Abstract
Small ankyrin 1, or sAnk1, is a small, alternatively spliced product of the erythroid ankyrin gene, ANK1, that is expressed in striated muscle and concentrated in the network sarcoplasmic reticulum (SR) surrounding the Z disks and M lines. We have characterized sAnk1 in muscle homogenates and SR vesicles, and have identified the region that targets it to the network SR. Selective extractions and partitioning into Triton X-114 show that sAnk1 behaves like the SR Ca-ATPase and so is an integral protein of the SR membrane. Mild proteolytic treatment of isolated SR vesicles indicates that sAnk1 is oriented with its hydrophilic, C-terminal sequence exposed to the solution, which is equivalent to the cytoplasmic face of the SR membrane in situ. SDS-PAGE in non-reducing gels suggests that sAnk1 is present as dimers and larger oligomers in the native SR. These results suggest that sAnk1 is oligomeric and oriented with its C-terminus exposed to the cytoplasm, where it may interact with proteins of the contractile apparatus. The N-terminal 29 amino acid hydrophobic sequence of sAnk1, which is predicted to span the SR membrane, is sufficient to target proteins to and anchor them in internal membranes of HEK 293 cells. It also targets reporter proteins to the network SR of skeletal myofibers and is thus the first example of a sequence that targets proteins to a particular compartment of the SR.
| Abbreviations | ||
| sAnk1 | = | small product of the ankyrin 1 gene |
| SERCA | = | Ca-dependent ATPase of the sarcoplasmic reticulum |
| SR | = | sarcoplasmic reticulum |
| TCEP | = | tris (carboxyethyl) phosphine |
Introduction
The factors that assemble, organize and stabilize the intracellular membranes of eukaryotic cells are still poorly understood. This is in part due to the fact that most internal membranes in eukaryotic cells do not have a regular or periodic arrangement. The transverse tubules and sarcoplasmic reticulum (SR) of striated muscle cells are notable exceptions, however. They provide an easily accessible experimental system to study how some intracellular membranes are constructed.
Cardiac and skeletal muscle cells are striated due to the regularly repeating patterns of thick and thin filaments that form each contractile unit, or sarcomere (reviewed in Craig [Citation1996]). Sarcomeres are roughly cylindrical in shape, ∼1 µm in diameter and ∼2.2 µm in length. They are arranged end-to-end to form myofibrils that can extend from one end of the muscle cell to the other. Myofibrils are also stacked side to side in the myoplasm, yielding structures that are aligned through much of the cellular interior. As the transverse, or ‘t’, tubules and the SR are arranged around each sarcomere in a stereotypical fashion, they too are regularly aligned in the myofiber. Here we focus on skeletal muscle and, in particular, on a small protein of the SR membrane that has the potential to interact with nearby contractile structures.
In mammalian skeletal muscle fibers, the t-tubules extend from the sarcolemma into the myoplasm, where they wrap around each sarcomere at the level of the A–I junction, the site at which the thin and thick filaments first overlap (reviewed in Franzini-Armstrong [Citation1996]). The SR of skeletal muscle does not extend to the sarcolemma but instead is limited to the myoplasm, where it is organized into different domains, each of which has its stereotypical location. The terminal cisternae of the SR, which interact most intimately with the t-tubules and are the source of the Ca2 + ions that are released to initiate contraction, are also at the level of the A–I junction in mammalian skeletal muscles. The terminal cisternae are connected by thin, tubular elements to the network SR, which is concentrated around the Z disk and, to a lesser extent, around the M line, at the ends and in the middle of each sarcomere, respectively. The network SR is responsible for most of the uptake of Ca2 + ions that occurs during muscle relaxation. Like the contractile apparatus itself, these membranes are present at each sarcomere and thus can be readily recognized and studied in most parts of the myofiber. The mechanism, that so unerringly aligns the network SR around the Z disk and M line of each sarcomere, is unknown.
A small protein that is appropriately positioned to play a role in this process is an alternatively spliced product of the ankyrin 1 gene, termed sAnk1 (for small ankyrin 1). Although ankyrin 1 (Ank1, also known as AnkR; for reviews see (Bennett & Baines [Citation2001]; Mohler et al. 2003) in erythrocytes and in skeletal muscle is ∼220 kDa and contains the typical ankyrin repeat and spectrin binding domains, sAnk1 is only ∼17 kDa in mass and contains neither domain. (Thus, an alternative term for it is AnkR17.5, according to a recent nomenclature: (Bennett & Baines [Citation2001])). Instead, it has an unique N-terminus consisting of a short, 29 amino acid hydrophobic region, followed by an additional unique sequence of 44 amino acids, and a sequence of 82 amino acids that it shares with the extreme C-terminus of some larger forms of ankyrin 1 (Birkenmeier et al. [Citation1993]; A). We showed previously that sAnk1 is selectively enriched in the network SR of skeletal muscle (Zhou et al. [Citation1997]). We postulated that, like its larger relative at the plasma membrane, sAnk1 links the SR membrane to the nearby actin-based cytoskeleton, which in skeletal muscle is the contractile apparatus. This requires that sAnk1 be firmly embedded in the SR membrane with its hydrophilic C-terminal sequence exposed to the myoplasm, where it would be available to interact with sarcomeric proteins. Here we present evidence to support this prediction. We also present evidence that sAnk1 is oligomeric in situ. We show further that the hydrophobic N-terminal 29 amino acid sequence of sAnk1 is sufficient to target a reporter protein to the network SR, making it the first sequence known to target proteins to a particular compartment of the SR.
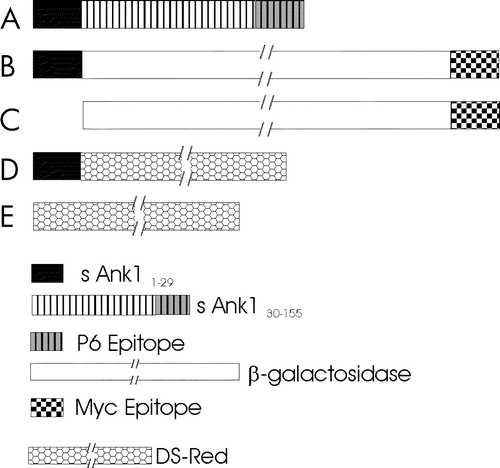
Figure 1. cDNAs encoding small ankyrin and constructs used in these experiments. (A) Small ankyrin consists of an N-terminal sequence of 29 amino acids that is highly hydrophobic, and a hydrophilic sequence of 126 amino acids, the last 15 of which were used to generate the p6 antibody. (B,C) Constructs encoding the lacZ gene product, β-galactosidase, with a C-terminal myc epitope tag, with (B) and without (C) the N-terminal 29 amino acid hydrophobic sequence of sAnk1 at the N-terminus. (D,E) Constructs encoding DS-Red, with (D) and without (E) the N-terminal 29 amino acid hydrophobic sequence of sAnk1 at the N-terminus. A key to the different sequences examined is given below (E) Not drawn to scale.
Materials and methods
Animals
Rats were purchased from Zivic Miller (Zelienople, PA). For collection of tissue for preparation of homogenates, rats were anesthetized with Metofane (Pitman-Moore, Mundelein, IL) followed by ketamine (80 mg kg−1) and xylazine (7 mg kg−1), and perfused through the heart with ∼100 ml PBS (10 mM NaP, 145 mM NaCl, pH 7.2) supplemented with a mixture of protease inhibitors (‘Complete’, EDTA-free, Roche Diagnostics, Mannheim, Germany). Similar procedures were followed prior to preparing cryosections, but the perfusion buffer contained 2% paraformaldehyde (see below). Adult New Zealand rabbits were purchased from Hazelton (Denver, PA). All procedures involving the use of living animals and animal tissue were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Isolation of SR vesicles and characterization of sAnk1
Vesicles of SR membrane, shown previously to be enriched in sAnk1 (Zhou et al. [Citation1997]), were isolated from hindlimb muscle of rabbits, as described (Eletr & Inesi [Citation1972]).
For studies of solubility, aliquots containing 40 µg of SR vesicle protein were incubated for 10 min at RT in 0.1 ml of PBS + 10 mM NaN3, 6 M urea in PBS, a solution buffered at pH 11 (50 mM ethanolamine, pH 11.0), or PBS supplemented with 0.5% Triton X-100. Samples were subjected to brief centrifugation in an Eppendorf desktop centrifuge (Brinkman Instruments, Westbury, NY) at 15,000×g or in an Airfuge (Beckman Instruments, Palo Alto, CA) at 178,000×g and the supernatants and pellets were analyzed by SDS-PAGE. Gels were either labeled with Coomassie blue, for identification of the SR Ca-ATPase (SERCA), or transferred electrophoretically to nitrocellulose for immunoblotting.
For studies of proteolytic susceptibility, aliquots containing 40 µg of SR vesicle protein were incubated for 20 min at RT in 0.1 ml of PBS containing 5–500 ng of trypsin or chymotrypsin, or in PBS alone. Samples were processed as above and either labeled with Coomassie blue (BioRad, Hercules, CA), to visualize SERCA, or transferred to nitrocellulose for immunoblotting for sAnk1 or calsequestrin. In an additional set of experiments, Triton X-100 was added to 2% (w/v), to solubilize the vesicles.
Partitioning into Triton X-114
The procedure of Bordier (Bordier [Citation1981]) was followed, with modifications. Aliquots containing 1 mg of SR vesicle protein and 10 µg bovine serum albumin (BSA) were incubated at 0°C for 15 min and then warmed to 37°C for 3–4 min in 1 ml PBS containing 1% Triton X-114. Insoluble material was removed by centrifugation at RT at 14,000 rpm for 3 min. The solution was then warmed to 37°C again for 4 min and subjected to a second centrifugation. The Triton X-114 and aqueous phases were analyzed as above, by Coomassie blue staining for SERCA and BSA, and by immunoblotting for sAnk1.
Creation of plasmids expressing sAnk1 and reporter proteins
The sequence encoding the first 29 amino acids of sAnk1 was cloned into the HindIII and BamHI restriction sites of the pcDNA3.1/Myc-His/lacZ vector (Invitrogen, Carlsbad, CA) using 5′-CCCAAGCTTACCGCCATGTGGACCTTCATCACGCAGC-TC-3′ and 5′-CGCGGATCCACGACTATATGCATCACATTCTGACA-3′ as the forward and reverse primers, respectively. This plasmid places expression of the fusion protein, or the epitope-tagged β-galactosidase alone, under the control of the CMV promoter (B). We used the same primers to insert the sequence encoding amino acids 1–29 of sAnk1 into DS-Red (D), using the EcoRI and BamHI sites in the DS-Red 2C1 plasmid (Clontech, Palo Alto, CA).
Transfection of myofibers in situ
Skeletal muscle fibers were transfected in vivo as described by Wolff et al. ([Citation1990]). Briefly, plasmid DNA constructs were solubilized in normal saline and 2% India ink to mark the site of injection. DNA was injected with a 28-gauge tuberculin syringe into the Tibialis anterior muscle of 2-week old Sprague–Dawley rats that had been anesthetized with Metofane. The rats were sacrificed under anesthesia 7–10 days later by perfusion with 2% paraformaldehyde, and the Tibialis anterior muscles were dissected out and snap frozen in a slush of liquid nitrogen. Frozen sections were prepared on a cryostat (Reichert-Jung, Cambridge Instruments, Deerfield, IL) at thicknesses of 8–20 µm (cross-sections) or 20 µm (longitudinal sections), and collected on slides coated with chrom–alum gelatin.
Immunofluorescence
For immunolabeling, sections were pretreated for 10 min in PBS + 10 mM NaN3 containing 1 mg ml−1 bovine serum albumin (PBS/BSA) and then incubated for 1 h with primary antibodies diluted in the same solution. The antibody to the C-terminal sequence of sAnk1, termed p6 (Zhou et al. [Citation1997]), was used at 2 µg ml−1. Mouse monoclonal antibodies to the myc epitope-tag (Invitrogen, Carlsbad, CA) were used at 1:500. Rabbit antibodies to β-galactosidase were from Chemicon (Temecula, CA) and were used at 1:1000. Non-immune rabbit IgG (5 µg ml−1) and MOPC 21 mouse IgG (5 µg ml−1) were used as controls in every experiment. After extensive washing, slides were incubated for 1 h with fluoresceinated goat anti-mouse IgG (FGAM, 10 µg ml−1) and tetramethylrhodaminylated goat anti-rabbit IgG (RGAR, 10 µg ml−1), both from Jackson Immunoresearch (West Grove, PA). All incubations were carried out at room temperature. Samples were washed extensively, and mounted in Vectashield (Vector Laboratories, Burlingame, CA) to reduce photobleaching.
Samples were first viewed under conventional epifluorescence optics and then under confocal optics, using a Zeiss 410 confocal laser scanning microscope (Carl Zeiss, Inc., Tarrytown, NY). Images were obtained at maximum resolution and assembled into montages with CorelDraw 9.
SDS-PAGE and immunoblotting
Most samples for analysis by SDS-PAGE were boiled in sample buffer containing 0.7 M 2-mercaptoethanol for 5 min (Laemmli [Citation1970]). In some experiments, samples were processed in sample buffer at 37°C for 15 min or at 100°C for 5 min, with or without reducing agents, or with an additional reducing agent, 4 mM tris (carboxyethyl) phosphine (TCEP), to supplement the 2-mercaptoethanol. Aliquots containing 30 µg protein/lane, for muscle homogenates, 80 µg protein/lane, for cell homogenates, or 15–20 µg protein/lane, for isolated SR vesicles, were subjected to electrophoresis (Laemmli [Citation1970]). Proteins in some gels were stained; others were transferred electrophoretically to nitrocellulose (Burnette [Citation1981]). Non-specific binding of antibodies was reduced by preincubating the nitrocellulose with Tris-buffered saline solution containing 0.5% Tween-20 and 3% non-fat milk, which was also used as the buffer in subsequent steps. Blots were incubated with a 1:100 dilution of antibodies to SERCA or calsequestrin, 1:2,500 for antibodies to the myc epitope, a 1:200 dilution of rabbit antibodies to DS-Red (Clontech), and at 100 ng ml−1 for anti-p6 antibodies, followed by species-specific goat anti-mouse or anti-rabbit IgG conjugated to alkaline phosphatase (Jackson Immunoresearch), diluted 1:10,000. Bound antibodies were detected by chemiluminescence (Western Light Detection, Tropix Laboratories, Bedford, MA).
Tissue culture and transfection
HEK 293 cells were kindly provided by Dr. William Randall (Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine). COS-7 cells were from the American Type Culture Collection. Cells were grown in DMEM and transfected with the various plasmid DNA constructs using the calcium phosphate precipitation method (Sambrook et al. [Citation1989]). Briefly, cells were grown to confluence and then subcultured onto 10 cm tissue culture dishes (Falcon, BD Biosciences, San Jose, CA) at ∼20% confluency. One day later, 3 h prior to transfection, cultures received fresh medium. Aliquots containing 10–30 µg of plasmid DNA were added as calcium phosphate precipitates to each culture. Fresh medium was then added and cultures were replaced in the incubator for 24 h. Medium was changed and cultures were harvested 48 h after transfection by scraping into PBS.
Materials
Unless otherwise indicated, all materials were purchased from Sigma Chemical Co. (St. Louis, MO) and were the highest grade available.
Results
Our experiments were designed to elucidate how sAnk1 is targeted and anchored to the SR, and how it is oriented with respect to the SR membrane. In one series of studies, we used isolated SR vesicles to determine the orientation of sAnk1 and nature of its association with the SR membrane. In another, we used transfection of cultured HEK 293 and COS-7 cells in vitro and skeletal muscle fibers in vivo to learn how sAnk1 is targeted to the SR.
Anchoring and orientation
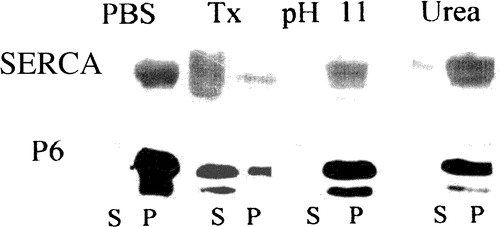
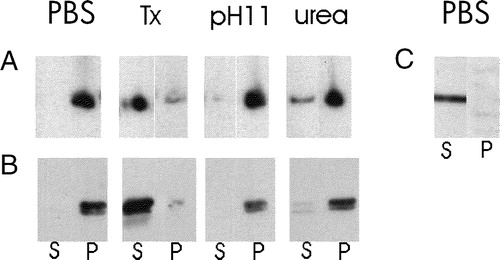
We hypothesized that the N-terminal hydrophobic sequence of sAnk1 was embedded in the lipid bilayer of the SR and so would cause sAnk1 to behave as an integral membrane protein. We tested this by subjecting isolated SR vesicles to extraction with 6 M urea and moderate alkaline conditions (pH 11), both of which have been used successfully to remove peripheral membrane proteins from other membranes. Under both conditions, sAnk1 remained firmly associated with the pelleted SR membrane fraction: no more sAnk1 was detectable in the supernatant after these extractions than was detectable after incubation in buffered saline (). In contrast, solutions containing Triton X-100 solubilized most of the sAnk1 from SR vesicles (, lane Tx S). These solubility properties of sAnk1 resemble those of SERCA (), which is also largely but not completely solubilized with Triton X-100 (Costello et al. [Citation1986]), suggesting that, like SERCA, sAnk1 is an integral protein of the SR membrane.
Figure 2. Extraction of proteins carrying the N-terminal hydrophobic sequence of sAnk1 from isolated SR vesicles. Isolated SR vesicles were incubated at RT in solutions of buffered saline (PBS), 6 M urea, 50 mM ethanolamine, pH 11, and buffered saline containing 2% Triton X-100 (Tx). After incubation, samples were subjected to centrifugation, and the distribution of sAnk1 between the supernatant and pellet fractions was examined by SDS-PAGE and immunoblotting with anti-p6 antibody. Parallel lanes were assayed for SERCA by SDS-PAGE and staining with Coomassie brilliant blue. sAnk1 and SERCA behaved similarly, as each were solubilized to significant extents by the detergent solution but not by other treatments.
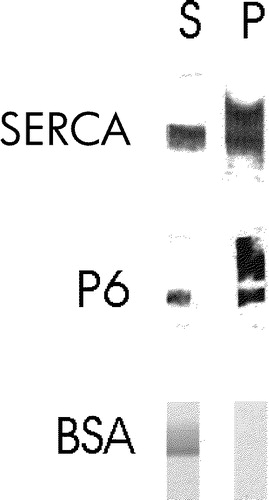
We confirmed the identity of sAnk1 as an integral membrane protein by examining its behavior in Triton X-114. The ability of proteins to associate with the Triton X-114 phase after solubilization with that detergent, followed by cooling to induce phase separation, has been taken as evidence of their ability to interact with the lipid bilayer, a characteristic usually associated with integral membrane proteins. Like SERCA, but unlike BSA, sAnk1 preferentially partitioned into the Triton X-114 fraction (, lanes P). We conclude that sAnk1 is an integral protein of the SR membrane.
Figure 3. sAnk1 partitions into Triton X-114. SR vesicles were extracted with a warm solution of Triton X-114, and the extracted materials were then cooled to induce phase separation. The aqueous and detergent phases were assayed for their content of sAnk1 by SDS-PAGE and immunoblotting, or for their content of SERCA by SDS-PAGE and staining with Coomassie brilliant blue. Both proteins partition into the detergent phase, consistent with their identification as integral membrane proteins. Bovine serum albumin remains in the aqueous phase.
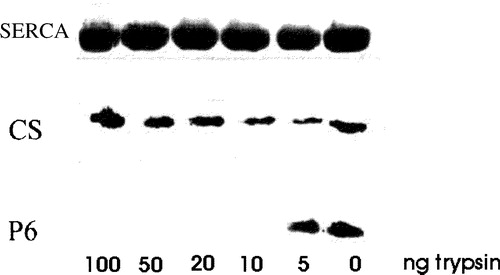
We next examined the orientation and state of oligomerization of sAnk1 in the SR. We used proteolysis of isolated SR vesicles to explore its orientation. Isolated SR vesicles typically are oriented ‘rightside out’, i.e., with what was originally the cytoplasmic surface of the SR membrane facing the medium, and what was originally the lumenal surface facing the interior of the vesicle (Scales & Inesi [Citation1976]; Ferguson et al. [Citation1985]; Franzini-Armstrong & Ferguson [Citation1985]). Calsequestrin, a protein of the SR lumen (Jorgensen et al. [Citation1985]; Thomas et al. [Citation1989]; McLeod et al. [Citation1991]), is therefore retained in the vesicle interior, where it is inaccessible to proteases added to the solution. Although SERCA is susceptible to proteolysis at concentrations of protease, relative to vesicular protein, of 1:100 or higher (Klip et al. [Citation1980]; Chu et al. [Citation1988]), SERCA in SR vesicles is resistant to degradation at lower relative protease concentrations (Chu et al. [Citation1988]). Using relative concentrations of trypsin ∼50 fold lower than those known to degrade SERCA (Klip et al. [Citation1980]; Chu et al. [Citation1988]), we found that neither SERCA nor calsequestrin in preparations of SR vesicles was degraded (). By contrast, the C-terminal epitope of sAnk1, recognized by the anti-p6 antibody, was degraded by these very low concentrations of proteases (10 ng ml−1: ). Permeabilization of the vesicles with Triton X-100, followed by digestion with trypsin, led to partial degradation of calsequestrin as well as the complete loss of sAnk1 (not shown). We obtained similar results with chymotrypsin at comparable concentrations (not shown). Thus, this epitope, and therefore the C-terminal, hydrophilic portion of sAnk1, is exposed to the solution, consistent with its exposure on the cytoplasmic surface of the SR in vivo.
Figure 4. Treatment of isolated SR vesicles with low concentrations of trypsin removes the p6 epitope. Isolated SR vesicles were treated for 10 min at RT with 0, 5, 10, 20, 50 and 100 ng ml−1 of trypsin in PBS, boiled in SDS-PAGE sample buffer and subjected to SDS-PAGE. Calsequestrin (CS) and the C-terminal region of sAnk1 containing the p6 epitope were labeled by immunoblotting; SERCA was visualized with Coomassie brilliant blue. Concentrations of trypsin above 5 ng ml−1 removed the p6 epitope from sAnk1 but had no effect on SERCA or calsequestrin, consistent with the presence of the C-terminus of sAnk1 on the cytoplasmic surface of the SR.
We also tested the possibility that, like phospholamban, another small, integral membrane protein of the SR (Jones et al. [Citation1985]; Jorgensen & Jones [Citation1987]), sAnk1 is multimeric in situ. We subjected muscle homogenates to SDS-PAGE and immunoblotting in the presence or absence of reducing agents, under stringent (95°C in sample buffer) and less stringent (warming in sample buffer at 37°C) conditions. We obtained similar results under both conditions, as well as in samples of muscle that were processed for SDS-PAGE within minutes of exsanguination. In samples exposed to reducing agents, much of the sAnk1 migrates with an apparent molecular mass of ∼21 kDa, but larger forms can be detected at ∼38 kDa, with much fainter bands at ∼58, 75, and 100 kDa (, lanes 1 and 3). The smallest band represents monomeric sAnk1, which usually migrates more slowly in SDS-PAGE than predicted for a polypeptide of ∼17 kDa (Zhou et al. [Citation1997]).
Figure 5. Oligomers of sAnk1 are present under non-reducing conditions. Fresh homogenates of rat hindlimb muscle were mixed with 0.7 M 2-mercaptoethanol in SDS-PAGE sample buffer supplemented with 4 mM TCEP, or SDS-PAGE sample buffer lacking all reducing agents. Each sample was then either boiled for 5 min, or incubated for 15 min in sample buffer at 37°C. After electrophoresis, samples were transferred to nitrocellulose paper and immunoblotted with anti-p6 antibodies. All reduced samples showed a major band at ∼21 kDa, with smaller amounts of label apparent at ∼38, 58, 75 and 100 kDa. All unreduced samples failed to show significant amounts of label at 21 kDa and instead showed major bands at ∼46 and ∼80 kDa, consistent with the presence of dimers and tetramers of sAnk1.
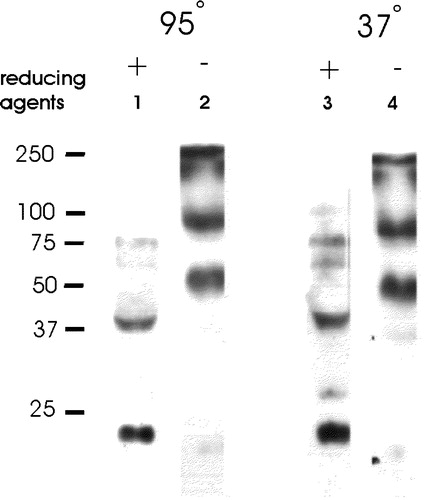
The higher bands could be larger polypeptides that share the C-terminal epitope recognized by anti-p6 antibodies, including larger, alternatively spliced products of the ANK1 gene (Birkenmeier et al. [Citation1993]), or multimers of sAnk1 itself that are not completely reduced. We were unable to generate antibodies to regions of sAnk1 N-terminal to the p6 epitope to distinguish between these alternatives. However, bacterially expressed fusion proteins containing the cytoplasmic sequence of sAnk1, examined after SDS-PAGE under non-reducing conditions, revealed the presence of disulfide-linked dimers (not shown). We therefore examined samples of isolated SR vesicles subjected to SDS-PAGE in the absence of reducing agents, to learn if this preparation also contains disulfide-linked oligomers of sAnk1.
The results of these experiments indicate that all the sAnk1 in isolated SR vesicles is oligomeric. In the absence of reducing agents, no sAnk1 remained at ∼21 kDa; instead, major bands carrying the p6 epitope were detectable at ∼46 and ∼80 kDa (, lanes 2 and 4). Additional labeling was also present near the top of the separating gel, but their apparent masses could not be determined. These results suggest that the sAnk1 present in the SR of freshly homogenized muscle is present as disulfide-linked oligomers, perhaps dimers, tetramers and even larger oligomers. These oligomers are likely to account for the minor bands seen at high apparent molecular weights in the presence of reducing agent.
Targeting to internal membranes
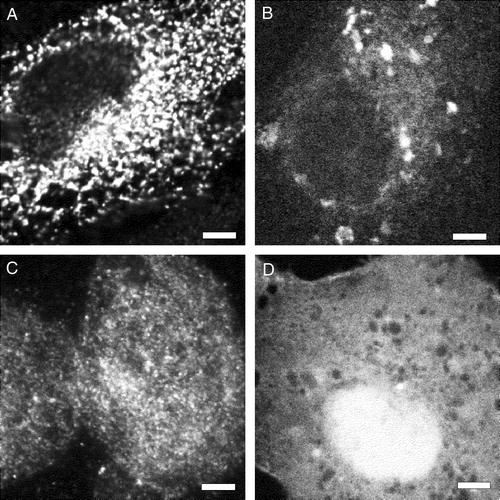
Previous experiments suggested that the hydrophobic sequence of sAnk1 was required to target it to the internal membranes of HEK 293 cells (Zhou et al. [Citation1997]), but they did not rule out the possibility that other portions of the sAnk1 sequence played a role in this process. To address this question, we created eukaryotic plasmid cDNAs encoding fusion proteins, consisting of DS-Red or β-galactosidase with the first 29 amino acids of sAnk1 linked to their N-termini (). The lac Z construct also contained a myc epitope tag at its C-terminus. Upon transfection of HEK 293 or COS-7 cells with the plasmids, each of the reporter proteins was concentrated in vesicular or reticular intracellular compartments (A,C). Proteins encoded by similar reporter constructs, lacking the N-terminal sequence of sAnk1 (), failed to incorporate into these structures and instead were more uniformly distributed in the cytoplasm (B,D). This suggests that the 29-amino acid hydrophobic N-terminus of sAnk1 is sufficient to target a cytoplasmic reporter protein to the internal membranes of HEK 293 and COS-7 cells.
Figure 6. The hydrophobic N-terminal sequence of sAnk1 targets a reporter protein to intracellular membranes in HEK 293 and COS-7 cells. The plasmid cDNAs encoding either myc-tagged β-galactosidase (A,C) or DS-Red (B,D), with (A,B) and without (C,D) the N-terminal hydrophic sequence of sAnk1 (see ), were introduced into HEK 293 (A,C) or COS-7 (B,D) cells. One day later, transfected COS-7 cultures were fixed and observed under fluorescence optics; HEK 293 cultures were fixed, permeabilized, labeled by immunofluorescence with antibodies to the myc tag or to β-galactosidase, and observed. The forms of DS-Red and lac Z containing the N-terminal hydrophobic sequence of sAnk1 were present in reticular or vesicular compartments within the cells (A,C), whereas the forms lacking this sequence were present more uniformly in the cytoplasm (B,D). Scale bar, 5 µm.
We used the same extraction procedures, that we used for endogenous sAnk1, to test the nature of the association with membranes of the reporter proteins containing the hydrophobic sequence of sAnk1. After brief homogenization, membranes from transfected HEK 293 cells were isolated by low speed centrifugation. The pellets were extracted with buffered saline, 6 M urea, alkaline solutions at pH 11, and buffered saline containing 2% Triton X-100. Only the latter was able to extract the reporter proteins from the membrane pellet in appreciable quantities (A,B). The DS-Red reporter protein lacking the N-terminal sequence of sAnk1 remained in the soluble fraction after homogenization in buffered saline (C). The myc-β-galactosidase lacking the N-terminal sequence of sAnk1 was also largely soluble, though some remained in the pellet fraction (not shown), perhaps because of non-specific association of β-galactosidase, which is tetrameric, with insoluble cellular material. These results show that a cytoplasmic protein can be firmly anchored to the internal membranes of HEK 293 and COS-7 cells by the N-terminal sequence of sAnk1. They suggest that the tight association of sAnk1 with the SR membrane is mediated primarily by its N-terminus and not by other, more C-terminal sequences.
Figure 7. Membrane association in mononucleate cells of lacZ and DS-Red constructs with and without the N-terminal hydrophobic sequence of sAnk1. (A,B) COS-7 cells (A) or HEK 293 cells (B) were transfected with plasmid cDNAs encoding DS-Red (A) or lac Z that carried a C-terminal myc epitope tag (B), each with the hydrophobic sequence of sAnk1 at the N-terminus (see ). COS-7 cells were also transfected with plasmid cDNA encoding DS-Red, without the N-terminal sequence of sAnk1 (C). One day after transfection, cells were homogenized and subjected to brief centrifugation. The pellets were washed with buffered saline and, after a brief centrifugation, the distribution of the reporter proteins was examined in the pellet and supernatant fractions. The DS-Red construct introduced into COS-7 cells carrying the N-terminal hydrophobic sequence of sAnk1 was enriched in the pellet and absent from the supernatant fractions (A, PBS). The construct lacking this sequence was only present in significant amounts in the supernatant fraction (C, PBS). The lac Z construct introduced into HEK 293 cells carrying the N-terminal hydrophobic sequence of sAnk1 behaved similarly to the DS-Red construct with the sAnk1 sequence, shown in A (panel B). The crude membrane fractions containing DS-Red or lac Z carrying the N-terminal hydrophobic sequence of sAnk1 were also extracted with solutions containing 2% Triton X-100 or 6 M urea, or buffered at pH 11. Only Triton X-100 solubilized the reporter proteins (A,B: Tx). These results suggest that the N-terminal hydrophobic sequence of sAnk1 is sufficient to confer DS-Red and lac Z with the solubility properties of integral membrane proteins, similar to sAnk1 itself.
Targeting to the network SR
We next asked if the same hydrophobic sequence of sAnk1 is sufficient to target the reporter protein to the SR of skeletal muscle fibers. We used intramuscular injection of the same plasmid cDNAs to transfect muscle fibers, following modifications of the methods developed by Wolff and his colleagues (Wolff et al. [Citation1990]; see Materials and methods). Although the transfection efficiency was typically very low (<1%), we were able to identify transfected myofibers in frozen cross-sections of the injected muscle by virtue of the fact that they labeled specifically with antibodies to the myc epitope tag (A,C). Double labeling of these same cross-sections with antibodies to endogenous sAnk1 showed the reporter protein containing the hydrophobic N-terminal sequence of sAnk1 to be present in the same reticular structures (B,D). Injection of the muscle fibers with the cDNA encoding the reporter protein lacking the hydrophobic N-terminus of sAnk1 resulted in a nearly uniform distribution of the exogenous protein (E) and little coincidence with endogenous sAnk1 (F).
Figure 8. The hydrophobic N-terminal sequence of sAnk1 targets a reporter protein to the network SR of skeletal muscle. The plasmid cDNAs encoding myc-tagged β-galactosidase with (A,C,G,J: 1–29) and without (E: con) the N-terminal hydrophobic sequence of sAnk1 (see ), were injected into muscle to transfect muscle fibers. Seven to 10 days later, muscle was perfusion fixed, collected, snap frozen and immunolabeled for the transgene product with antibodies to the myc epitope or to β-galactosidase (A,C,E,G,I), as well as for endogenous sAnk1, recognized with the anti-p6 antibody (B,D,F,H,J: P6). (A–F) Cross-sections of transfected skeletal muscle fibers. Lac Z carrying the N-terminal hydrophobic sequence of sAnk1 (A,C, arrows) codistributed with the endogenous sAnk1 in the SR (B,D, arrows). Lac Z lacking this sequence (E) did not target to the SR (F). (G–J) Longitudinal sections of muscle showing transfected skeletal muscle fibers were labeled as in A–F. The protein encoded by the transgene concentrates in structures that surround the M lines (white arrows) and Z disks (grey arrows) of the myofibers (G,I), which also contain sAnk1 (H,J). Whereas sAnk1 concentrates around Z disks with lower amounts around M lines (Zhou et al. [Citation1997]), the reporter protein appears to concentrate around M lines with lower amounts around Z disks. Scale bars, 5 µm.
![Figure 8. The hydrophobic N-terminal sequence of sAnk1 targets a reporter protein to the network SR of skeletal muscle. The plasmid cDNAs encoding myc-tagged β-galactosidase with (A,C,G,J: 1–29) and without (E: con) the N-terminal hydrophobic sequence of sAnk1 (see Figure 1), were injected into muscle to transfect muscle fibers. Seven to 10 days later, muscle was perfusion fixed, collected, snap frozen and immunolabeled for the transgene product with antibodies to the myc epitope or to β-galactosidase (A,C,E,G,I), as well as for endogenous sAnk1, recognized with the anti-p6 antibody (B,D,F,H,J: P6). (A–F) Cross-sections of transfected skeletal muscle fibers. Lac Z carrying the N-terminal hydrophobic sequence of sAnk1 (A,C, arrows) codistributed with the endogenous sAnk1 in the SR (B,D, arrows). Lac Z lacking this sequence (E) did not target to the SR (F). (G–J) Longitudinal sections of muscle showing transfected skeletal muscle fibers were labeled as in A–F. The protein encoded by the transgene concentrates in structures that surround the M lines (white arrows) and Z disks (grey arrows) of the myofibers (G,I), which also contain sAnk1 (H,J). Whereas sAnk1 concentrates around Z disks with lower amounts around M lines (Zhou et al. [Citation1997]), the reporter protein appears to concentrate around M lines with lower amounts around Z disks. Scale bars, 5 µm.](/cms/asset/87066d36-ca5c-4f52-a8fd-e65b9fef3be6/imbc_a_124409_f0008_b.jpg)
These results suggest that the N-terminal 29 amino acid sequence of sAnk1 is sufficient to target proteins to the SR of skeletal muscle, but they do not prove that the targeted reporter protein is actually in the network SR, which is limited to regions surrounding the Z disks and the M lines of each sarcomere (Zhou et al. [Citation1997]). To test this, we prepared longitudinal sections of the muscles that had been injected with the reporter construct carrying the N-terminal sequence of sAnk1. This experiment was tedious, due to the scarcity of transfected fibers and the difficulty of obtaining sections of these fibers parallel to their long axes. Nevertheless, we were able to visualize a number of transfected fibers in longitudinal sections. In each, the reporter protein containing the 29 amino acid hydrophobic sequence of sAnk1 was present at the level of the Z disks and M lines (G,I), as suggested by double labeling with antibodies to endogenous sAnk1 (H,J). Although we were not able to confirm the relationship of the individual lines of labeling with the contractile structures, our previous studies indicate that sAnk1 is concentrated more extensively around the Z lines of fast twitch muscle fibers but is also present around the M lines (Zhou et al. [Citation1997]). Notably, however, the reporter protein showed heavier concentrations where labeling for the endogenous sAnk1 was fainter, presumably around the M lines (white arrows), and lighter concentrations where labeling for the endogenous sAnk1 was brighter (grey arrows), presumably around the Z disks. These results suggest that, although other factors may influence its distribution within this membrane compartment, the N-terminal 29 amino acids of sAnk1 are sufficient to target a protein specifically to the network SR of skeletal muscle.
Discussion
Our aim in these experiments was to learn how sAnk1, a small, alternatively spliced form of ankyrin 1 expressed in striated muscle, becomes localized to the network SR, and how it is anchored to and organized within the SR membrane. Our results indicate that sAnk1 oligomerizes in situ, behaves as an integral membrane protein, and is anchored to the membrane through its hydrophobic, N-terminal sequence, with its hydrophilic C-terminus exposed on the cytoplasmic surface of the SR. We also show that the hydrophobic N-terminal region of sAnk1 is sufficient to target it and other proteins to internal membranes of mononucleate cells. Finally, experiments with transfected skeletal muscle fibers identify the N-terminal sequence of sAnk1 as the first sequence to target a protein to the network SR.
Several pieces of evidence strongly indicate that sAnk1 is an integral membrane protein that resembles SERCA in its ability to associate with the lipid bilayer. First, following extraction with Triton X-114 and cooling, sAnk1 partitions into the Triton X-114 phase together with SERCA, the prototypical integral membrane protein of the SR. Furthermore, like SERCA, sAnk1 is released from SR membrane vesicles by detergents but not by treatments that strip membranes of peripheral proteins. Two reporter proteins that associate with intracellular membraneous compartments via the hydrophobic N-terminal sequence of sAnk1 have similar solubility properties, consistent with the idea that these properties, which sAnk1 shares with SERCA, are conferred by its N-terminal hydrophobic sequence.
The results of proteolysis experiments with very low concentrations of trypsin further indicate that the p6 epitope, located at the C-terminus of sAnk1 (Zhou et al. [Citation1997]), extends from the cytoplasmic face of the SR membrane, which in isolated SR vesicles is exposed to the medium (Scales & Inesi [Citation1976]; Ferguson et al. [Citation1985]; Franzini-Armstrong & Ferguson [Citation1985]). This is consistent with the fact that this epitope is accessible to labeling in frozen sections of skeletal muscle that have not been exposed to detergent (Zhou et al. [Citation1997]; ). It is, however, inconsistent with the typical role of N-terminal hydrophobic stretches of amino acids as signal sequences for translocation across the membrane of the rough endoplasmic reticulum. Although clearly hydrophobic enough to serve as a signal sequence, the N-terminus and nearby residues of sAnk1 appear to serve as a ‘stop transfer signal’, that simultaneously anchors the nascent protein to the membrane and dissociates it from the translocase. As sAnk1 also lacks the typical consensus recognition sequence, A-X-A, recognized by the signal peptidase (von Heijne [Citation1998]), it can remain anchored in the membrane through its N-terminal sequence. It is therefore not surprising that sAnk1 is oriented with respect to the SR membrane with its C-terminal sequence exposed to the cytoplasm (). Indeed, an algorithm for analyzing the transmembrane orientation of proteins, HMMTOP (Tusnady & Simon [Citation2001]), predicts this orientation. It also predicts that the most N-terminal amino acids of sAnk1, WT (the N-terminal methionine is likely to be removed during translation), are exposed on the lumenal side of the SR membrane. Although this is consistent with our earlier results (Zhou et al. [Citation1997]), it must still be tested experimentally.
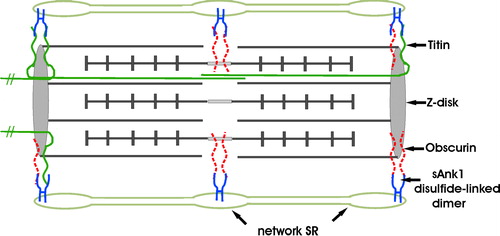
Figure 9. Model of sAnk1 in the network SR membrane, and its relationship to its ligands, obscurin and titin. sAnk1 is anchored to the network SR membrane by its N-terminal hydrophobic sequence. Molecular modeling predicts that, following removal of its N-terminal methionine residue, only 2 amino acids are exposed on the lumenal surface of the SR membrane. The entire sequence C-terminal to the transmembrane domain is exposed to the cytoplasm, where it can interact with obscurin and titin at the level of the Z disks, or obscurin alone at the level of the M lines. sAnk1 is pictured as disulfide-linked dimers, although tetramers, and even larger oligomers, may also be present. These dimers may bind to two molecules of obscurin simultaneously at either Z disks or M lines, with one molecule of obscurin and one molecule of titin at Z lines, or with two molecules of titin at Z-lines. The titins that are anchored at the Z lines of the sarcomere pictured here, but that extend towards the M lines of nearby sarcomeres, are not drawn as complete molecules. Not drawn to scale. This figure is reproduced in color in Molecular Membrane Biology online.
Our results further suggest that sAnk1 in the native SR is present as disulfide-linked dimers and larger oligomers. In reducing gels, sAnk1 runs primarily as a monomer but larger forms are readily detectable by immunoblotting (). These might be dimers and larger multimers, larger proteins, including products of the Ank1 gene, that carry the p6 epitope (e.g., Birkenmeier et al. [Citation1993]) or that cross-react with the antibody to this epitope, or covalently-linked complexes of sAnk1 with other proteins of the SR membrane. We have tried unsuccessfully to generate antibodies to more N-terminal sequences of sAnk1 to start to distinguish among these alternatives. Instead, we used SDS-PAGE in the absence of reducing agents, which revealed that most of the sAnk1 in native SR migrates at molecular masses of 48 and 80 kDa. Although these experiments cannot rule out an association of sAnk1 with other SR proteins, they are most readily explained by the ability of sAnk1 to form disulfide-linked dimers and tetramers. Notably, sAnk1 has two cysteines, one in its juxtamembrane sequence at position 22, the other at position 34, just C-terminal to the sequence we used to create the 29 amino acid N-terminal hydrophobic tail. As we have observed disulfide-linked dimers to form in vitro in bacterially expressed fusion proteins containing only the latter cysteine residue (our unpublished observation), this cysteine probably mediates the formation of stable sAnk1 dimers. Tetramers of sAnk1, which seem likely to account for the bands at ∼80 kDa in , may form as a result of disulfide bonding of the more juxtamembrane cysteine residues, but experimental evidence for this is still lacking.
sAnk1 resembles two other small, integral proteins of the SR membrane, phospholamban and sarcolipin (Jones et al. [Citation1985]; Odermatt et al. [Citation1997]). The former is expressed in cardiac muscle, the latter primarily in fast-twitch skeletal muscle. Like these proteins (Jones et al. [Citation1985]; Simmerman et al. [Citation1996]; Hellstern et al. [Citation2001]), sAnk1 is an integral, single-pass SR protein that oligomerizes in situ. sAnk1 has an N-terminal hydrophobic sequence, however, and has its C-terminal sequence facing the cytoplasm. By contrast, phospholamban and sarcolipin are typical ‘tail-anchored’ proteins, with C-terminal hydrophobic membrane anchors and N-terminal sequences facing the cytoplasm. Although the oligomeric state of sarcolipin is not known, phospholamban uses leucine zipper motifs in its transmembrane sequence, rather than disulfide bonds, to oligomerize into pentamers (Simmerman et al. [Citation1996]; but see Cornea et al. [Citation2000]). Several computer algorithms that we have used have failed to recognize a potential leucine zipper or any other structural motif in the transmembrane sequence of sAnk1. Finally, as its ability to regulate the SERCA activity of cardiac muscle resides in its monomeric form (Kimura et al. [Citation1997]; Cornea et al. [Citation2000]), phospholamban must exist in the SR membrane as both monomers and pentamers. The results of our non-reducing gels suggest that very little endogenous sAnk1 is present as the monomer.
Oligomerization of sAnk1 in situ may have functional consequences. We have identified two ligands of the cytoplasmic sequence of sAnk1, obscurin and titin (Bagnato et al. [Citation2003]; Kontrogianni-Konstantopoulos & Bloch [Citation2003]; Kontrogianni-Konstantopoulos et al. [Citation2003]). Whereas only obscurin is present surrounding the M lines of striated muscle (Kontrogianni-Konstantopoulos & Bloch [Citation2003]), both protein ligands are located at Z disks (Kontrogianni-Konstantopoulos & Bloch [Citation2003]; Kontrogianni-Konstantopoulos et al. [Citation2003]). Oligomers of sAnk1 in the network SR near Z disks could bind to both ligands simultaneously, bringing them into close proximity. Alternatively, sAnk1 oligomers could bind to two or more molecules of the same type, perhaps enhancing the apparent affinity of binding for each. Both possibilities are pictured in . Oligomerization may also influence the ability of the hydrophobic sequence of sAnk1 to target this molecule to the appropriate domains of the SR.
The mechanisms responsible for targeting integral proteins to the network SR, or to the other internal membrane compartments of striated muscle, including the terminal cisternae or the transverse tubules, are still poorly understood. Our previous results (Zhou et al. [Citation1997]) indicated that the 29 amino acid, hydrophobic N-terminal sequence of sAnk1 was necessary for targeting of sAnk1 to intracellular membranes, probably the endoplasmic reticulum, of HEK 293 cells, but they did not prove that it was sufficient. Here we show that it is sufficient to target a reporter protein not only to intracellular membrane-bound compartments of mononucleate cells in culture, but also to the network SR of mature skeletal myofibers. Significantly, the reporter protein did not appear at detectable levels at other membrane systems in transfected myofibers, including the nuclear membrane, the sarcolemma, t-tubules or mitochondria. It remains to be determined if the specificity resides in the putative lumenal sequence (WT), the transmembrane domain (FITQLLVTLVLLGFFLV), or the cytoplasmic, juxtamembrane sequence (SCQNVMHIV) expressed in the reporter construct. Nevertheless, these results suggest that targeting specificity resides in the 29 most N-terminal amino acids of sAnk1. To our knowledge, this is the first sequence demonstrated to target a protein to a specific compartment of the SR membrane.
Surprisingly, however, the reporter protein carrying the hydrophobic sequence of sAnk1 became more concentrated in the network SR where endogenous sAnk1 was less concentrated. This may be due to the absence of additional targeting information contained within the cytoplasmic sequence of sAnk1, or to competition between the two molecules for access to the membrane of the network SR. Alternatively, other factors, such as the large size of the reporter protein, which may inhibit its access to membranes that are closely apposed to the contractile structures, may also come into play. Further studies of sAnk1 sequences expressed with several different reporter proteins should provide additional insights into the mechanisms that target it and other proteins to the network SR.
sAnk1 is therefore an unusual protein, with novel properties. Although it shares sequence with larger forms of ankyrin 1, sAnk1 is nevertheless distinct in having a unique, hydrophobic N-terminus that targets it specifically to and anchors it within the network SR. The cytoplasmic portion of sAnk1 contains sequence unique to sAnk1 as well as a region shared with larger forms of Ank1, both of which are likely to contribute to its ability to bind titin and obscurin (Bagnato et al. [Citation2003]; Kontrogianni-Konstantopoulos & Bloch [Citation2003]; Kontrogianni-Konstantopoulos et al. [Citation2003]). We speculate that this binding may anchor the network SR to the contractile apparatus at Z disks and M lines and so help to align the network SR with these structures ().
This paper was first published online on prEview on 23 September 2005.
We are grateful to Dr Aikaterini Kontrogianni-Konstantopoulos for useful discussions and for preparing the template we used for . This work was supported by a fellowship to NCP from the Robert Woods Johnson Foundation, and by a grant to RJB from the National Institutes of Health (RO1 HL64304).
References
- Bagnato P, Barone V, Giacomello E, Rossi D, Sorrentino V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J Cell Biol 2003; 160: 245–253
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 2001; 81: 1353–1392
- Birkenmeier CS, White RA, Peters LL, Hall EJ, Lux SE, Barker JE. Complex patterns of sequence variation and multiple 5′ and 3′ ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem 1993; 268: 9533–9540
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 1981; 256: 1604–1607
- Burnette WN. ‘Western blotting’: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 1981; 112: 195–203
- Chu A, Sumbilla C, Scales D, Piazza A, Inesi G. Trypsin digestion of junctional sarcoplasmic reticulum vesicles. Biochem 1988; 27: 2827–2833
- Cornea RL, Autry JM, Chen Z, Jones LR. Reexamination of the role of the leucine/isoleucine zipper residues of phospholamban in inhibition of the Ca2 + pump of cardiac sarcoplasmic reticulum. J Biol Chem 2000; 275: 41487–41494
- Costello B, Chadwick C, Saito A, Chu A, Maurer A, Fleischer S. Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J Cell Biol 1986; 103: 741–753
- Craig R. The structure of the contractile filaments. Myology: Basic and Clinical2nd edn, AG Engel, C Franzini-Armstrong. McGraw-Hill, New York 1996; 134–175
- Eletr S, Inesi G. Phase changes in the lipid moieties of sarcoplasmic reticulum membranes induced by temperature and protein conformational changes. Biochim Biophys Acta 1972; 290: 178–185
- Ferguson DG, Franzini-Armstrong C, Castellani L, Hardwicke PM, Kenney LJ. Ordered arrays of Ca2 + -ATPase on the cytoplasmic surface of isolated sarcoplasmic reticulum. Biophys J 1985; 48: 597–605
- Franzini-Armstrong C. The sarcoplasmic reticulum and the transverse tubules. Myology: Basic and Clinical2nd edn, AG Engel, C Franzini-Armstrong. McGraw-Hill, New York 1996; 176–199
- Franzini-Armstrong C, Ferguson DG. Density and disposition of Ca2 + -ATPase in sarcoplasmic reticulum membrane as determined by shadowing techniques. Biophys J 1985; 48: 607–615
- Hellstern S, Pegoraro S, Karim CB, Lustig A, Thomas DD, Moroder L, Engel J. Sarcolipin, the shorter homologue of phospholamban, forms oligomeric structures in detergent micelles and in liposomes. J Biol Chem 2001; 276: 30845–30852
- Jones LR, Simmerman HK, Wilson WW, Gurd FR, Wegener AD. Purification and characterization of phospholamban from canine cardiac sarcoplasmic reticulum. J Biol Chem 1985; 260: 7721–7730
- Jorgensen AO, Jones LR. Immunoelectron microscopical localization of phospholamban in adult canine ventricular muscle. J Cell Biol 1987; 104: 1343–1352
- Jorgensen AO, Shen AC, Campbell KP. Ultrastructural localization of calsequestrin in adult rat atrial and ventricular muscle cells. J Cell Biol 1985; 101: 257–268
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J Biol Chem 1997; 272: 15061–15064
- Klip A, Reithmeier RA, MacLennan DH. Alignment of the major tryptic fragments of the adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem 1980; 255: 6562–6568
- Kontrogianni-Konstantopoulos A, Bloch RJ. The hydrophilic domain of small ankyrin 1 interacts with the two NH2-terminal immunoglobulin domains of titin. J Biol Chem 2003; 278: 3985–3991
- Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell 2003; 14: 1138–1148
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- McLeod AG, Shen ACY, Campbell KP, Michalak M, Jorgensen AO. Frog cardiac calsequestrin: identification, characterization, and subcellular distribution in two structurally distinct regions of peripheral sarcoplasmic reticulum in frog ventricular myocardium. Circ Res 1991; 69: 344–359
- Mohler PJ, Gramolini AO, Bennett V. Ankyrins. J Cell Sci 2002; 115: 1565–1566
- Odermatt A, Taschner PE, Schere SW, Beatty B, Khanna VK, Cornblath DR, Chaudhry V, Yee WC, Schrank B, Karpati G, et al. Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated wtih SERCA1: absence of structural mutations in five patients with Brody disease. Genomics 1997; 45: 541–553
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York 1989
- Scales D, Inesi G. Assembly of ATPase protein in sarcoplasmic reticulum membranes. Biophys J 1976; 16: 735–751
- Simmerman HK, Kobayashi YM, Autry JM, Jones LR. A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J Biol Chem 1996; 271: 5941–5946
- Thomas K, Navarro J, Benson RJ, Campbell KP, Rotundo RL, Fine RE. Newly synthesized calsequestrin, destined for the sarcoplasmic reticulum, is contained in early/intermediate Golgi-derived clathrin-coated vesicles. J Biol Chem 1989; 264: 3140–3145
- Tusnady GE, Simon I. The HMMTOP transmembrane topology predictor server. Bioinform 2001; 17: 849–850
- von Heijne G. Life and death of a signal peptide. Nature 1998; 396: 111–112
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990; 247: 1465–1468
- Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol 1997; 136: 621–631