Abstract
Objective: The objective of the present work was to investigate a possible mechanism of NF-κB signaling pathway and autophagy in the regulation of osteoblast differentiation, and provide experimental basis for the study of tooth eruption disorder.
Methods: Mouse osteoblast-like (MC3T3-E1) cells were inoculated with a cell density of 70%. According to the grouping experimental design, Western blot and monodansylcadaverine (MDC) detection were conducted after dosing for 24 h. The cells were divided into the following five groups: blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group; 25 µg/mL SN50 group and 50 µg/mL SN50 group.
Results: Western blot analysis revealed that the expression of LC3 protein was present in the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group and 50 µg/mL SN50 group, with no significant differences among these groups. However, the expression of LC3 protein was significantly lower in the 25 µg/mL SN50 group. MDC detection showed that, in the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group and 50 µg/mL SN50 group, there was obvious green fluorescence in the cytoplasm of the osteoblasts. However, in the 25 µg/mL SN50 group, it was found that there were significantly fewer green fluorescent particles.
Conclusion: The osteoblast itself had a strong function of autophagy. The appropriate concentration of SN50 in blocking the NF-κB pathway of the osteoblast was associated with the obvious inhibition of autophagy. However, the relationship between NF-κB signaling pathway and autophagy in the process of tooth eruption requires further study.
1. Introduction
The eruption of teeth is critically dependent upon the creation of an eruption pathway through the alveolar bone (Heikinheimo et al., Citation2015; Shastri et al., Citation2014). The dynamic balance between osteoblasts and osteoclasts is the basis for the formation of normal tooth eruption channels. The authors’ previous study studied serum levels of cleidocranial dysplasia (CCD) patients and found that the level of bone-specific alkaline phosphatase (B-ALP) and tartrate resistant acid phosphatase-5b (TRCAP-5b) were both heightened (Li et al., Citation2011). This suggested that the differentiation of out-of-step osteoblasts and osteoclasts may be the main cause of the disorder. Although significant progress has been made in the understanding of tooth eruption disorder, scholars have yet to obtain a full understanding of the molecular mechanisms of this syndrome. However, it is known that the NF-κB signaling pathway plays a critical role in bone dynamic balance, and in the formation, development and remodeling of bone matrix by regulating the expression of RANK/RANKL and other molecular signaling of osteoclast (Huh et al., Citation2014; Wu et al., Citation2015; Zhou et al., Citation2015). Therefore, it is also the key signaling pathway in the formation of tooth eruption channels. Membrane-bound RANKL was expressed by osteoblast/stromal cells and soluble RANKL released in the bone microenvironment. Along the NF-κB signaling pathway, RANKL binds to its receptor RANK expressed in osteoclasts and stimulates the development and fusion of osteoclast precursors to become large multinuclear cells. When these differentiated cells reach a critical size they become activated mature osteoclasts (Kim et al., Citation2012; Zhong et al., Citation2016). Recent studies have found that the mechanism of NF-κB inhibitor triggering neuronal differentiation may be related to its function in inhibition of cell cycle and activation of autophagy (Vucicevic et al., Citation2014). However, the possible role between the NF-κB signaling pathway and autophagy in osteoblast differentiation has never been explored. Therefore, this study aimed to find a possible mechanism of NF-κB signaling pathway and autophagy, and to provide an innovative theoretical and experimental basis for the prevention and treatment of tooth eruption disorder.
2. Material and methods
2.1. Materials
ə-MEM nutrient medium (Corning, NY), import fetal bovine serum (Waltham, MA), hexadecadrol (Shanghai, China), β-sodium glycerophosphate (Shanghai, China), ascorbyl phosphate (Shanghai, China), I-form collagenase (Shanghai, China), 0.25% trypsinase (Shanghai, China), NF-κB SN50 (Shanghai, China), PVDF membrane (Shanghai, China), RIPA lysis buffer (Shanghai, China), prestained protein marker (Shanghai, China), monodansylcadaverine (MDC) kit (Waltham, MA), BCA protein assay kit (Shanghai, China), ECL-PLUS/Kit (Waltham, MA), SDS–PAGE protein electrophoresis apparatus (Shanghai, China), protein translocation (Shanghai, China), refrigerated high-speed centrifuge (Waltham, MA), conventional centrifuge (Waltham, MA), micropipettor (Shanghai, China), CO2 incubator (Osaka, Japan), inverted microscope (Shanghai, China), stereomicroscope and fluorescence microscope (Tokyo, Japan).
2.2. Methods
2.2.1. Osteoblast cell line in vitro culture
Osteoblas-like (MC3T3-E1) cells were resuscitated, with the MEM method at 37 °C, with 5% CO2 incubator in static culture. When the cells were growing well, single cell suspension was prepared by digestion with 0.25% trypsin, and cell suspension was prepared with the help of complete medium. The cells (2 × 104/cm2) were then inoculated onto 24-hole culture plates and allowed to develop colonies.
2.2.2. MDC detection
Adherence of cells were grown in the 24 hole plate to the required convergence, discarding the culture medium, and washing two times with 300 μL 1 × wash buffer.
The concentration of the liquid was diluted to concentration of the work according to the volume ratio of 1 × buffer and MDC is 9:1. Calculated the volume of the dye solution needed in an experiment, prepared the MDC stain working fluid and mixed gently. Added 100 μL MDC dyeing working fluid with each hole, immunofluorescence cell staining for 30 min at room temperature, discarded the stain, washed three times with 300 μL 1 × buffer. Cover the coverslip with 100 μL collection buffer, observed the green fluorescent particles under the fluorescence microscope, counted and take pictures.
2.2.3. Western blot
The MC3T3-E1cell samples were harvested, washed two times with precooled PBS and then lysed in extraction buffer. The cell samples were then centrifugated at 4 °C for 15 min. Protein concentrations of the cell lysates were quantitated using the BCA Protein Assay Kit. Based on the molecular weight of the target protein, different concentrations of gum were prepared. Equal amounts of protein samples were then separated using sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto polyvinylidene fluoride (PVDF) membrane. After blocking in sealing solution for 1 h, the membrane was incubated with rabbit anti-LC3 polyclonal antibody overnight at 4 °C. After four washes, the blots were subsequently incubated with peroxidase-conjugated secondary antibody for 1.5 h at room temperature. Chemiluminescent detection was performed using the ECL western blotting substrate kit. GAPDH was detected on the same membrane and used as loading control.
First antibody information:
Second antibody information:
2.3. Statistical analysis
All experimental data are presented as the mean ± standard error of mean (SEM). Statistical significance was determined by the one-way analysis of variance test. Significance was considered at p < .05.
3. Results
3.1. Western blot
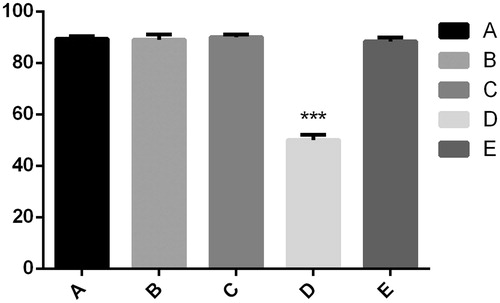
Western blot analysis showed that, the expression of LC3 protein was observed in the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group and 50 µg/mL SN50 group, with no significant differences among the groups. However, the expression of LC3 protein was significantly lower in the 25 µg/mL SN50 group ( and ).
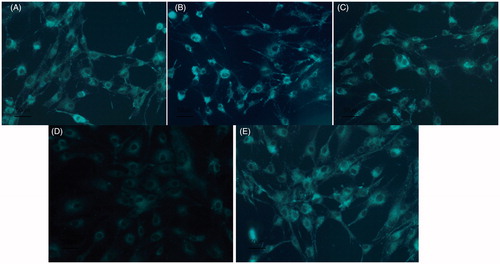
Figure 1. A–E respectively on behalf of the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group; 25 µg/mL SN50 group and 50 µg/mL SN50 group. F–J is repeated A–E.
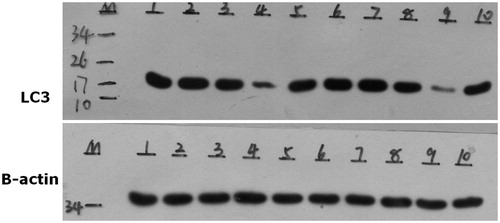
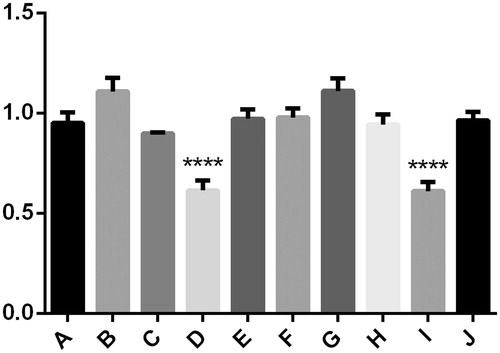
Figure 2. There was no significant difference between group A, B, C and E. However, the expression of LC3 protein was significantly lower in group D, ****p < .05 compared to other cases. The same results appeared in group F–J, the expression of LC3 protein was significantly lower in group I, ****p < .05 compared to other cases.
3.2. MDC-staining results
MDC detection showed that, in the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group and 50 µg/mL SN50 group, there was obvious green fluorescence in the cytoplasm of the osteoblast. In the 25 µg/mL group SN50, there were significantly fewer green fluorescent particles ( and ).
4. Discussion
Results from the present study indicated that the mouse osteoblast-like MC3T3-E1 cells had a strong function of autophagy. And the appropriate concentration (25 µg/mL) of SN50 in blocking the NF-κB pathway of osteoblast was associated with the obvious inhibition of autophagy, as demonstrated by Western blot and MDC. This further confirmed that the NF-κB signaling pathway and autophagy played an important role in the regulation of osteoblast differentiation, which provided a better understanding of the dynamic balance of bone mass and experimental basis for the study of tooth eruption disorder.
The importance of the experiment can also be understood from the fact that the process of tooth eruption necessitates the osteoblasts and osteoclasts to maintain a dynamic balance in their function. If unbalanced, it can lead to tooth eruption disorder (Cobourne & Sharpe, Citation2013; Oosterkamp et al., Citation2014). CCD is a representative disease, which is a permanent delay in most of teeth (Dutra et al., Citation2013; Lee et al., Citation2013). At present, most research on tooth eruption disorder has focused on the changes of the number and differentiation of osteoclasts in the process of tooth eruption (Gleizal et al., Citation2014; Hørberg et al., Citation2015; Shiyan et al., Citation2016). Conversely, research on osteoblasts, which are closely related to bone metabolism, is rare. Little is known about how osteoblasts induce the aggregation and differentiation of osteoclasts and promote the formation of tooth eruption channels. And studies on the molecular mechanism of the signaling pathway and autophagy in the process of tooth eruption are scarce. Therefore, the aim of this study was to investigate whether NF-κB signaling pathway and autophagy influence osteoblasts, and to explore the possible underlying mechanisms. Also, the authors sought to better understand the mechanism of bone mass dynamic balance in the process of tooth eruption and provide some experimental basis for elucidating the possible causes of tooth eruption disorder.
A large number of studies have suggested that the combination of RNAK expression on the surface of osteoclast precursor cells and the expression of RNAKL in osteoblasts together encompass the most important signaling pathway to activate osteoclast as well as promote the differentiation and maturation of osteoclast precursor cells (Tyagi et al., Citation2016; Zhan et al., Citation2013). Membrane-bound RANKL, mainly expressed by osteoblasts, bone marrow stromal cells and immune cells, which are usually in the cytoplasm in the form of a compound of trimer, can be activated by many factors. RANK, primarily located on the surface of the osteoclast precursor cell membrane, is the only target receptor that interacts with RANKL (Jimi & Fukushima, Citation2016; Quan et al., Citation2015). When RANKL binds to RANK, it stimulates the development and fusion of osteoclast precursors to become large multinuclear cells. When these cells are large enough, activated mature osteoclasts are formed (Kim et al., Citation2012). It is known that regulation of alveolar bone resorption is a critical event in tooth eruption. However, there are few domestic or foreign studies in literature on N-κB signaling pathway in dental eruption.
A 32-amino acid peptide hormone, calcitonin (CT) secreted mainly from the thyroid gland in response to increases in blood calcium levels, plays an important role in bone homeostasis (De Crea et al., Citation2014; Qin et al., Citation2007; Rizzoli et al., Citation2015). One biological activity of CT includes lowering serum calcium concentrations in pathological states of increasing bone resorption primarily by inhibiting osteoclast-mediated bone resorption. Previously, an experiment was conducted to discover whether calcitonin affects RANKL during mouse tooth eruption in vivo or in vitro (Rouhani et al., Citation2016). It was discovered that the number of osteoclasts in the CT group was less than that in control group. And, there was a significant difference of RANKL mRNA between the CT and control groups, which could mean that CT could also inhibited osteoclast differentiation through inhibiting RANKL expression (Qin et al., Citation2007). If that was the case, it would further confirm that the NF-κB signaling pathway plays a critical role in teeth eruption. However, in the process of tooth eruption, it is still not known how the expression of RANK/RANKL is changed, how to maintain the dynamic balance of bone mass in the presence of a mechanism, or how osteoblasts behave in the process.
Autophagy is an important mechanism of cell self-preservation (Mintern & Harris, Citation2015; Wei et al., Citation2015). Widely existing in eukaryotic cells, lysosomal dependent degradation pathway plays an important role in maintaining the survival of cells and the stability of the inner environment. As known, LC3 is located in the precursor and the autophagy body, always used as the marker protein of the autophagy, and positive-reaction for cleaved caspase-3 could reveal the execution of apoptosis. Once the autophagy occurs, the autophagosome fuses with a lysosome to form an autolysosome, thereby promoting the degradation of autophagosomal contents by lysosomal acid proteases (Someya et al., Citation2016; Wu et al., Citation2016; Xu et al., Citation2014). Autophagy, as a defense mechanism for the removal of toxic multimeric complexes and aggregated proteins in neurodegenerative diseases, may also occur in osteoblast (Menzies et al., Citation2017). However, with the exception of study demonstrating that activation of autophagy during SN50-induced differentiation of neural stem cells (NSCs) was evidenced by the presence of an increased number of acidic vacuoles in the cytoplasm and increased expression of membrane form of microtubule associate protein LC-3 (Vucicevic et al., Citation2014), the role of autophagy induction by SN50 in osteoblast survival and death has not yet been studied. Therefore, the possible mechanism of NF-κB signaling pathway and autophagy in the regulation of osteoblast differentiation was investigated in this study, and the results of which can provide experimental basis for further research of tooth eruption disorder.
Toward this end, experiment was carried out. Different concentrations of NF-κB signaling pathway blockers SN50 were added to osteoblasts in vitro. Western blot and MDC detection were conducted after dosing for 24 h. Western blot analysis showed that, the expression of LC3 protein was observed in the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group and 50 µg/mL SN50 group, with no significant differences among the groups. However, the expression of LC3 protein was significantly lower in the 25 µg/mL SN50 group. The formation of autophagy vesicles was traced by MDC-fluorescence-staining-method, and the activation of the autophagy was observed under a laser scanning confocal microscope. The experimental results showed that there was obvious green fluorescence in the cytoplasm of the osteoblast in the blank control group; 6.25 µg/mL SN50 group; 12.5 µg/mL SN50 group and 50 µg/mL SN50 group. However, in the SN50 25 µg/mL group, there were significantly fewer green fluorescent particles.
5. Conclusion
Through this study, the following conclusions were drawn: (i) Osteoblasts themselves have a strong autophagy function, which will provide a new direction for the study of the dynamic balance of bone and tooth eruption disorder. (ii) When the NF-κB signaling pathway was blocked, there was a significant change in the autophagy function of the osteoblasts, which proved that the NF-κB signaling pathway and autophagy was closely related. This will provide a solid foundation for the molecular study of osteoblasts. (iii) In SN50, as the NF-κB pathway blocking agent, only the proper concentration (25 µg/mL) played a significant role in autophagy, which showed that SN50 had obvious biological effectiveness. This will provide basis for the further study of SN50 function. (iv) When the NF-κB pathway was blocked by SN50, autophagy of osteoblast-like cell MC3T3-E1 was inhibited. Studies have shown that NF-κB plays a double role in autophagy, in some cells the activation of NF-κB promotes autophagy (Guo et al., Citation2016; Liu et al., Citation2016), while in other cells it inhibits the autophagy (Gao et al., Citation2016). This study suggested that NF-κB may promote autophagy in osteoblasts. The results obtained in this research further indicates that the NF-κB signaling pathway and autophagy play an important role in the regulation of osteoblast differentiation, and will provide ideas for the further study of the relationship between NF-κB pathway and autophagy.
Although it has been reported that autophagy signaling pathways can be used as an entry point for the treatment of many diseases, which is valuable in both theory and clinical applications, the relationship between autophagy and related signaling pathways is still not clear (Hsieh et al., Citation2016; Krętowski et al., Citation2016; Poillet-Perez et al., Citation2015; Tsukahara et al., Citation2015). This study investigated the possible mechanism in the differentiation of osteoblasts through a preliminary study on NF-κB signaling pathways and autophagy. The findings further explain the biological mechanism of the formation of the tooth eruption channel, and provide a new theoretical and experimental basis for the prevention and treatment of tooth eruption disorder. It is the hope of the authors that this study, even performed on preliminary research, will contribute to an understanding of the problem.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Cobourne MT, Sharpe PT. 2013. Diseases of the tooth: the genetic and molecular basis of inherited anomalies affecting the dentition. Wiley Interdiscip Rev Dev Biol 2:183–212.
- DE Crea C, Raffaelli M, Maccora D, Carrozza C, Canu G, Fadda G, et al. 2014. Calcitonin measurement in fine-needle aspirate washouts vs. cytologic examination for diagnosis of primary or metastatic medullary thyroid carcinoma. Acta Otorhinolaryngol Ital 34:399–405.
- Dutra EH, Chen IP, Reichenberger EJ. 2013. Dental abnormalities in a mouse model for craniometaphyseal dysplasia. J Dent Res 92:173–179.
- Gao H, Lin L, Haq IU, Zeng SM. 2016. Inhibition of NF-κB promotes autophagy via JNK signaling pathway in porcine granulosa cells. Biochem Biophys Res Commun 473:311–316.
- Gleizal A, Bourlet J, Saint Amand J, Zulma C, Bachelet JT. 2014. Oro-dental development and anomalies. Rev Prat 64:1445–1456.
- Guo X, Jiang H, Yang J, Chen J, Yang J, Ding JW, et al. 2016. Radioprotective 105 kDa protein attenuates ischemia/reperfusion-induced myocardial apoptosis and autophagy by inhibiting the activation of the TLR4/NF-κB signaling pathway in rats. Int J Mol Med 38:885–893.
- Heikinheimo K, Kurppa KJ, Laiho A, Peltonen S, Berdal A, Bouattour A, et al. 2015. Early dental epithelial transcription factors distinguish ameloblastoma from keratocystic odontogenic tumor. J Dent Res 94:101–111.
- Hørberg M, Lauesen SR, Daugaard-Jensen J, Kjær I. 2015. Linear scleroderma en coup de sabre including abnormal dental development. Eur Arch Paediatr Dent 16:227–231.
- Hsieh MJ, Chien SY, Lin JT, Yang SF, Chen MK. 2016. Polyphyllin G induces apoptosis and autophagy cell death in human oral cancer cells. Phytomedicine 23:1545–1554.
- Huh JE, Lee WI, Kang JW, Nam D, Choi DY, Park DS, et al. 2014. Formononetin attenuates osteoclastogenesis via suppressing the RANKL-induced activation of NF-κB, c-Fos, and nuclear factor of activated T-cells cytoplasmic 1 signaling pathway. J Nat Prod 77:2423–2431.
- Jimi E, Fukushima H. 2016. NF-κB signaling pathways and the future perspectives of bone disease therapy using selective inhibitors of NF-κB. Clin Calcium 26:298–304.
- Kim HN, Lee JH, Jin WJ, Lee ZH. 2012. α-Tocopheryl succinate inhibits osteoclast formation by suppressing receptor activator of nuclear factor-κB ligand (RANKL) expression and bone resorption. J Bone Metab 19:111–120.
- Krętowski R, Borzym-Kluczyk M, Stypułkowska A, Brańska-Januszewska J, Ostrowska H, Cechowska-Pasko M. 2016. Low glucose dependent decrease of apoptosis and induction of autophagy in breast cancer MCF-7 cells. Mol Cell Biochem 417:35–47.
- Lee KE, Seymen F, Ko J, Yildirim M, Tuna EB, Gencay K, Kim JW. 2013. RUNX2 mutations in cleidocranial dysplasia. Genet Mol Res 12:4567–4574.
- Li Y-f, Qin H, Xu H-z. 2011. Bone-specific alkaline phosphatase and tartrate-resistant acid phosphatase levels in patients with cleidocranial dysplasia: one case report. J Clin Rehabil Tissue Eng Res 15:7742–7744.
- Liu Q, Sun Y, Lv Y, Le Z, Xin Y, Zhang P, Liu Y. 2016. TERT alleviates irradiation-induced late rectal injury by reducing hypoxia-induced ROS levels through the activation of NF-κB and autophagy. Int J Mol Med 38:785–793.
- Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, et al. 2017. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93:1015–1034.
- Mintern JD, Harris J. 2015. Autophagy and immunity. Immunol Cell Biol 93:1–2.
- Oosterkamp BC, Ockeloen CW, Carels CE, Kuijpers-Jagtman AM. 2014. Tooth eruption disturbances and syndromes. Ned Tijdschr Tandheelkd 121:233–238.
- Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M. 2015. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol 4:184–192.
- Qin H, Yang F-S, Wu L-A, Fang J. 2007. Effects of calcitonin on osteoclast during tooth eruption in mouse. Chin J Conserv Dent 17:681–683.
- Quan GH, Wang H, Cao J, Zhang Y, Wu D, Peng Q, et al. 2015. Calycosin suppresses RANKL-Mediated osteoclastogenesis through Inhibition of MAPKs and NF-κB. Int J Mol Sci 16:29496–29507.
- Rizzoli R, Sigaud A, Azria M, Herrmann FR. 2015. Nasal salmon calcitonin blunts bone microstructure alterations in healthy postmenopausal women. Osteoporos Int 26:383–393.
- Rouhani A, Mardani-Kivi M, Bazavar M, Barzgar M, Tabrizi A, Hashemi-Motlagh K, Saheb-Ekhtiari K. 2016. Calcitonin effects on shoulder adhesive capsulitis. Eur J Orthop Surg Traumatol 26:575–580.
- Shastri D, Tandon P, Singh GP, Singh A. 2014. A newer simultaneous space creation, eruption, and adjacent root control spring for the management of impacted tooth. Contemp Clin Dent 5:555–557.
- Shiyan H, Nanquan R, Shuhao X, Xiaobing L. 2016. Research progress on the cellular and molecular mechanisms of tooth eruption. Hua Xi Kou Qiang Yi Xue Za Zhi 34:317–321.
- Someya S, Leeuwenburgh C, Martin AD. 2016. Intraoperative hemidiaphragm electrical stimulation reduces oxidative stress and upregulates autophagy in surgery patients undergoing mechanical ventilation: exploratory study. J Transl Med 14:305.
- Tsukahara T, Matsuda Y, Haniu H. 2015. The role of autophagy as a mechanism of toxicity induced by multi-walled carbon nanotubes in human lung cells. Int J Mol Sci 16:40–48.
- Tyagi AK, Prasad S, Majeed M, Aggarwal BB. 2016. Calebin A downregulates osteoclastogenesis through suppression of RANKL signalling. Arch Biochem Biophys 593:80–89.
- Vucicevic L, Misirkic-Marjanovic M, Paunovic V, Kravic-Stevovic T, Martinovic T, Ciric D, et al. 2014. Autophagy inhibition uncovers the neurotoxic action of the antipsychotic drug olanzapine. Autophagy 10:2362–2378.
- Wei T, Kang Q, Ma B, Gao S, Li X, Liu Y. 2015. Activation of autophagy and paraptosis in retinal ganglion cells after retinal ischemia and reperfusion injury in rats. Exp Ther Med 9:476–482.
- Wu GJ, Lin CJ, Lin YW, Chen RM. 2016. Data analyses of honokiol-induced autophagy of human glioma cells in vitro and in vivo. Data in Brief 9:667–672.
- Wu C, Wang W, Tian B, Liu X, Qu X, Zhai Z, et al. 2015. Myricetin prevents titanium particle-induced osteolysis in vivo and inhibits RANKL-induced osteoclastogenesis in vitro. Biochem Pharmacol 93:59–71.
- Xu W, Jiang H, Hu X, Fu W. 2014. Effects of high-mobility group box 1 on the expression of Beclin-1 and LC3 proteins following hypoxia and reoxygenation injury in rat cardiomyocytes. Int J Clin Exp Med 7:5353–5357.
- Zhan X, Zhang C, Dissanayaka WL, Cheung GS, Jin L, Yang Y, et al. 2013. Storage media enhance osteoclastogenic potential of human periodontal ligament cells via RANKL-independent signaling. Dent Traumatol 29:59–65.
- Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. 2016. NF-κB Restricts inflammasome activation via elimination of damaged mitochondria. Cell 164:896–910.
- Zhou C, Liu W, He W, Wang H, Chen Q, Song H. 2015. Saikosaponin a inhibits RANKL-induced osteoclastogenesis by suppressing NF-κB and MAPK pathways. Int Immunopharmacol 25:49–54.