Abstract
Syntaxin 10 is a soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) protein localized to the trans-Golgi network (TGN), where two other members of the syntaxin family, syntaxins 6 and 16, also reside. The role of syntaxin 10 in regulating TGN protein traffic is not yet defined. Syntaxin 10 co-localizes well with syntaxins 6 and 16 at the TGN in interphase cells, and appears to interact with both syntaxin 6 and 16 as evidenced by co-immunoprecipitation analyses. However, unlike syntaxin 6 and 16, neither syntaxin 10 antibodies nor its cytosolic domain inhibits endosome-TGN transport of shiga toxin. Syntaxin 16 knockdown with small interfering RNA (siRNA) affects the TGN localization of syntaxin 6 but not syntaxin 10, and clearly inhibits endosome-TGN transport. On the other hand, knockdown of syntaxin 10 expressions had no significant effect on the TGN localization of syntaxin 6 and 16, and did not inhibit endosome-TGN transport. Unlike syntaxin 16, syntaxin 10 does not interact specifically with Vps45, the Sec1/Munc18 (SM) family member at the TGN. On the other hand, syntaxin 10 reciprocally co-immunoprecipitated endosomal syntaxin 12/13, and knockdown of syntaxin 10 expressions affects the surface expression of transferrin receptor (TfR) and seems to induce the formation of an immobile TfR pool. Therefore, in spite of its co-localization and possible interaction with syntaxin 16, syntaxin 10 is not part of the syntaxin 16-based SNARE complex involved in endosome-TGN transport, and may have a hitherto unrecognized function in the TGN-endosome boundary.
| Abbreviations | ||
| NSF | = | N-ethylmaleimide sensitive factor |
| SNAP | = | soluble NSF attachment proteins |
| SNARE | = | SNAP receptor |
Introduction
Soluble N-ethylmaleimide sensitive factor (NSF) attachment protein (SNAP) receptors (SNAREs) function is to determine the specificity of the docking and fusion of vesicles to the correct target membranes Citation[1], Citation[2]. Initially identified as molecules involved in mediating synaptic vesicle docking and fusion and in the yeast secretory pathway Citation[3], SNAREs have been shown to mediate membrane fusion events in different membrane compartments of eukaryotic cells. These include homotypic membrane fusions between the endoplasmic reticulum (ER), endosomes and the vacuole, as well as heterotypic membrane fusions between transport vesicles or carriers with target membrane. The neuronal SNARE syntaxin 1A was first characterized as a neuronal specific protein involved in the regulation of neurotransmitter release Citation[4]. Its localization on the presynaptic plasma membrane makes it a target (t)-SNARE. Most, if not all, mammalian syntaxin-related molecules have now been identified and characterized. These molecules have distinct steady state subcellular distributions, in line with their predicted functions of regulating compartment specific vesicle fusion processes Citation[5–14].
The trans-Golgi network (TGN) is a critical gateway in protein transit for both the exocytic and endocytic pathway Citation[15–17]. In the exocytic or anterograde path, it is associated with sorting of specialized hydrolytic enzymes to the lysosomes and is the point of initiation of specialized polarized transport to the different domains of the plasma membrane in epithelial and neuronal cell types. In a retrograde sense, it receives traffic from both the late endosome and the early/recycling endosome (EE/RE). The TGN is either a part of, or plays an active role in transport processes such as retrograde transport of toxin molecules to the ER Citation[18], transit from the exocytic pathway to the nucleus and retrotranslocation to the ubiquitin-proteosome pathway Citation[15]. Regulation of membrane traffic in and out of the TGN is therefore under delicate and stringent regulatory controls.
Of all the fourteen known mammalian syntaxins, only four are associated with the Golgi apparatus. Three of these, syntaxins 6, 10 and 16 are localized to the TGN Citation[19–23]. Syntaxin 6 appears to be fairly dynamic with reference to its membrane localizations and shown to be involved in multiple membrane fusion events Citation[24]. These include homotypic fusion of immature secretory granules Citation[25], homotypic fusion of early endosomes Citation[26], exocytosis in neutrophils Citation[27] and the regulation of GLUT4 trafficking in 3T3 adipocytes Citation[28]. Syntaxin 16 is an example of mammalian syntaxin with distinct structurally and functionally related homologues in both yeast (Tlg2p) Citation[29] and higher plants (AtTlg2) Citation[30]. Both Tlg2p and AtTlg2 have been implicated in TGN transport in their respective hosts. We have also recently demonstrated that both syntaxins 6 and 16 are part of a TGN SNARE complex that mediates transport from the EE/RE to the TGN Citation[23]. In this context, these syntaxins are most likely to function together with two other SNARE molecules, VAMP4 Citation[31] and Vti1a Citation[32], Citation[33].
We have previously cloned syntaxin 10 and showed that its transcripts are rather specifically enriched in heart and skeletal muscle. Amongst syntaxins, syntaxin 10 has the highest degree of homology with syntaxin 6 (61.4% identity). Immunofluorescence labeling with specific antibodies suggests that it is localized to the TGN Citation[20]. We now report further investigations on syntaxin 10's cellular localization, function and interaction. We showed that although syntaxin 10 may interact with the syntaxin 16-based TGN SNARE complex, it is not involved in mediating endosome to TGN transport. We also showed that unlike syntaxin 16, syntaxin 10 does not interact with Vps45 but interacts with the endosomal syntaxin 12/13, and may have a role in TGN-endosomal transport.
Materials and methods
Syntaxin 10 Citation[20] and syntaxin 16 Citation[21–23] cDNA clones and polyclonal antibodies have been described previously. Mouse antisera against syntaxin 16 were obtained by immunizing Balb-c mice with hexahistidine-tagged fusion protein corresponding to the cytoplasmic domain of syntaxin 16. The hybridoma cell lines 9E10 and Okt9, which produces the monoclonal antibody against the myc-epitope tag and transferrin receptor (TfR), respectively, were purchased from the American Type Culture Collection. The mouse monoclonal antibody against the Shiga B fragment was described previously Citation[23]. Polyclonal antibodies against syntaxin 6, Vps45 and syntaxin 12/13 were from Synaptic Systems (Germany). Small interfering RNAs (siRNA) against syntaxin 10 and syntaxin 16 were synthesized by Dharmacon Research Inc. (USA). Streptolysin O was obtained from Dr S. Bhakdi (Johannes-Gutenberg-Universität, Mainz, Germany).
DNA manipulations were carried out according to established protocols Citation[34]. DNA polymerase chain reactions for generating constructs were carried out using high fidelity Pfu polymerase (Promega, USA). Small interfering RNA oligomers were generated against the following target sequence:
Syntaxin 10: 5′-AAGGACCATATGGTCAGCCCA-3′
Syntaxin 16: 5′-AAGCAGCGATTGGTGTGACAA-3′
Cells were maintained in RPMI or DME medium supplemented with 10% FBS. Transfections of constructs were performed using Lipofectamine 2000 and that of the siRNA using Oligofectamine (Invitrogen) according to manufacturer's protocols. For co-immunoprecipitation and Western immunoblot analysis, cells were lysed in lysis buffer (50 mM Tris (pH 8), 10 mM EDTA and 150 mM NaCl) for 1 h. Lysates were precleared with mouse or rabbit serum agarose and incubated with the respective antibodies followed by protein A-sepharose or protein G-sepharose (Amersham, USA).
Shiga toxin B fragment production and in vitro transport assay
Bacterial periplasm was extracted from transformed bacteria by osmotic shock and concentrated by binding to Q-sepharose beads. Shiga B was isolated from periplasmic extract by chromatography on Mono-Q column and stored at −70°C.
Shiga toxin B fragment uptake and transport assay is performed using semi-intact HeLa cells (ATCC). Briefly, cells were starved for 1 h in minimum essential medium without sulfate (Gibco), incubated with 1 µM Shiga toxin B proteins for 1 h at 18°C followed by 30-min chase in fresh medium. Cells were then permeabilized with 20 µg/ml streptolysin O, scrapped, pelleted at 1000 g and re-suspended in 150 µl of membrane buffer (25 mM Hepes, pH 7.3, 250 mM sucrose and 1 mM EDTA). Endosome to TGN transport was reconstituted in a standard transport assay which is modified from reference Citation[23] with 35Sulfate (Amersham). Details of the transport assay protocol had been described Citation[35]. Membrane pellets were recovered and dissolved in 20-µl sample buffer. Proteins were separated on 15% modified Laemmli SDS-PAGE gel, and transferred onto nitrocellulose membranes. Radioactive bands was imaged and analyzed with a phosphoimager.
Vesicular stomatitis virus infection and vesicular stomatitis virus G protein (VSVG) transport
Vesicular stomatitis virus infection is performed essentially as described previously Citation[36], with minor variations. Briefly, cells grown on coverslips were infected with VSVGts045 for 1 h in serum free medium at 32°C, and subsequently in complete medium for another 5 h. Cycloheximide was added to a final concentration of 20 µg/ml, 45 min before termination of the incubations. Cells were then fixed and processed for immunofluorescence staining as outlined below.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed as described previously Citation[20], Citation[23], Citation[36]. Cells plated on cover slips, subjected to various treatments, were fixed with 4% paraformaldehyde followed by sequential incubation with the primary antibodies and FITC or Texas red-conjugated secondary antibodies. Fluorescence labeling was visualized using an Axiophot microscope (Carl Zeiss, Inc., USA) equipped with epifluorescence optics and the MRC1024 (BioRad, USA) confocal laser optics or Carl Zeiss 510 confocal imaging system.
Results
Syntaxin 10 colocalizes and interacts with components of a TGN SNARE complex
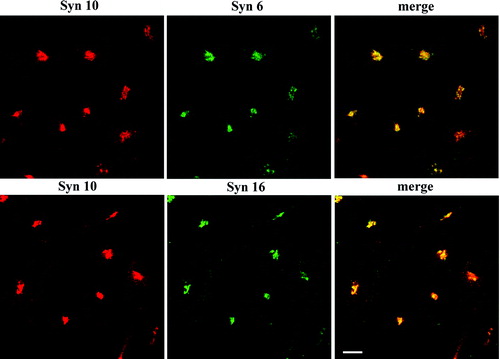
We have shown previously that syntaxin 10 antibodies label the TGN, and its labeling pattern collapses into the microtubule-organizing center upon BFA treatment Citation[20]. To confirm co-localization between syntaxin 10 and other known syntaxins at the TGN, we performed double immunofluorescence labeling with mice antibodies against syntaxin 6 and syntaxin 16. As shown in , syntaxin 10 co-localized well with both the other known TGN syntaxins in perinuclear structures.
Figure 1. Syntaxin 10 co-localizes with syntaxin 6 and 16 at the TGN. Cells were fixed with 4% paraformaldehyde and incubated with primary antibody against syntaxin 10 (rabbit), mouse monoclonal antibody against syntaxin 6, or mouse anti-syntaxin 16 antisera, followed by Texas-red or FITC-labeled secondary antibodies. Bar = 10 µm.
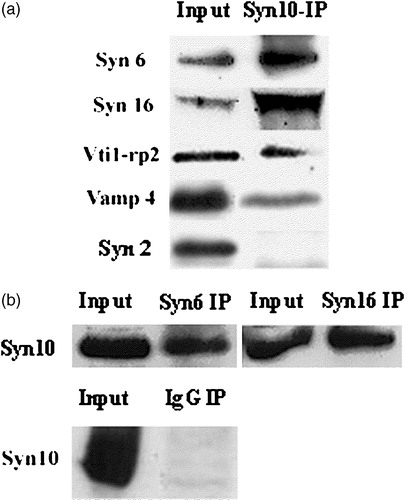
We have shown previously that a SNARE complex containing both syntaxin 6 and 16 is responsible for mediating a direct transport pathway from the EE/RE to the TGN, bypassing the late endosome Citation[23]. We therefore ask if syntaxin 10 interacts physically with syntaxin 6 and 16 in vivo. As shown in , syntaxin 10 reciprocally co-immunoprecipitates both syntaxin 6 and 16, and to a lesser extent Vti1-rp2/Vti1a and a myc-tagged form of VAMP4, but not syntaxin 2 (which is localized to the cell surface). The above results suggest that syntaxin 10 co-localizes, and could potentially interact with components of a previously described TGN SNARE complex in vivo.
Figure 2. Syntaxin 10 interacts in vivo with TGN SNAREs: (a). HeLa cell lysates were subjected to immunoprecipitation with syntaxin 10 antibody. Immunoprecipitates were eluted, resolved on 12% SDS-PAGE gels, and subjected to Western immunoblot analyses with antibodies against syntaxin 6, syntaxin 16, Vti1-rp2 and syntaxin 2. For co-precipitation analysis with VAMP4, a construct of N-terminal myc-tagged VAMP4 is transiently transfected into 293T cells and subjected to immunoprecipitations as above and western immunoblot analysis with an anti-myc monoclonal antibody (9E10); (b). Reciprocal immunoprecipitation analyses. HeLa cell lysates were subjected to immunoprecipitation with syntaxin 6, syntaxin 16 antibodies and an irrelevant rabbit anti-mouse IgG (Pierce), respectively. Western immunoblot analyses were then performed with syntaxin 10 antibodies.
Syntaxin 10 is not involved in transport from the early endosome to the TGN
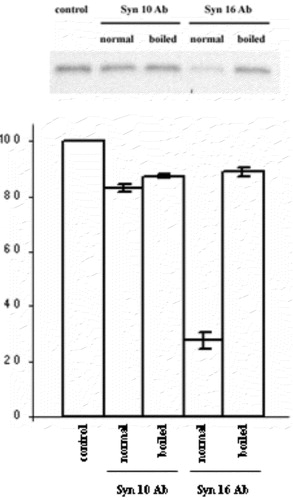
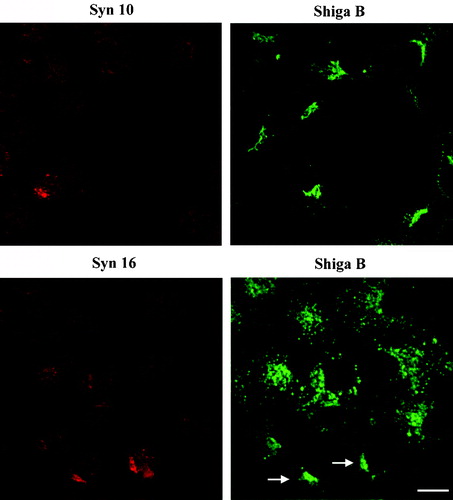
In view of its possible involvement in retrograde transport to the TGN based on its localization and SNARE partners, we ask if syntaxin 10 is also involved in mediating EE/RE-TGN transport. To do this, we employed a previously established protocol in assessing the transport in HeLa cells of an exogenously added engineered Shiga toxin B (sulf)2 (Shiga B), whose sulfation indicates transport to the TGN. In contrast to syntaxin 16 antibodies, which clearly inhibited Shiga B sulfation, syntaxin 10 antibodies have no significant effect (). This is confirmed especially by the fact that heat-inactivated syntaxin 10 antibodies showed no significant difference in terms of activity from active antibodies.
Figure 3. Syntaxin 10 antibody does not inhibit Shiga toxin B-(sulf)2 sulfation. Syntaxin 10 or syntaxin 16 antibodies, either heat-inactivated at 100°C for 2 min (boiled) or otherwise (normal), were added to standard transport assay set ups as described in Materials and Methods. Control indicates a standard reaction without any antibodies. 35S-labeled Shiga B were resolved with a 15% modified Laemmli peptide separation PAGE gel, and blotted onto nitrocellulose membranes. The membrane is then subjected to phosphoimaging analysis. A representative autoradiogram is shown. The graph represents quantitative analysis of several independent experiments. Band intensities were quantified by densitometry, and expressed as percentage of the respective controls (which were set arbitrarily as 100%). P<0.01 in the case of syntaxin 16 antibody added, compared to control.
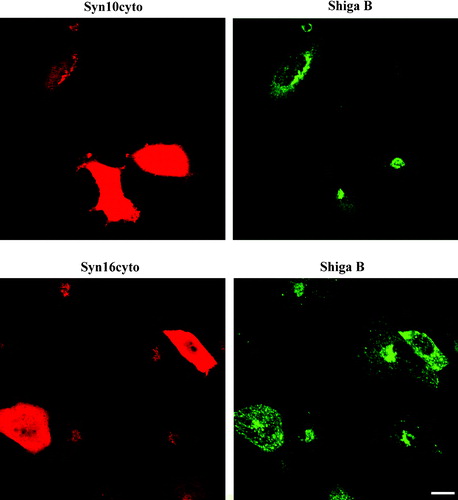
To confirm this finding by a different approach, we transiently transfected HeLa cells with constructs expressing the cytoplasmic domains of syntaxin 10 (syn10cyto) and 16 (syn16cyto), and compared the effect of the over-expression of these truncated cytoplasmic forms of the syntaxins on Shiga B transport by indirect immunofluorescence microscopy. Expressions of syntaxin cytoplasmic domains have been used previously to effect a dominant negative inhibition of SNARE functions, presumably via competitive inhibition of endogenous SNARE pairings. As shown in , over-expression of syn16cyto clearly inhibits the transport of Shiga B to the perinuclear TGN. Shiga B labeling in syn16cyto expressing cells appears to be spotty, reminiscent of early endosomal labeling observed during early time points of Shiga B internalization (not shown). On the other hand, over-expression of syn10cyto has no effect on Shiga B's eventual transport to the perinuclear TGN.
Figure 4. Dominant-negative form of syntaxin 10 does not inhibit Shiga B transport to the TGN. Cells transiently transfected with constructs expressing the cytoplasmic domain of syntaxin 10 (Syn10cyto) or syntaxin 16 (Syn16cyto) were fixed with 4% paraformaldehyde and incubated with antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against Shiga B, followed by Texas-red or FITC-labeled secondary antibodies. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
A quantitative or normalized comparison of the relative potency of the different dominant negative mutants of the syntaxins is not particularly feasible. We therefore sought to confirm the roles of syntaxin 10 and 16 in Shiga B EE/RE-TGN transport by small-interfering RNA (siRNA)-mediated knockdown of their expressions. In doing this, we were also able to assess the effect of the loss of each of syntaxins on TGN architecture and the TGN localization of the other syntaxins. Prolonged (more than two days) incubations with the siRNAs resulted in cell detachment and some disruption of internal membranes (not shown). However, incubation with siRNAs for 36–48 h resulted in fairly effective knockdown of the respective proteins without obvious impairment of cell health (). The extent of knockdowns in entire transfected populations typically range from 50 to 80%. However, with the analysis of individual cells stained for the syntaxins by immunofluorescence microscopy, we are able to recognize cells and cell patches with more significant knockdowns (more than 80%). In a sense, the cells with inefficient knockdown served well as internal controls in our subsequent observations.
Figure 5. Effects of knockdown of syntaxin expressions on TGN morphology and on the localization of other TGN syntaxins: (a). Cell were transiently transfected with either siRNA oligomers for syntaxin 10, syntaxin 16 or glutathione S-transferase (as a control) for 48 h and the protein levels in the cell lysates were analyzed by immunoblotting for syntaxin 10 and 16, respectively. Immunoblotting for tubulin indicates roughly equivalent protein loading in each lane; (b). Cells were transiently transfected with the respective siRNA oligomers for 48 h. Cell were then fixed with 4% paraformaldehyde and incubated with primary antibody against syntaxins 10 or 16 (rabbit), with mouse monoclonal antibody against syntaxin 6, or mouse anti-syntaxin 16 antiserum, followed by Texas-red or FITC-labeled secondary antibodies. Arrows indicate cells where syntaxin 16 knockdown is incomplete or minimal. Note that the syntaxin 6 staining pattern in these cells remained perinuclear. Comparatively, the perinuclear staining of syntaxin 6 in cells with significant syntaxin 16 knockdown became diffused (arrowheads). However, the perinuclear staining of syntaxin 10 is largely unaffected in syntaxin 16 knockdown cells. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
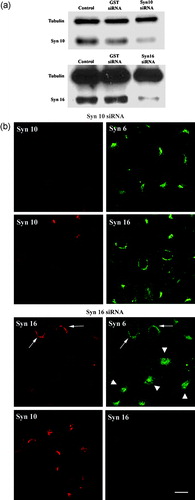
As shown in , knockdown of syntaxin 10 by siRNA did not significantly affect the TGN labeling of either syntaxin 6 or 16. On the other hand, knockdown of syntaxin 16 expressions by siRNA significantly perturbed syntaxin 6 localizations. Importantly, the TGN localization of syntaxin 10 remained unaffected. In our experimental conditions of syntaxin 16 knockdown, the proteins levels (and their inferred stability) of both syntaxins 6 and 10, as assessed by immunoblot analyses, were not significantly altered (not shown).
When Shiga B is then incubated with siRNA treated cells, we found that syntaxin 10 knockdown had no effect on Shiga B transport to the TGN (). On the other hand, syntaxin 16 knockdown resulted in an arrest of Shiga B in spotty structures resembling early endosomes, reminiscent of that observed in syn16cyto expressing cells (). The above results convincingly showed that in spite of syntaxin 10's TGN localization, it does not seem to play a role in mediating EE/RE-TGN transport. An essential role for syntaxin 16 in EE/RE-TGN transport shown previously Citation[23] is, on the other hand, further corroborated by the above analyses.
Figure 6. Knockdown of syntaxin 10 expressions has no effect on Shiga B transport to the TGN. Cells were transiently transfected with syntaxin 10 (Syn10) or syntaxin 16 (Syn 16) siRNA oligomers for 48 h. Cells were then incubated with Shiga B fragment (5 µg/ml) for 1 h and then fixed with 4% paraformaldehyde. They were incubated with primary antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against Shiga B, followed by Texas-red or FITC-labeled secondary antibodies. In cells with efficient syntaxin 16 knockdown, Shiga B staining remained spotty and early-endosome-like. Arrows indicated cells where syntaxin 16 knockdown was not particularly effective, and syntaxin 16 staining is still visible in these cells. Note that Shiga B transport to the perinuclear TGN patch was not impaired in these cells. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
Neither syntaxin 10 nor syntaxin 16 knockdown affects anterograde exocytosis of VSVG and both have only moderate effects on the mannose 6-phosphate receptor (M6PR)
We next investigate if syntaxin 10 is involved instead in the anterograde transport out of TGN. The VSVG traverses the exocytic pathway en route to the cell surface, and is a popular marker for exocytic transport. We infected HeLa cells, either treated or untreated with siRNA against the syntaxins 10 and 16, with VSVts045 and examined by immunofluorescence microscopy for any impairment in VSVG cell surface expression. This impairment may be manifested as an arrest in transport at the TGN, for example, and would be easily recognized by accumulation of VSVG staining at the perinuclear region. As shown in , neither the knockdown of syntaxin 10 expressions, nor that of syntaxin 16, had any significant effect on VSVG surface expression. There is also no significant entrapment of VSVG in the TGN or other internal membranes. Over-expression of the cytoplasmic domains of syntaxins 10 and 16 also did not alter VSVG surface expression (data not shown). Both these syntaxins, therefore, do not appear to be directly involved in TGN-plasma membrane transport.
Figure 7. Knock down of syntaxin 10 or syntaxin 16 expressions has no effect on VSVG transport to the cell surface and little effect on the localization of the mannose 6-phosphate receptor: (a) Left panels: Cells were transiently transfected with syntaxin 10 (Syn10) or syntaxin 16 (Syn 16) siRNA oligomers for 48 h and then infected with VSVts045 for 6 h as described in Materials and Methods. Cells were then fixed with 4% paraformaldehyde and incubated with primary antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against VSVG, followed by Texas-red or FITC-labeled secondary antibodies. Note that although not all cells are infected with the virus and therefore expressing VSVG, neither normal nor syntaxin 10/16 knockdown cells are impaired in terms of surface VSVG expression. Bar = 10 µm. (b). Right panels: Cells were transfected with syntaxin 10 (Syn10) or syntaxin 16 (Syn 16) siRNA oligomers for 48 h and double-labeled with antibody against syntaxins 10 or 16 (rabbit) and mouse monoclonal antibody against M6PR, followed by Texas-red or FITC-labeled secondary antibodies. Bar = 10 µm; (b). Changes in the morphology of M6PR with varying degree of syntaxin 10 knockdown. M6PR staining (green) is more diffuse in cells with higher degree of syntaxin 10 (red) knock down, but remains distinctly perinuclear. Figures 7A and 7B are reproduced in colour in Molecular Membrane Biology online.
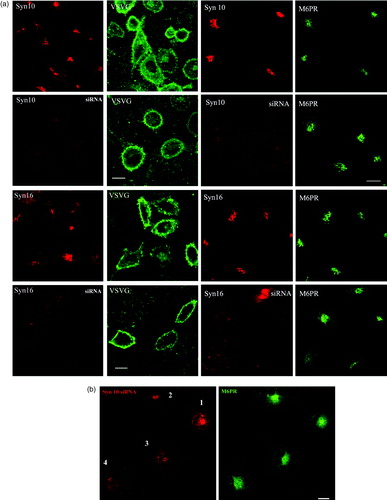
We have also examined if the steady state distribution of M6PR is affected by the syntaxin knockdowns. As shown in Figures , knockdown of both syntaxin 10 and 16 resulted in a more diffused staining of M6PR. However, the latter remains largely perinuclear.
Syntaxin 10 interacts with syntaxin 12/13 and may have a distinct role in endosomal trafficking
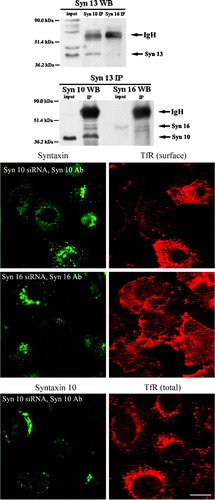
Our co-immunoprecipitation studies also reveal that syntaxin 10 interacts with the endosomal syntaxin 12/13 Citation[11], Citation[37]. As shown in , there is robust reciprocal co-immunoprecipitation between syntaxin 10 and syntaxin 12/13. Whereas syntaxin 16 antibodies appear to also co-immunoprecipitate some syntaxin 12/13, the reciprocal co-immunoprecipitation of syntaxin 16 by syntaxin 12/13 antibody is not particularly convincing. Since syntaxin 12/13 had been shown to play a role in the cycling of plasma membrane proteins Citation[37], we checked if the knockdown of syntaxin 10 expressions could perturb the cellular localization of TfR, which recycles between the plasma membrane and the endosome. As shown in , knockdown of syntaxin 10 expressions, but not that of syntaxin 16, significantly decreased the cell surface TfR levels. The reduction in TfR surface expression is concomitant with a corresponding increase in intracellular TfR. Taken together, these observations suggest that knockdown of syntaxin 10 expression may impair the expression of TfR to the cell surface. This effect on the recycling receptor TfR is rather specific, as a similar knockdown does not diminish the cell surface levels of the non-recycling epidermal growth factor (EGF) receptor (data not shown).
Figure 8. Syntaxin 10 interacts with syntaxin 12/13 and its depletion alters the steady state localization of TfR: (a). HeLa cell lysates were subjected to immunoprecipitation with antibodies against syntaxin 12/13, 10 or 16. Immunoprecipitates were eluted, resolved on 8% SDS-PAGE gels, and subjected to Western immunoblot analyses with antibodies against Vps45. Input is 1/7.5 of the lysate used for the immunoprecipitation. Representative blots are shown; (b). Cells were transiently transfected with the respective siRNA oligomers for 48 h. Cell were then incubated with culture supernatants (diluted 1:10 with basal RPMI medium) of the TfR monoclonal antibody producing Okt9 hybridoma for 20 min on ice. For the assessment of cell surface TfR levels (surface), cells were then washed extensively, fixed with 4% paraformaldehyde and incubated with primary antibodies against syntaxins 10 or 16, followed by Texas-red or FITC-labeled secondary antibodies. For the assessment of internal TfR levels (internal), cells were washed extensively, immersed in complete RPMI medium, transferred to 37°C, and incubated for 30 min. Cell were then washed, fixed with 4% paraformaldehyde and incubated with primary antibodies against syntaxin 10, followed by Texas-red or FITC-labeled secondary antibodies. Arrowheads indicate cells where syntaxin 10 or 16 knockdown is incomplete or minimal. Bar = 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
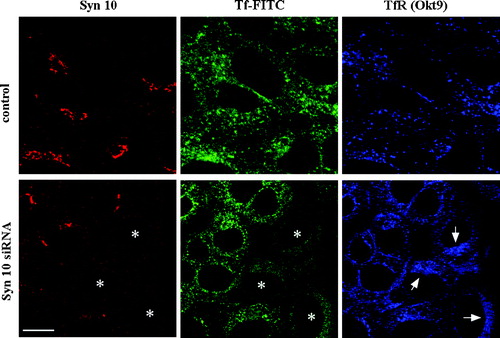
We sought to further investigate the reason for the decrease in cell surface TfR and the corresponding increase in intracellular TfR in syntaxin 10 knockdown cells. To see if this is due to some inhibition or delay in the overall endosome to cell surface recycling of TfR, we measured the rate of internalization and recycling of biotinylated transferrin, but could see no significance difference in the recycling kinetics between syntaxin knock down cells and controls. To understand this apparent discrepancy, a triple labeling of syntaxin 10, transferrin-FITC and TfR was performed after transferrin-FITC internalization at 18°C for 1 h and further incubated in fresh medium at 37°C to allow recycling to occur for varying time points. Clearing of internalized transferrin-FITC occurs in about 15 min (not shown). As shown in (at 5 min), transferrin-FITC in normal cells co-localized and their intensity correlated well with the labeling of TfR itself. However, in cells depleted of syntaxin 10, there is a distinct difference between transferrin-FITC and TfR labeling. While there is a noticeable decrease in transferrin-FITC in these cells compared to control, TfR labeling in these cells are increased and as in , appear to cluster at the perinuclear region. The reduction in trasferrin-FITC level in the syntaxin knock down cells can be attributed to the reduction in surface TfR. Further, although recycling kinetics are not obviously impaired in these cells, it appeared that a significant fraction of intracellular TfR in these cells did become somewhat immobilized.
Figure 9. Syntaxin 10 knock down increases intracellular accumulation TfR at a perinuclear region. Cells were transiently transfected with syntaxin 10 (Syn10) siRNA oligomers for 48 h. Transfected cells were allowed to internalized transferrin-FITC for 1 h at 18°C, followed by another 30 min of incubation in plain medium at 18°C. Cells were then shifted to 37°C for varying times before fixing and labeled with mouse monoclonal antibody against transferrin (Okt9) and the rabbit polyclonal antibody against syntaxin 10. This is followed by incubations with Cy5 conjugated goat anti-mouse IgG and Texas-red conjugated goat anti-rabbit IgG. Shown are cells after 5 min incubation at 37°C, where the transferrin-FITC staining is still obvious. Asterisks (*) indicate 3 cells with clear syntaxin 10 knockdown and arrows point to the perinuclear accumulation of TfR (labeled by Okt9) in these cells. Bar = 10 µm.
Discussion
SNARE molecules localized to the same compartment by biochemical or microscopic means have thus far been shown to interact with each other physically and functionally. Some SNAREs are in fact known to be present in more than one SNARE complex, and participate in more than one transport steps. We showed in this report that although syntaxin 10 is localized to the TGN and could potentially interact in vivo with syntaxins 6 and 16, it does not seem to play a role in EE/RE-TGN transport. This is supported by the lack of inhibition by syntaxin 10 specific antibodies of a biochemical assay of EE/RE-TGN transport, and corroborated by morphological assessments based on the expression of dominant negative forms of the molecule and knockdown of its expression by siRNA.
Both N-terminal epitope-tagged and untagged forms of all three TGN syntaxins are fairly faithfully TGN-localized when over-expressed in cells (not shown). In cells over-expressing epitope tagged or untagged forms of these syntaxins, there appear to be some amount of delay in transport along the exocytic pathway, and some increased labeling of the ERGIC-like spotty structures (not shown). Syntaxin 6 is a particularly ‘mobile’ syntaxin molecule, and has been shown to function in numerous transport steps Citation[24]. Both syntaxin 6 and syntaxin 16 have also been shown to follow anterograde traffic out from the TGN to the cell surface Citation[28].
The effect of syntaxin 16 siRNA on the localization of syntaxins 6 and 10 are indicative of the nature of their interaction. While the localization of syntaxin 6 is apparently disrupted in syntaxin 16 knockdown cells, the localization of syntaxin 10 is unaffected. This is suggestive of a more robust or more permanent association between syntaxin 6 and 16 at the TGN, and in view of its mobility, the apparent steady state localization of syntaxin 6 at the TGN may be due largely to its association with syntaxin 16. Since syntaxin 10 localization is unaffected by syntaxin 16 knockdown, their interactions revealed by co-immunoprecipitation may reflect transient associations or promiscuous association between SNAREs in detergent lysates Citation[38].
Several syntaxins are known to be present in several distinct SNARE complexes, which is usually taken to represent their versatility in engaging in multiple transport steps. The ER-Golgi syntaxin 5 is present in three complexes that are probably functionally distinct. The first described is the one with three other SNAREs Sec22B, Bet1, and membrin/GS27 Citation[39], the second involves Ykt6, GS28 and Bet 1 Citation[40], and the third comprises of GS15, GS28 and Ykt6 Citation[41]. Likewise, it was reported that syntaxin 7, syntaxin 8, vti1b and endobrevin/VAMP-8 form a complex that functions in the fusion of late endosomes Citation[42]. Syntaxin 7 may also form another discrete SNARE complex with syntaxin 6, Vti1b and either VAMP7 or VAMP8 in melanocytes Citation[43]. We have demonstrated, rather exhaustively, that although syntaxin 10 can be shown to interact in vivo with syntaxins 6 and 16, it has no function in endosome to TGN transport. It is clear that in spite of its 61% identity to syntaxin 6, they do not have the same function. At the moment, it is not yet clear what the physiological function of syntaxin 10 may be. Neither syntaxin 10 nor syntaxin 16 appears to be absolutely required for anterograde exocytosis from the TGN to the cells surface, as neither over-expression of the respective cytoplasmic domains nor siRNA-mediated knockdown of these syntaxins has any significant effect on the surface expression of VSVG.
No TGN SNARE has thus far been implicated in the more classical retrograde transport pathway from the late endosome to the TGN, which is clearly a SNAP-dependent event Citation[44]. It is not inconceivable that syntaxin 10 may play a role in this pathway. However, because both syntaxin 10 and syntaxin 16 knock down in our hands appear to have a similarly modest effect on the steady state distribution of M6PR (which returns to the TGN via the late endosome), syntaxin 10 is unlikely to play an exclusive or essential role in that pathway.
On the other hand, syntaxin 10's interaction with syntaxin 12/13 is suggestive of a possible role for syntaxin 10 in another aspect of endosomal trafficking, that of recycling between the endosome and the cell surface. The fact that TfR surface level diminishes, and the receptor molecules accumulate intracellularly in cells where syntaxin 10 expression has been knocked down lends further support to this notion. This result may seem counterintuitive, considering the steady state localization of syntaxin 10 at the TGN. However, that a component of the cellular vesicular transport machinery being functional in multiple cellular locations, or in locations that they are not normally associated with at steady state, is not without precedence. COPI components have a known function in the endosome, although their membrane association appears to be almost exclusively cis-Golgi Citation[45], Citation[46]. Sec13, a component of the ER budding COPII machinery, has been shown to be associated with nucleoporins and may be shuttled into the nucleus Citation[47]. Syntaxin 6 has been shown to function in multiple pathways in spite of it's primarily TGN localization Citation[24]. The functional scope of the cis-Golgi localized syntaxin 5 has recently been expanded to more distal part of the Golgi apparatus as its interaction with GS15 was recognized Citation[41], while VAMP8 has been shown to function at both the early and late endosomes Citation[48]. Likewise, VAMP7/TI-VAMP has been associated with late endosome-lysosome transport Citation[49] as well as neurite outgrowth processes that would involve SNARE interactions at the growth cone plasma membrane Citation[50].
Of course, in the case of syntaxin 10, more work would be required to clearly define the exact step or steps in TfR recycling in which it might function. The available syntaxin 12/13 antibodies label poorly in immunocytochemical analyses, prohibiting a meaningful assessment of the extent of co-localization between syntaxin 10 and syntaxin 12/13. We did, however, observe that uptake of TfR antibody alters the staining pattern of syntaxin 10, and there is significant co-localization between the two upon antibody internalization (data not shown). Our biochemical analysis using biotinylated transferrin did not reveal any obvious difference in terms of kinetics of recycling between syntaxin 10 knockdown and control cells. Triple labeling for syntaxin 10, transferrin-FITC and TfR however revealed that a significant fraction of TfR became concentrated at a loose perinuclear patch in syntaxin 10 knockdown cells, which may well be the late endosome. It therefore appears that syntaxin 10 depletion may affect TfR distribution in the cell by inducing the formation of a non-recycling pool. The exact mechanism that led to the formation of this pool remains to be investigated.
In human tissues, syntaxin 10 appears to be enriched in heart and skeletal muscles Citation[20]. It would be of interest to examine if it has a specific function in these tissues by targeted gene disruption approaches. At the moment, the possibility of doing this is hampered by the fact that it is not clear if a syntaxin 10 orthologue exists in rodents. Our syntaxin 10 antibodies do not cross-react with proteins in rodent cells. Search of mouse and rat genomic and expressed sequence tags (ESTs) have thus far reveal only hits with about 50% identity to human syntaxin 10, and these encode the homologous syntaxin 6. Syntaxin 10 does not appear to be primate specific, as matching porcine (gb: BI347067) and bovine (gb: BI683435) ESTs can be found in the database. The corresponding rodent syntaxin 10 might yet surface when annotation of the rodent genomes becomes more complete, and with it the opportunity to exploit the more established rodent systems for genetic, developmental and physiological analysis. This paper was first published online on prEview on 29 June 2005.
Work on syntaxins in BLT's laboratory is supported by a grant (R-183-000-106-112) from the Academic Staff Research Fund, National University of Singapore.
References
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science 1994; 272: 227–234
- Hay JC, Scheller RH. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol 1997; 9: 505–512
- Bennett MK. SNAREs and the specificity of transport vesicle targeting. Curr Opin Cell Biol 1995; 7: 581–586
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 1992; 257: 255–259
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell 1993; 74: 863–873
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localized to distinct membrane compartments. J Biol Chem 1998; 273: 10317–10324
- Bock JB, Maten HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature 2001; 409: 839–841
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem 1996; 271: 17961–17965
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell 1996; 7: 2019–2027
- Low SH, Chapin SJ, Weimbs T, Kömüves LG, Bennett MK, Mostov K. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell 1996; 7: 2007–2018
- Tang BL, Tan AE, Lim LK, Lee SS, Low DY, Hong W. Syntaxin 12, a member of the syntaxin family localized to the endosome. J Biol Chem 1998; 273: 6944–6950
- Hatsuzawa K, Hirose H, Tani K, Yamamoto A, Scheller RH, Tagaya M. Syntaxin 18, a SNAP receptor that functions in the endoplasmic reticulum, intermediate compartment, and cis-Golgi vesicle trafficking. J Biol Chem 2000; 275: 13713–13720
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol 1994; 4: 220–233
- Teng, FY, Wang, Y, Tang, BL. 2001. The syntaxins. Genome Biol, 2: reviews 3012
- Tekirian TL. The central role of the trans-Golgi network as a gateway of the early secretory pathway: physiologic vs nonphysiologic protein transit. Exp Cell Res 2002; 281: 9–18
- Traub LM, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr Opin Cell Biol 1997; 9: 527–533
- Rohn WM, Rouille Y, Waguri S, Hoflack B. Bi-directional trafficking between the trans-Golgi network and the endosome/lysosome system. J Cell Sci 2000; 113: 2093–2101
- Johannes L, Goud B. Surfing on a retrograde wave: how does shiga toxin reach the ER?. Trends Cell Biol 1998; 8: 158–162
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell 1997; 8: 1261–1271
- Tang BL, Low DH, Tan AE, Hong W. Syntaxin 10: a member of the syntaxin family localized to the trans-Golgi network. Biochem Biophys Res Commun 1998; 242: 345–350
- Tang BL, Low DH, Lee SS, Tan AE, Hong W. Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem Biophys Res Commun 1998; 242: 673–679
- Simonsen A, Bremnes B, Ronning E, Aasland R, Stenmark H. Syntaxin-16, a putative Golgi t-SNARE. Eur J Cell Biol 1998; 75: 223–231
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol 2002; 156: 653–664
- Wendler F, Tooze S. Syntaxin 6: The promiscuous behavior of a SNARE protein. Traffic 2001; 2: 606–611
- Wendler F, Page L, Urbe S, Tooze SA. Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol Biol Cell 2001; 12: 1699–1709
- Mills IG, Jones A, Clague MJ. Involvement of endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol 1998; 16: 881–884
- Martin-Martin B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood 2000; 96: 2574–2583
- Shewan AM, Van Dam EM, Martin S, Tang BL, Hong W, Bryant NJ, James DE. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN38: Involvement of an acidic targeting motif. Mol Biol Cell 2003; 14: 973–986
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem 1998; 273: 11719–11727
- Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV. At VP545 complex formation at the trans-Golgi network. Mol Biol Cell 2000; 11: 2251–2264
- Steegmaier M, Klumperman J, Foletti DL, Yoo JS, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell 1999; 10: 1957–1972
- Xu Y, Wong SH, Tang BL, Subramaniam VN, Zhang T, Hong W. A 29-kDa Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (Vti1-rp2) implicated in protein trafficking in the secretory pathway. J Biol Chem 1998; 273: 21783–21789
- Kreykenbohm V, Wenzel D, Antonin W, Atlachkine V, Von Mollard GF. The SNAREs vti1a and vti1b have distinct localization and SNARE complex partners. Eur J Cell Biol 2002; 81: 273–280
- FM Ausubel, Brent, R, Kingston, RE, Moore, DD, Seidman, JG, Smith, JA, Struhl, K. eds. Current Protocols in Molecular Biology. New York:Greene Publishing and Wiley Interscience; 1993.
- Tai G, Lu L, Wang TL, Tang BL, Goud B, Johannes L, Hong W. Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network. Mol Biol Cell 2004; 15: 4011–4022
- Tang BL, Wong SH, Qi XL, Low SH, Hong W. Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J Cell Biol 1993; 120: 325–328
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol 1998; 143: 957–971
- Tsui MM, Banfield DK. Yeast Golgi SNARE interactions are promiscuous. J Cell Sci 2000; 113: 145–152
- Xu D, Joglekar AP, Williams AL, Hay JC. Subunit structure of a mammalian ER-Golgi SNARE complex. J Biol Chem 2000; 275: 39631–39639
- Zhang T, Hong W. Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport. J Biol Chem 2001; 276: 27480–27487
- Xu Y, Martin S, James DE, Hong W. GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol Biol Cell 2002; 13: 3493–3507
- Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J 2000; 19: 6453–6464
- Wade N, Bryant NJ, Connolly LM, Simpson RJ, Luzio JP, Piper RC, James DE. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP) 8, and VAMP7 in b16 melanoma cells. J Biol Chem 2001; 276: 19820–19827
- Itin C, Rancano C, Nakajima Y, Pfeffer SR. A novel assay reveals a role for soluble N-ethylmaleimide-sensitive fusion attachment protein in mannose 6-phosphate receptor transport from endosome to the trans-Golgi network. J Biol Chem. 1997; 272: 27737–27744
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol 1996; 133: 29–41
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol 1997; 139: 1747–1759
- Enninga J, Levay A, Fontoura BM. Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol Cell Biol 2003; 23: 7271–7284
- Antonin W, Holroyd C, Tikkanen R, Honing S, Jahn R. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol Biol Cell 2000; 11: 3289–3298
- Advani RJ, Yang B, Prekeris R, Lee KC, Klumperman J, Scheller RH. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol 1999; 146: 765–775
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol 2000; 149: 889–900