyltransferase, as well as n-Octanoic acid. J Biochem 146:675–682.
- Takakura N, Banno Y, Terao Y, Ochida A, Morimoto S, Kitamura S, et al. 2013. Aromatic ring compound. US Patent 9238639
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, et al. 2000. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911.
- Taylor MS, Dempsey DR, Hwang Y, Chen Z, Chu N, Boeke JD, Cole PA. 2015. Mechanistic analysis of ghrelin-O-acyltransferase using substrate analogs. Bioorg Chem 62:64–73.
- Taylor MS, Ruch TR, Hsiao PY, Hwang Y, Zhang P, Dai L, et al. 2013. Architectural organization of the metabolic regulatory enzyme ghrelin O-acyltransferase. J Biol Chem 288:32211–32228.
- Teubner BJ, Garretson JT, Hwang Y, Cole PA, Bartness TJ. 2013. Inhibition of ghrelin O-acyltransferase attenuates food deprivation-induced increases in ingestive behavior. Horm Behav 63:667–673.
- Teuffel P, Wang L, Prinz P, Goebel-Stengel M, Scharner S, Kobelt P, et al. 2015. Treatment with the ghrelin-O-acyltransferase (GOAT) inhibitor GO-Coa-Tat reduces food intake by reducing meal frequency in rats. J Physiol Pharmacol 66:493–503.
- Togliatto G, Trombetta A, Dentelli P, Gallo S, Rosso A, Cotogni P, et al. 2015. Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell mir-126 expression. Diabetes 64:1370–1382.
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, et al. 2010. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59:2145–2151.
- Tschop M, Smiley DL, Heiman ML. 2000. Ghrelin induces adiposity in rodents. Nature 407:908–913.
- Van Der Ploeg L, Laken H, Sharma S, Datta R, Halem H, Dong J, et al. 2014. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sci 109:20–29.
- Wellman MK, Patterson ZR, Mackay H, Darling JE, Mani BK, Zigman JM, et al. 2015. Novel regulator of acylated ghrelin, cf801, reduces weight gain, rebound feeding after a fast, and adiposity in mice. Front Endocrinol 6:144.
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448–452.
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. 2001. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992
- Yada T, Damdindorj B, Rita RS, Kurashina T, Ando A, Taguchi M, et al. 2014. Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes Obes Metab 16 Suppl 1:111–117.
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. 2008a. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132:387–396.
- Yang J, Zhao TJ, Goldstein JL, Brown MS. 2008b. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA 105:10750–10755.
- Yao J, Yuan Y, Zheng F, Zhan CG. 2016. Unexpected reaction pathway for butyrylcholinesterase-catalyzed inactivation of “hunger hormone” ghrelin. Sci Rep 6:22322.
- Yoh J, Nishi Y, Hosoda H, Tajiri Y, Yamada K, Yanase T, et al. 2011. Plasma levels of n-decanoyl ghrelin, another acyl- and active-form of ghrelin, in human subjects and the effect of glucose- or meal-ingestion on its dynamics. Regul Pept 167:140–148.
- Zhao F, Darling JE, Gibbs RA, Hougland JL. 2015. A new class of ghrelin O-acyltransferase inhibitors incorporating triazole-linked lipid mimetic groups. Bioorg Med Chem Lett 25:2800–2803.
- Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, et al. 2010. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA 107:15868–15873.
- Zhou A, Webb G, Zhu X, Steiner DF. 1999. Proteolytic processing in the secretory pathway. J Biol Chem 274:20745–20748.
- Zhu X, Cao Y, Voogd K, Steiner DF. 2006. On the processing of proghrelin to ghrelin. J Biol Chem 281:38867–38870.