Abstract
Insolubility in non-ionic detergents such as Triton X-100 at low temperature is a widely used biochemical criterion for characterization of membrane domains. In view of the emerging role of membrane organization in the function of G-protein coupled receptors, we have examined detergent insolubility of the 5-HT1A receptor in CHO cells using a novel GFP fluorescence approach developed by us. Using this approach, we have explored the membrane organization of the serotonin1A receptor tagged to enhanced yellow fluorescent protein (5-HT1AR-EYFP) stably expressed in CHO-K1 cells under conditions of varying detergent concentration, reduced membrane cholesterol and agonist stimulation. Our results show that a small yet significant fraction of the 5-HT1A receptor exhibits detergent insolubility, which increases upon depletion of membrane cholesterol. Stimulation of 5-HT1AR-EYFP by its endogenous ligand, serotonin, did not cause a significant change in the detergent insolubility of the receptor. Taken together, our results on detergent insolubility of 5-HT1AR-EYFP provide new insights into the membrane organization of the 5-HT1A receptor and could be relevant in the analysis of membrane organization of other G-protein coupled receptors.
| Acronyms | ||
| 5-hydroxytryptamine | = | 5-HT |
| 5-hydroxytryptamine-1A receptor | = | 5-HT1A receptor |
| 5-hydroxytryptamine-1A receptor tagged to EYFP | = | 5-HT1AR-EYFP |
| β1-adrenergic receptor | = | β1AR |
| β2-adrenergic receptor | = | β2AR |
| adenylate cyclase | = | AC |
| bicinchoninic acid | = | BCA |
| Chinese hamster ovary cells | = | CHO cells |
| detergent resistant membrane | = | DRM |
| dialkylindocarbocyanine | = | DiI |
| 1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate | = | DiIC16 |
| enhanced yellow fluorescent protein | = | EYFP |
| 1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate | = | FAST DiI |
| green fluorescent protein | = | GFP |
| G-protein coupled receptor | = | GPCR |
| glycosylphosphoinositol | = | GPI |
| methyl-β-cyclodextrin | = | MβCD |
Introduction
The G-protein coupled receptor (GPCR) superfamily comprises the largest class of molecules involved in signal transduction across the plasma membrane, providing a mechanism of communication between the exterior and the interior of the cell Citation[1]. These receptors can be activated by ligands as chemically diverse as biogenic amines, peptides, glycoproteins, lipids, nucleotides and even photons, thereby mediating diverse physiological processes such as neurotransmission, cellular metabolism, secretion, cellular differentiation, growth, and inflammatory and immune responses. GPCRs which represent ∼1% of the mammalian genome are major targets for the development of novel drug candidates in all clinical areas Citation[1]. It is estimated that up to 50% of clinically prescribed drugs act as either agonists or antagonists at GPCRs which points out their immense therapeutic potential Citation[2]. Several ligands of GPCRs are found among the top 100 globally selling drugs.
Since a significant portion of integral membrane proteins such as GPCRs remains in contact with the membrane lipid environment Citation[3] and important sites for protein function often lie in the transmembrane regions, the organization and function of membrane proteins could depend on the surrounding membrane lipid environment. Lipid–protein interaction in membranes has attracted a lot of attention in relation to its role in assembly, stability and function of membrane proteins Citation[3], Citation[4]. In this context, the crucial role of organization of lipids and proteins in membranes and its relevance in membrane function assumes significance Citation[3–6]. There is growing evidence for the concept of membranes being organized into domains with defined lipid and protein compositions. These domains, sometimes referred to as ‘rafts’, are believed to serve as platforms for signaling by concentrating certain lipids (such as cholesterol and sphingolipids) and proteins while excluding others Citation[5–7]. Work from several laboratories has suggested that organization of membranes into domains could play a key role in a number of processes such as membrane trafficking, sorting, signal transduction, and pathogen entry Citation[7–9]. The role of membrane domains in GPCR functioning represents a challenging aspect of GPCR research. Receptor–G-protein interactions may be dependent on their organization in membranes and not solely on the binding sites present on the interacting proteins Citation[10]. The restricted mobility of receptor, G-protein, and effector on the cell surface in addition to the selectivity of receptor–G-protein interaction, is now believed to be an important determinant of spatiotemporal regulation of GPCR signaling Citation[10], Citation[11]. In this context, we have recently shown that dynamics of G-protein coupled receptors could be modulated by G-protein activation Citation[12]. The heterogenous distribution of GPCRs into domains has given rise to new challenges and complexities in receptor signaling, since signaling in such a case has to be understood in the context of the three dimensional organization of various signaling components which include receptors and G-proteins Citation[11].
The serotonin1A (5-HT1A) receptor binds the neurotransmitter serotonin (5-HT) and is an important representative of the G-protein coupled receptor superfamily. This receptor is one of the best studied among the serotonin receptor subtypes and is involved in a variety of cognitive, behavioral, and developmental functions Citation[13]. The 5-HT1A receptor serves as an important target in the development of therapeutic agents for neuropsychiatric disorders such as anxiety and depression. Although pharmacological features and behavioral aspects related to the 5-HT1A receptor have been well reported, membrane organization and dynamics of the 5-HT1A receptor are relatively unexplored. We have addressed this issue using the biochemical tool of detergent insolubility in this paper. Resistance to solubilization by mild non-ionic detergents such as Triton X-100 at low temperature represents an extensively used biochemical criterion to identify, isolate and characterize certain types of membrane domains Citation[14], Citation[15]. Detergent insolubility has been increasingly used as a hallmark of the presence of ‘rafts’, a class of membrane domains enriched in sphingolipids and cholesterol Citation[6]. Several glycosylphosphoinositol-anchored (GPI-anchored) proteins, few transmembrane proteins and certain classes of G-proteins have been found to reside in detergent resistant membrane domains, popularly referred to as detergent resistant membranes Citation[15]. Early work which demonstrated the phenomenon of insolubility of membrane components in cold non-ionic detergents such as Triton X-100 Citation[14] has later been explained on the basis of phase separation in membranes Citation[5]. This is reinforced by results from model membrane studies which show that enrichment with lipids such as sphingolipids (with high melting temperature) and cholesterol serves as an important determinant for the phenomenon of detergent resistance Citation[16]. The tight acyl chain packing in cholesterol-sphingolipid rich membrane regions is thought to confer detergent resistance to membrane regions enriched in these lipids and to the proteins which reside in them.
In order to monitor membrane organization of the 5-HT1A receptor, we have previously developed a novel green fluorescent protein-based approach to directly determine detergent insolubility of membrane proteins Citation[17]. This method is based on quantitating fluorescence of the membrane protein before and after detergent treatment and is free from possible artifacts induced by antibodies in immunoblotting experiments. Using this approach, we have explored the membrane organization of the serotonin1A receptor tagged to enhanced yellow fluorescent protein (5-HT1AR-EYFP) stably expressed in CHO-K1 cells under conditions of reduced membrane cholesterol and agonist stimulation. In addition, we have addressed the relevance of detergent concentrations in detergent insolubility experiments.
Materials and methods
Materials
Gentamycin sulfate, MβCD, penicillin, serotonin, sodium bicarbonate and streptomycin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). D-MEM/F-12 (Dulbecco's Modified Eagle Medium: nutrient mixture F-12 (Ham) (1:1)), fetal calf serum, and geneticin (G 418) were from Invitrogen Life Technologies (Grand Island, NY, USA). Amplex Red cholesterol assay kit was from Molecular Probes (Eugene, OR, USA). BCA reagent kit for protein estimation was obtained from Pierce (Rockford, IL, USA). All other chemicals used were of the highest purity available. Water was purified through a Millipore (Bedford, MA, USA) Milli-Q system and used throughout. Photoetched grid coverslips were purchased from Bellco (Vineland, NJ, USA).
Construction of the vector for EYFP-tagged 5-HT1A receptor and isolation of stable transfectants
The vector coding for the human 5-HT1A receptor tagged at the C-terminal end to EYFP was used to transfect CHO-K1 (Chinese hamster ovary) cells. The construction of the vector, transfection of CHO-K1 cells and isolation of transfectants stably expressing the fusion protein were carried out as described earlier Citation[12].
Cells and cell culture
CHO-K1 cells or CHO-K1 cells stably expressing the serotonin1A receptor tagged to enhanced yellow fluorescent protein (referred to as CHO-5-HT1AR-EYFP) were used. Cells were grown in D-MEM/F-12 (1:1) supplemented with 2.4 g/l of sodium bicarbonate, 10% fetal calf serum, 60 µg/ml penicillin, 50 µg/ml streptomycin, 50 µg/ml gentamycin sulfate in a humidified atmosphere with 5% CO2 at 37°C. CHO-5-HT1AR-EYFP cells were maintained in the above-mentioned conditions with 300 µg/ml geneticin. These cells, when used for detergent extraction and fluorescence microscopy experiments, were grown in glass bottom dishes made by replacing the bottoms of 35 mm plastic tissue culture dishes with photoetched grid coverslips as described earlier Citation[17].
Extraction with Triton X-100
CHO-5-HT1AR-EYFP cells were plated in glass bottom dishes and grown in DMEM/F-12 medium for 2 days. In order to deplete cells of cholesterol, cells were incubated with 5 mM MβCD in HEPES-Hanks buffer for 30 min. In the case of agonist stimulation experiments, cells were incubated with 10 µM serotonin in HEPES-Hanks buffer for 30 min. Cells were then washed and imaged in HEPES-Hanks buffer to record the fluorescence intensity before detergent extraction. Cells were then incubated with 0.05% (w/v) cold Triton X-100 for 10 min on ice. The detergent solution was removed and cells were carefully washed in cold HEPES-Hanks buffer before imaging the same group of cells whose fluorescence intensity was recorded before detergent extraction. Control experiments where cells were processed through all steps of the extraction procedure using cold HEPES-Hanks buffer without any detergent were carried out in parallel.
Fluorescence microscopy and image analysis
CHO-5-HT1AR-EYFP cells were imaged using a Meridian Ultima 570 confocal laser scanning microscope system attached to an inverted Olympus fluorescence microscope. The same group of cells was imaged before and after detergent extraction. Optical sections of the cells were recorded using a 60×, 1.4 NA oil-immersion objective using the 514 nm line of an Ar laser at a z-slice thickness of 0.5 µm. Fluorescence emission was collected using the 505–535 nm bandpass filter. Image analysis was carried out using the Meridian DASY Master Program v4.19. Sections of the cells largely representing the cell plasma membrane were selected and projected together resulting in a single combined image of the chosen sections. Outlines of each cell (or a small group of cells) were drawn out and integrated fluorescence intensities were determined using the Meridian DASY Master program. Integrated fluorescence intensities of each cell or a group of cells before and after detergent extraction were monitored. Detergent insolubility of the receptor was estimated by determining the residual fluorescence of cells upon detergent treatment.
Estimation of cholesterol and inorganic phosphate of cell membranes
Cell membranes were prepared and the total protein content was determined using the BCA assay kit as described earlier Citation[12]. Cholesterol content of the membranes isolated from CHO-5-HT1AR-EYFP cells was estimated using the Amplex Red cholesterol assay kit as described earlier Citation[18]. Concentration of lipid phosphate of membranes was determined subsequent to total digestion by perchloric acid using Na2HPO4 as standard Citation[18]. DMPC was used as an internal standard to assess lipid digestion. Samples without perchloric acid digestion produced negligible readings.
Statistical analysis of data
Frequency distribution analysis was performed using Microcal Origin software version 5.0 (OriginLab Corp., Northampton, MA, USA). Data obtained were used to plot graphs (shown in and ) with GRAFIT program version 3.09b (Erithacus Software, Surrey, UK). Significance levels were estimated by one-way ANOVA using Microcal Origin software version 5.0 (OriginLab Corp., Northampton, MA, USA).
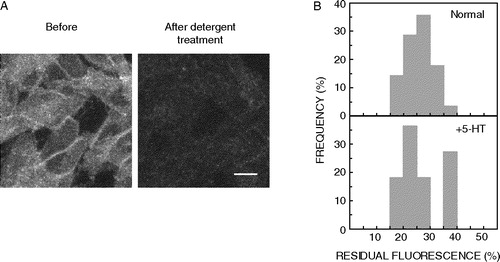
Figure 1. Effect of cholesterol depletion on detergent insolubility of 5-HT1AR-EYFP. (A) CHO-5-HT1AR-EYFP cells under normal and cholesterol depleted conditions are shown before and after treatment with 0.05% (w/v) cold Triton X-100 respectively (reproduced from Citation[17]). Cholesterol depletion was performed by treating the cells with 5 mM MβCD. Images represent combined mid-plane confocal sections of the same group of cells before and after detergent extraction. Fluorescence intensity of the same group of cells before and after detergent treatment was quantitated using the Meridian DASY Master program, and detergent insolubility of the 5-HT1A receptor was assessed by determining the residual fluorescence. Scale bar represents 10 µm. See Materials and methods for other details. (B) Frequency distribution profiles of detergent insolubility of 5-HT1AR-EYFP determined under normal and cholesterol-depleted conditions. Detergent insolubility of 5-HT1AR-EYFP was estimated by measuring the residual fluorescence following detergent extraction as described in Materials and methods. The plots shown represent 28 different data points of residual fluorescence measurements each for the normal and cholesterol-depleted conditions. The frequency of occurrence of various values has been normalized to the total number of measurements. See Materials and methods for other details.
![Figure 1. Effect of cholesterol depletion on detergent insolubility of 5-HT1AR-EYFP. (A) CHO-5-HT1AR-EYFP cells under normal and cholesterol depleted conditions are shown before and after treatment with 0.05% (w/v) cold Triton X-100 respectively (reproduced from Citation[17]). Cholesterol depletion was performed by treating the cells with 5 mM MβCD. Images represent combined mid-plane confocal sections of the same group of cells before and after detergent extraction. Fluorescence intensity of the same group of cells before and after detergent treatment was quantitated using the Meridian DASY Master program, and detergent insolubility of the 5-HT1A receptor was assessed by determining the residual fluorescence. Scale bar represents 10 µm. See Materials and methods for other details. (B) Frequency distribution profiles of detergent insolubility of 5-HT1AR-EYFP determined under normal and cholesterol-depleted conditions. Detergent insolubility of 5-HT1AR-EYFP was estimated by measuring the residual fluorescence following detergent extraction as described in Materials and methods. The plots shown represent 28 different data points of residual fluorescence measurements each for the normal and cholesterol-depleted conditions. The frequency of occurrence of various values has been normalized to the total number of measurements. See Materials and methods for other details.](/cms/asset/7b907fd7-e8c6-46f8-b439-981c5a4c3a23/imbc_a_142156_f0001_b.jpg)
Results
Monitoring detergent insolubility of the 5-HT1A receptor using a GFP fluorescence-based approach
CHO-K1 cells stably expressing the 5-HT1A receptor tagged to EYFP (enhanced yellow fluorescent protein; previously known as GFP-10C), a red-shifted emission variant of GFP, were used to assess detergent insolubility of the 5-HT1A receptor. These cells display typical plasma membrane localization of 5-HT1AR-EYFP characterized by greater fluorescence intensity at the cell periphery (see A). We have earlier shown that EYFP fusion to the 5-HT1A receptor does not affect the ligand binding properties, G-protein coupling and signaling functions of the receptor Citation[12]. CHO-K1 cells stably expressing the 5-HT1AR-EYFP therefore represent a reliable system to explore the membrane organization of the 5-HT1A receptor. In a typical experiment, a group of cells were imaged before and after treatment with the non-ionic detergent Triton X-100 at 4°C (see A). Fluorescence intensity of the same group of cells before and after detergent treatment was quantitated and detergent insolubility of the 5-HT1A receptor was assessed by determining the residual fluorescence as described in Materials and methods. This analysis showed that ∼26% of 5-HT1AR-EYFP fluorescence is retained upon extraction with 0.05% (w/v) Triton X-100. This represents the fraction of the 5-HT1A receptor that is resistant to detergent treatment under these conditions.
In order to validate this fluorescence microscopic approach toward determination of detergent insolubility of membrane components, we previously utilized specific lipid (phase-sensitive dialkylindocarbocyanine (DiI) probes) and protein markers (transferrin receptor) whose organization in membranes and ability to be extracted by cold non-ionic detergents have been well documented Citation[19]. Results obtained from these experiments showed that this method is capable of distinguishing ordered domains labeled by DiIC16 from the fluid regions of the membrane characterized by FAST DiI labeling Citation[17]. These results, along with the observation of low detergent insolubility of transferrin receptor, validated the novel observation of detergent insolubility of the 5-HT1A receptor in particular and GFP fluorescence-based approach in general Citation[17].
Since solubilization of membrane components is dependent on detergent/protein or detergent/lipid ratios Citation[20], Citation[21], it is possible that the fraction of any receptor insoluble in a given detergent could be dependent on the concentration or amounts of the detergent used. This aspect, which could mislead investigators on the detergent insolubility status of any membrane protein, adds to concerns on employing detergent insolubility to characterize membrane domains Citation[22]. Therefore, in the case of experiments involving detergent treatment to assess insolubility of membrane components, it is prudent to consider the relative proportions of detergent and protein used while estimating detergent insolubility Citation[18], Citation[22]. This is particularly important in the case of microscopy-based detergent extraction experiments where the ability to visualize effects of detergent treatment may necessitate one to use a lower concentration of detergent. Keeping this in mind, we used different concentrations of Triton X-100 to assess detergent insolubility of the 5-HT1AR-EYFP. We extracted cells with varying concentrations of Triton X-100 and imaged them to quantitate the residual fluorescence to determine the fraction of detergent insoluble 5-HT1A receptor under these conditions (see ). These results suggest a reduction in the insoluble fraction of the 5-HT1AR-EYFP from ∼26% to ∼14%, upon increasing the detergent concentration from 0.05 to 0.075% (w/v). However, upon further increase to 0.1% Triton X-100, there is no significant change in this fraction. Thus, we observed ∼14 and ∼13% of residual fluorescence when cells were treated with 0.075 and 0.1% (w/v) Triton X-100 respectively (). These results demonstrate that the fraction of insoluble receptor would depend on the ratio of detergent to protein used in a given experiment as is known from membrane protein solubilization experiments. It is important to note here that we observed qualitatively similar effects whether lower concentrations such as 0.05 Citation[17] or higher concentrations such as 0.1% (data not shown) Triton X-100 were used to assess detergent insolubility of DiI probes. In other words, differential solubilization of DiIC16 and FAST DiI was observed both at low and high concentrations of detergent used which further confirms and validates the present method for estimating detergent insolubility of 5-HT1AR-EYFP. Based on these observations, we conclude that a small fraction of 5-HT1AR-EYFP is insoluble in cold Triton X-100 in the concentration range of Triton X-100 used here.
Table I. Detergent insolubility of 5-HT1AR-EYFP at various concentrations of Triton X-100a.
Effect of cholesterol depletion on detergent insolubility of 5-HT1AR-EYFP
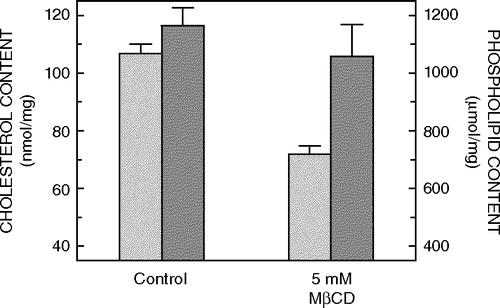
Cholesterol is often found enriched in detergent-insoluble fractions isolated from many natural sources Citation[14]. It is considered an essential constituent of such fractions and studies carried out in model membrane systems have addressed the specific molecular requirements for sterols in detergent resistance Citation[16], Citation[23]. To explore the role of cholesterol in detergent insolubility of 5-HT1AR-EYFP, cholesterol depletion was carried out using methyl-β-cyclodextrin (MβCD), and detergent insolubility was tested. CHO-5-HT1AR-EYFP cells were treated with 5 mM MβCD, which selectively extracts cholesterol from membranes by including it in a central nonpolar cavity. As shown in , this treatment resulted in ∼30% reduction in membrane cholesterol without any significant change in the total phospholipid content. No significant change in membrane distribution of 5-HT1AR-EYFP was observed upon depletion of membrane cholesterol (see A). CHO-5-HT1AR-EYFP cells thus depleted of cholesterol were extracted with cold Triton X-100, and the fluorescence from the same group of cells was monitored before and after treatment with 0.05% (w/v) Triton X-100 (A). Analysis of residual EYFP fluorescence showed that, on an average, ∼34% of 5-HT1AR-EYFP fluorescence is retained upon detergent extraction of cholesterol-depleted CHO-5-HT1AR-EYFP cells.
Figure 2. Effect of treatment of CHO-5-HT1AR-EYFP cells in culture with 5 mM MβCD on the lipid composition of membranes. Cholesterol (light gray bars) and phospholipid (dark gray bars) contents were assayed as described in Materials and methods. The lipid composition of control membranes isolated from CHO-5-HT1AR-EYFP cells, which have not been treated with MβCD, is shown for comparison. Values have been normalized with respect to the total protein content. Data represent the means±SE of at least three independent experiments. See Materials and methods for other details.
Independent sets of data obtained from these experiments were analyzed as a histogram. Frequency distribution profiles for various measurements of residual fluorescence for normal and cholesterol-depleted cells are shown in B. The frequency distribution profile for detergent insolubility in the case of cholesterol-depleted cells appears to be broader and characterized by the presence of data points with higher residual fluorescence values compared to the distribution observed for normal cells. As a result, there is a small but significant (p<0.05) increase in the mean residual fluorescence in the case of cells depleted of cholesterol. Thus, while normal cells display ∼26%, cholesterol-depleted cells exhibit ∼34% of residual fluorescence upon detergent extraction.
As a control, we performed experiments in which either normal or cholesterol-depleted cells were carried through the incubation and washing steps using cold HEPES-Hanks buffer without any detergent. No significant change in fluorescence intensity was observed upon quantitation of fluorescence intensity in these control cells (data not shown).
Detergent insolubility of 5-HT1AR-EYFP upon stimulation by serotonin
Agonist-induced translocation of receptors into or out of membrane domains has been described as a mode of modulating receptor–G-protein-effector interaction, and thereby membrane signaling, of several G-protein coupled receptors Citation[24]. Changes in the membrane organization of the receptor under conditions of stimulation have often been examined by the biochemical phenomenon of detergent insolubility. We monitored detergent insolubility of 5-HT1AR-EYFP upon stimulating the CHO-5-HT1AR-EYFP cells with the endogenous ligand serotonin (5-HT). A shows images of cells stimulated with 10 µM serotonin before and after treatment with Triton X-100. The fluorescence of the same group of cells was monitored before and after detergent extraction, and the data were analyzed as described above. Frequency distribution profiles for detergent insolubility of 5-HT1AR-EYFP under conditions of serotonin-stimulation are shown in B along with the corresponding distribution profile for normal (unstimulated) cells for comparison. CHO-5-HT1AR-EYFP cells display ∼27% of residual fluorescence in the presence of 5-HT. Statistical analysis indicates that there is no significant difference in the detergent insolubility of 5-HT1AR-EYFP when cells were stimulated with 5-HT (p<0.05). These results suggest that there is no significant alteration in the membrane organization of 5-HT1AR-EYFP as apparent from the lack of any appreciable difference in the extent of detergent insolubility of 5-HT1AR-EYFP upon stimulation by serotonin.
Figure 3. Detergent insolubility of 5-HT1AR-EYFP upon stimulation by 10 µM serotonin. (A) CHO-5-HT1AR-EYFP cells were incubated with the endogenous ligand 5-HT, followed by treatment with 0.05% (w/v) cold Triton X-100. Cells were imaged as described in Materials and methods. The images represent combined mid-plane confocal sections of the same group of cells before and after detergent extraction. Scale bar represents 10 µm. See Materials and methods for other details. (B) Frequency distribution profiles of detergent insolubility of 5-HT1AR-EYFP under normal and ligand stimulation (+5-HT) conditions are shown. Detergent insolubility of 5-HT1AR-EYFP was estimated by measuring the residual fluorescence following detergent extraction as described in Materials and methods. The plots shown represent 14 data points of residual fluorescence measurements in the case of serotonin stimulation, and 28 data points in the case of unstimulated (normal) cells. The frequency of occurrence of various values has been normalized to the total number of measurements made. See Materials and methods for other details.
Discussion
The concept of organization of membranes into domains is being increasingly appreciated due to its possible implications on cellular functions Citation[6–8]. Insolubility of membrane components in non-ionic detergents such as Triton X-100 has been a widely utilized biochemical tool to identify and characterize membrane domains Citation[14], Citation[15]. This is primarily due to the experimental ease and convenience of the method over other approaches involving advanced microscopic and imaging techniques or sensitive fluorescence-based approaches. However, the phenomenon of detergent insolubility is often complicated by concerns over the use of a membrane-perturbing agent such as Triton X-100 to understand membrane organization Citation[22], Citation[25]. Nevertheless, detergent insolubility continues to be an important and useful tool to study membrane domains. Information obtained from this extensively used biochemical approach can often form the basis for a more detailed analysis of membrane domains. We have earlier shown using a GFP fluorescence-based method that a small fraction of the 5-HT1A receptor tagged to EYFP is insoluble in Triton X-100 Citation[17]. In order to gain further insight into the membrane organization of the 5-HT1A receptor and its possible functional consequences, we have monitored detergent insolubility of 5-HT1AR-EYFP at different concentrations of Triton X-100, and under conditions of reduced membrane cholesterol levels and agonist stimulation. Our results demonstrate that the fraction of insoluble receptor would depend on the ratio of detergent to protein used in a given condition. Based on control experiments involving DiI probes, we conclude that a small fraction of the receptor is insoluble irrespective of the concentration of the detergent used.
Cholesterol is an important component of eukaryotic cell membranes and is often found distributed nonrandomly in biological and model membranes Citation[23], Citation[26], Citation[27]. The tight packing of membrane regions enriched in lipids such as cholesterol and sphingolipids (with high melting temperature) is thought to confer detergent resistance of these regions and to the proteins which reside in them Citation[7], Citation[16], Citation[23]. According to this model, depletion of cholesterol is believed to cause disruption of such domains resulting in an increased extraction of proteins residing in the domain Citation[28]. Several examples are indeed known where decreased detergent insolubility of membrane proteins has been observed upon depletion of membrane cholesterol Citation[29], Citation[30]. In addition, the lateral mobility of certain proteins generally found in detergent resistant membrane domains has been reported to be increased upon lowering membrane cholesterol content Citation[31], Citation[32], further supporting this model. Interestingly, in contrast to these observations, there are reports indicating a decrease in lateral mobility of membrane components upon lowering membrane cholesterol levels Citation[33], Citation[34]. This is consistent with the observation that cholesterol depletion from lipid vesicles originally present in a uniform liquid phase leads to separation of phases as monitored by the distribution of fluorescent lipid probes Citation[35]. More importantly, similar observations were reported by Hao et al. in several mammalian cell types Citation[33]. These authors showed that while the cell surface was uniformly labeled under normal conditions by lipid probes with preferential phase partitioning properties, reduction in membrane cholesterol content induced formation of visible micrometer-scale domains on the cell surface Citation[33]. These results, along with evidence from model membrane studies Citation[35], have given rise to the proposal that cholesterol, while maintaining domain organization in membranes, could also be involved in reducing immiscibility of domains. Hence, reduction in cholesterol levels may induce domain segregation Citation[7].
Our results indicate a small but significant increase in the detergent insolubility of 5-HT1AR-EYFP upon depletion of membrane cholesterol (B). We interpret these results on the basis of the above model of formation of large sized ordered domains upon cholesterol depletion. In such a scenario, cholesterol depletion could lead to segregation of ordered domains on the cell surface, into which a slightly greater fraction of 5-HT1AR-EYFP may be included, resulting in an increase in the relative detergent insoluble fraction of the 5-HT1AR-EYFP. shows a schematic representation of such a possibility. It should be mentioned here that the requirement of cholesterol for detergent insolubility of membrane components has recently been critically assessed suggesting that the presence of long chain saturated lipids (lipids that pack into ordered domains) and not cholesterol, contributes to detergent insolubility Citation[18]. In addition, there have been reports indicating that detergent insolubility is unaltered upon cholesterol depletion Citation[36], Citation[37], and diffusion properties of membrane components are not correlated with their DRM localization Citation[38]. Thus, it is possible that cholesterol depletion leads to differential effects on detergent insolubility of membrane components Citation[33].
Figure 4. Schematic representation of possible membrane reorganization (shown as top view) induced by cholesterol depletion and its implications on detergent insolubility of 5-HT1AR-EYFP in membranes. Membrane organization under normal (A) and cholesterol-depleted (B) conditions is shown. The EYFP-tagged serotonin1A receptor (5-HT1AR-EYFP) is shown as filled squares. Current understanding of membrane organization involves heterogeneities (domains/rafts) on the cell surface (shown as circles), with boundaries depicted as being discontinuous due to the tendency of cholesterol to form intermixed domains Citation[7] (shown in panel A). As a result of this, while the presence of such domains could be inferred through the phenomenon of detergent resistance, they may not be detected as separate entities through light microscopy. Due to the role of cholesterol in domain miscibility, lowering its levels from membranes could lead to segregation and coalescence of microdomains into large micrometer-scale ordered domains Citation[33] (shown in panel B). The eventual distribution of 5-HT1AR-EYFP in such domains could determine the fraction of detergent insoluble receptor. Since our results suggest a small increase in detergent insoluble receptor upon cholesterol depletion, it could be speculated that a greater number of receptors may be included into the large micrometer-scale ordered domains (shown in panel B).
![Figure 4. Schematic representation of possible membrane reorganization (shown as top view) induced by cholesterol depletion and its implications on detergent insolubility of 5-HT1AR-EYFP in membranes. Membrane organization under normal (A) and cholesterol-depleted (B) conditions is shown. The EYFP-tagged serotonin1A receptor (5-HT1AR-EYFP) is shown as filled squares. Current understanding of membrane organization involves heterogeneities (domains/rafts) on the cell surface (shown as circles), with boundaries depicted as being discontinuous due to the tendency of cholesterol to form intermixed domains Citation[7] (shown in panel A). As a result of this, while the presence of such domains could be inferred through the phenomenon of detergent resistance, they may not be detected as separate entities through light microscopy. Due to the role of cholesterol in domain miscibility, lowering its levels from membranes could lead to segregation and coalescence of microdomains into large micrometer-scale ordered domains Citation[33] (shown in panel B). The eventual distribution of 5-HT1AR-EYFP in such domains could determine the fraction of detergent insoluble receptor. Since our results suggest a small increase in detergent insoluble receptor upon cholesterol depletion, it could be speculated that a greater number of receptors may be included into the large micrometer-scale ordered domains (shown in panel B).](/cms/asset/ddeb390b-a094-47cd-96e6-ceb3a94f03d3/imbc_a_142156_f0004_b.jpg)
It has been proposed that G-protein coupled receptors are not uniformly present on the plasma membrane but are concentrated in specific membrane microdomains, some of which are presumably enriched in cholesterol Citation[10]. For example, coupling efficacy of β1 and β2-adrenergic receptors (β1AR and β2AR) and prostaglandin E2 receptors to adenylate cyclase (AC6) correlates with their colocalization or lack of it with AC6 in caveolae Citation[39]. Upon exposure to agonist, β2AR, but not β1AR, is found to translocate out of caveolin-rich fractions Citation[24]. Such an agonist-dependent spatial segregation of the receptor and effector on the cell surface could explain lower efficacy of β2AR coupling to its effector AC6 compared to β1AR Citation[39]. Similar agonist dependent association of receptors and cognate G-proteins has been shown in the case of bradykinin receptors Citation[40]. In the context of the role of membrane organization in the function of GPCRs, we examined membrane organization of the 5-HT1A receptor upon agonist stimulation. Our results on the detergent insolubility of 5-HT1AR-EYFP in the presence of its endogenous ligand, serotonin (5-HT), do not point to any specific change in the membrane organization of the 5-HT1A receptor as detected by the phenomenon of detergent insolubility (B). In addition, stimulation by 5-HT has not been found to result in any significant difference in the fluorescence distribution of 5-HT1AR-EYFP Citation[12]. We have previously shown using fluorescence recovery after photobleaching (FRAP) that the lateral mobility of 5-HT1AR-EYFP shows a significant increase in the presence of serotonin Citation[12]. Based on all these results, it appears that while the membrane dynamics (diffusion) of the 5-HT1A receptor could be modulated in the presence of serotonin, fluorescence distribution and detergent insolubility measurements do not indicate any apparent cell surface reorganization of 5-HT1AR-EYFP when stimulated by serotonin.
In summary, we report here the detergent insolubility of the 5-HT1A receptor at various concentrations of detergent, and under conditions of cholesterol depletion and ligand stimulation. Our results provide new insights into the membrane organization of the 5-HT1A receptor and are relevant in the analysis of membrane organization of other G-protein coupled receptors.
This work was supported by Research Grants from Life Sciences Research Board, and Council of Scientific and Industrial Research, Government of India, to AC, and CAEN grant from the International Society for Neurochemistry to SK. AC is an Honorary Faculty Member of the Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore (India). SK thanks the Council of Scientific and Industrial Research for the award of a Senior Research Fellowship. We gratefully acknowledge Thomas J. Pucadyil for the data presented in and for helpful discussions. We thank Dr Sadashiva Karnik for the kind gift of the 5-HT1AR-EYFP construct and Ms Nandini Rangaraj for help with confocal microscopy. We thank members of our laboratory for critically reading the manuscript.
References
- Perez DM. The evolutionarily triumphant G-protein-coupled receptor. Mol Pharmacol 2003; 63: 1202–1205
- Karnik SS, Gogonea S, Patil S, Saad Y, Takezako T. Activation of G-protein-coupled receptors: A common molecular mechanism. Trends Endocrinol Metab 2003; 14: 431–437
- Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 2003; 1612: 1–40
- Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim Biophys Acta 2004; 1666: 2–18
- Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 1998; 164: 103–114
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Mukherjee S, Maxfield FR. Membrane domains. Annu Rev Cell Dev Biol 2004; 20: 839–866
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1: 31–39
- van der Goot FG, Harder T. Raft membrane domains: From a liquid-ordered membrane phase to a site of pathogen attack. Semin Immunol 2001; 13: 89–97
- Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: Implications for molecular pharmacology. Br J Pharmacol 2004; 143: 235–245
- Hur E-M, Kim KT. G protein-coupled receptor signalling and cross-talk: Achieving rapidity and specificity. Cell Signal 2002; 14: 397–405
- Pucadyil TJ, Kalipatnapu S, Harikumar KG, Rangaraj N, Karnik SS, Chattopadhyay A. G-protein-dependent cell surface dynamics of the human serotonin1A receptor tagged to yellow fluorescent protein. Biochemistry 2004; 43: 15852–15862
- Pucadyil TJ, Kalipatnapu S, Chattopadhyay A. The serotonin1A receptor: A representative member of the serotonin receptor family. Cell Mol Neurobiol 2005; 25: 553–580
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992; 68: 533–544
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol Membr Biol 1999; 16: 145–156
- Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem 1998; 273: 1150–1157
- Kalipatnapu S, Chattopadhyay A. A GFP fluorescence-based approach to determine detergent insolubility of the human serotonin1A receptor. FEBS Lett 2004; 576: 455–460
- Pucadyil TJ, Chattopadhyay A. Exploring detergent insolubility in bovine hippocampal membranes: A critical assessment of the requirement for cholesterol. Biochim Biophys Acta 2004; 1661: 9–17
- Mukherjee S, Soe TT, Maxfield FR. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J Cell Biol 1998; 144: 1271–1284
- Kalipatnapu S, Chattopadhyay A. Membrane protein solubilization: recent advances and challenges in solubilization of serotonin1A receptors. IUBMB Life 2005; 57: 505–512
- Arni S, Keilbaugh SA, Ostermeyer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem 1998; 273: 28478–28485
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct 2003; 32: 257–283
- Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 2000; 39: 843–849
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. J Biol Chem 2000; 275: 41447–41457
- Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J 2002; 83: 2693–2701
- Liscum L, Underwood KW. Intracellular cholesterol transport and compartmentation. J Biol Chem 1995; 270: 15443–15446
- Simons K, Ikonen E. How cells handle cholesterol. Science 2000; 290: 1721–1725
- Edidin M. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol 2001; 11: 492–496
- Field KA, Holowka D, Baird B. FcεRI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci USA 1995; 92: 9201–9205
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 1998; 141: 929–942
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol 2000; 148: 997–1008
- Shvartsman DE, Kotler M, Tall RD, Roth MG, Henis YI. Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts. J Cell Biol 2003; 163: 879–888
- Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci USA 2001; 98: 13072–13077
- Vrljic M, Nishimura SY, Moerner WE, McConnell HM. Cholesterol depletion suppresses the translational diffusion of class II major histocompatibility complex proteins in the plasma membrane. Biophys J 2005; 88: 334–347
- Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J 2003; 85: 3074–3083
- Rivas MG, Gennaro AM. Detergent resistant domains in erythrocyte membranes survive after cell cholesterol depletion: an EPR spin label study. Chem Phys Lipids 2003; 122: 165–169
- Romanenko V, Fang Y, Travis A, Levitan I. Partitioning of Kir2.1 channels into Triton-insoluble membrane domains is independent of the level of cellular cholesterol. J Gen Physiol 2004; 124: 15a
- Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol 2004; 165: 735–746
- Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 2001; 276: 42063–42069
- de Weerd WFC, Leeb-Lundberg LMF. Bradykinin sequesters B2 bradykinin receptors and the receptor-coupled Gα subunits Gαq and Gαi in caveolae in DDT1 MF-2 smooth muscle cells. J Biol Chem 1997; 272: 17858–17866