Abstract
Differential solubilization of membrane components by cold 1% Triton X-100 extraction is common practice in cell biology and membrane research, used to define components of, or localization within membrane domains called lipid rafts. In this study, extraction of biological membranes was continuously monitored in single cells by confocal microscopy. The distributions of fluorescently-tagged proteins that label raft and non-raft membranes, cytosolic and cytoskeletal proteins were continuously monitored upon addition of the detergent. Membranes containing the non-raft membrane protein VSVG-GFP were immediately extracted from the plasma membrane, whereas raft-membrane proteins were predominantly resistant to the detergent. The morphological characteristics of differential membrane solubilization consisted of the formation of pores that expand and percolate as the detergent-mediated solubilization proceeds. Pore expansion and percolation was much slower and more restricted in non-polarized MDCK cells than in COS-7 cells. Heterologous overexpression in COS-7 cells of the fluorescently-tagged human MAL, a tetra-spanning, lipid-raft-associated protein, significantly slowed and limited membrane pore expansion and percolation. Extensive percolation resulting in large holes in the membrane was observed for the raft-associated, GPI-GFP-labeled membranes in COS-7 cells. Quantitative analysis carried out using pixel intensity variance as an indicator of membrane pore expansion demonstrated that the MAL protein is capable of modifying the plasma membrane, thereby increasing its resistance to detergent-induced pore formation.
| Abbreviations | ||
| detergent-resistant membrane | = | DRM |
| pixel fluorescence intensity variance | = | PFIVar |
| Madin Darby Canine Kidney | = | MDCK |
| fluorescent protein | = | FP |
| Triton X-100 | = | TX-100 |
Introduction
A central hypothesis in the field of intracellular trafficking is that membrane domains serve as the platform for signaling, as well as transport and sorting processes (Ikonen [Citation2001]). Sphingolipid rafts are the most studied case of membrane domains involved in targeting proteins to the apical membrane in polarized epithelial cells (Brown & London [Citation1998]). The predominant method for defining the content of these domains has been differential solubilization by cold 1% Triton X-100 (TX-100) extraction (Brown & London [Citation1998]). This method has been used extensively in countless systems to determine whether a protein is associated to a raft or not. Numerous other studies, involving biophysical and fluorescence-microscopic methods, have either provided new information on the nature of lipid rafts (Sharma et al. [Citation2004]), or otherwise questioned their existence (Kenworthy & Edidin [Citation1998]), as well as their relevance to specific processes (Glebov & Nichols [Citation2004]). The interpretation that the insolubility of a membrane protein indicates that it resides within a lipid-raft domain is currently under strong criticism (Munro [Citation2003]). A key argument against cold 1% TX-100 extraction is that the end result of differential solubilization is an artificial separation of two phases that did not exist prior to introduction of the detergent (Heerklotz [Citation2002]). However, one of the initial observations substantiating the existence of lipid rafts was the coupling between increased detergent resistance and anterograde trafficking of glycosylphosphatidylinositol (GPI)-anchored proteins (Brown & Rose [Citation1992]). An alternative, more cautious view, is that the resistance to cold 1% TX-100 extraction corresponds to a specific feature of a protein related to its capacity to interact with a distinct lipid or protein environment (Munro [Citation2003]) and form a stable or dynamic domain at a distinct time and location (Mayor & Rao [Citation2004]).
MAL, also known as VIP17 (Cheong et al. [Citation1999]), is a lipid-raft-associated tetra-spanning protein expressed in epithelial cells, in activated T cells and in myelinating oligodendrocytes (Alonso & Weissman [Citation1987]; Puertollano et al. [Citation1997]). Furthermore, MAL is an essential part of the apical sorting machinery (Martin-Belmonte et al. [Citation2000]), as shown by depletion with antisense RNA. These data, together with the fact that MAL is a relatively small (17 kD), highly hydrophobic protein that is mostly embedded within the membrane, led us to hypothesize that its function is comprised of membrane-domain formation and maintenance via interaction with lipids, thereby affecting the local biophysical state of the membrane.
In this study, we analyzed the consequences of heterologous expression of fluorescently-tagged MAL on the morphology of detergent-resistant cellular membranes during the course of the solubilization process. Real-time analysis of cold TX-100 extraction was performed on cells expressing fluorescently-tagged cytosolic and cytoskeletal membrane proteins, in order to visualize with high spatial and temporal resolution, the events that produce the separation of raft and non-raft membranes. We found that TX-100 instantly generates submicroscopic pores large enough to allow outflow of a cytosolic fluorescent protein. These pores rapidly percolate and fuse with each other to form large holes in the plasma membrane (PM). Interestingly, heterologous overexpression of MAL slowed and restricted pore expansion and percolation. Moreover, in MDCK cells, where MAL is expressed endogenously, pore expansion and percolation were considerably restricted for overexpressed GPI-FP relative to COS-7 cells.
Materials and methods
Reagents and constructs
Human VIP17/MAL was isolated from a human brain cDNA library by PCR with the primers 5′ TCCCCGCGGTTATGAAGACTTC3′ and 5′CCGCTCGAGGTATGGCCCCCG3′. The isolated cDNA was sequenced and inserted into the XhoI and SacII sites in ECFP-C1, EYFP-C1 (Clontech, Palo Alto, CA) and diHcRED-C1 generated by Ellenberg's laboratory (Gerlich et al. [Citation2003]), consisting of two dimerizing diHcRED molecules in tandem. GPI-YFP (Nichols et al. [Citation2001]) and VSVGtsO45-YFP (Ward et al. [Citation2001]) were prepared as described elsewhere. Actin-EGFP and tubulin-EGFP were from Clontech.
Cell culture and transient transfection
COS-7 (African green monkey) and MDCK cells were grown at 37C in a humidified atmosphere with 5% CO2. Cell cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% V/V bovine serum (FBS) and penicillin/streptomycin (Biological Industries, Bet-Haemek, Israel). For microscopy, cells were sub-cultured in glass coverslip chambers (Nalgene Nunc Int. Rochester, NY, USA). Cells were grown to 20–30% confluence and then transiently transfected with 1.5 µg DNA/chamber using FuGENE-6 reagent (Roche Applied Science, Mannheim, Germany). CLSM images were obtained ∼15–18 h after transfection.
Confocal laser scanning microscopy
Cells were imaged in DMEM without phenol-red but with supplements, including 20 mM HEPES, pH 7.4. Transfection and imaging were carried out in Labtek chambers (Nunc). A Zeiss LSM PASCAL equipped with an Axiovert 200 inverted microscope, and Ar 458 nm, 488 nm, 514 nm and HeNe 543 lasers were used for ECFP, EGFP, EYFP, and the tandem dimer DiHcRed, respectively. Dual-color imaging of cells co-expressing ECFP- and DiHcRED-tagged proteins was carried out using simultaneous scanning with Ar 458 nm and HeNe 543 nm lasers. Emissions were separated by NFT filter with a 515-nm cutoff: CFP signal was detected after a 475–525 nm bandpass filter, and DiHcRED signal was detected after a 560-nm longpass filter. The confocal and time-lapse images were captured using a 63× NA 1.4 objective. Image capture was carried out using the standard time-series option (Zeiss), in the presence of an electronic temperature-controlled air-stream incubator. The entire microscope room was cooled to 15–18°C using an air conditioning system. Temperature on the microscope stage was monitored using electronic temperature-controlled air-stream incubator. Images and movies were generated and analyzed using the Zeiss LSM software and NIH Image and ImageJ software (W. Rasband, NIH, Bethesda, MD).
Western-blot analysis
COS-7 cells expressing VSVG-FP, GPI-FP or MAL-FP were lysed with 1% (V/V) Triton X-100 (TX-100) in PBS. After 15-min incubation, either on ice or at 17°C, the supernatant was collected, the plates were washed with cold PBS and the raft-containing membranes were scraped out of the plates with 1% TX-100 in PBS. Both supernatant and membrane fractions were boiled for 1 min with sample buffer, separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). Membranes were blocked and probed for GFP expression (monoclonals 7.1 + 13.1, Roche). The secondary peroxidase-conjugated antibody was sheep anti-mouse IgG (NA 931, Amersham Pharmacia Biotech). Detection was carried out using an ECL SuperSignal kit (Pierce).
Results
Real-time image analysis of differential extraction
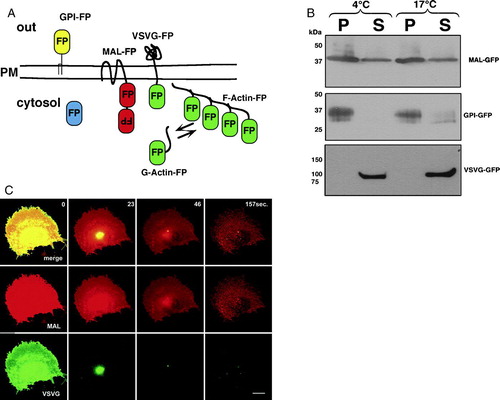
To examine the differential extraction process in real time, we used cells expressing various types of fluorescently-tagged proteins as illustrated in A. These included the lipid-raft-associated proteins GPI-FP and MAL-FP, the non-raft VSVG-FP, and cytoskeletal (actin and tubulin) and cytosolic-soluble FPs. Because the solubilization was carried out at temperatures between 17 and 19°C, and not at 4°C, we analyzed the solubilization pattern of MAL-FP, GPI-FP and VSVG-FP expressed in COS-7 cells at both temperatures using western-blot analysis (B). As shown, VSVG-FP was completely dissolved in TX-100 at both temperatures, whereas GPI-FP and MAL-FP were mostly detergent-resistant. Note that the soluble fraction of MAL could be attributed to overexpressed MAL-FP in intracellular membranes (data not shown). C demonstrates a typical experiment in which real-time analysis of differential extraction was performed on membranes of a COS-7 cell co-expressing the raft marker MAL-DiHcRED and the non-raft protein VSVG-GFP. The VSVG-GFP signal was rapidly eliminated, leaving the membranes labeled with MAL.
Figure 1. Western-blot and real-time analysis of differential TX-100 extraction. (A) Schematic representation of the fluorescent protein (FP)-tagged proteins that were used throughout this study: the raft-associated membrane proteins GPI-FP and MAL-FP, the non-raft-associated membrane protein VSVG-FP, cytosolic FP, and the cytoskeletal protein Actin-FP. The colors designate the cyan, yellow and green fluorescent variants of FP and the tandem dimer red fluorescent protein (DiHcRED. (B) Western-blot analysis with anti-GFP antibody of TX-100 insoluble (P) and soluble (S) membranes from cells expressing MAL-CFP, GPI-GFP or VSVG-GFP carried out at 4°C and 17°C. (C) Representative images (experiment repeated 7 times) from a time-lapse sequence of a single COS-7 cell co-expressing the proteins MAL-DiHcRED (middle panel, red) and VSVG-GFP (lower panel, green) after addition of 1% (V/V) TX-100 in PBS (see also QuickTime movie S1C in the online supplementary information). Bars = 20 µm
Characterization of detergent-mediated membrane pores
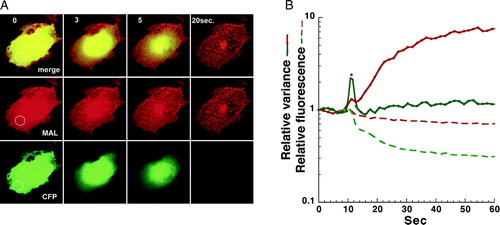
As the solubilization process proceeded, dark patches began to appear on the membrane. These patches were essentially pores in the membrane, as demonstrated in . Membrane perforation was detected by the efflux of cytosolic CFP co-expressed with the DiHcRED-tagged detergent-resistant MAL (). Membrane pore expansion was detected by the increase in PFIVar values of the DiHcRED-tagged MAL in the region of interest (ROI) in A. By comparison, the variance of pixel fluorescence intensity (PFIVar) values of the CFP remained low during the efflux of CFP. The fluorescence intensity detected the efflux of the cytosolic CFP and some solubilization of MAL, the latter probably localized intracellularly due to overexpression (see also Supplementary in online supplementary information).
Figure 2. Dual-color analysis to visualize cytosol efflux during extraction. (A) Images of a representative COS-7 (experiment repeated 9 times) cell co-expressing the raft proteins MAL-DiHcRED (red) and cytosolic-soluble CFP (green) after addition of 1% (V/V) TX-100 in PBS at 17°C (experiment repeated 7 times). Bar = 20 µm (see also QuickTime movie S3A in the online supplementary information). (B) Analysis on a semi-logarithmic scale of the fluorescence intensity (dashed lines) and variance (continuous lines) of the fluorescent intensities of MAL-DiHcRED (red) and the cytosolic CFP (green) in the region marked off in A.
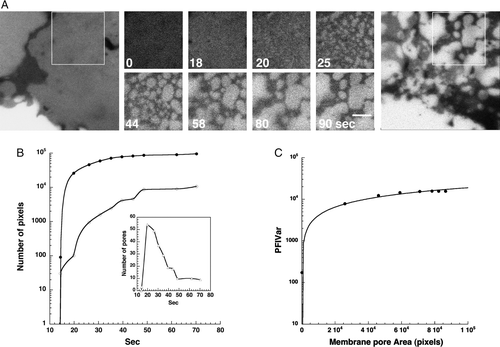
Based on analysis of 20 separate experiments, each carried out on a single cell expressing GPI-GFP at high spatial and temporal resolution, pores were initially very small, but they rapidly expanded and percolated until they reached an apparent steady state, within 1–2 min (A). Large pores in detergent-resistant cellular membranes, the end result of this process, have been previously reported (Mayor & Maxfield [Citation1995]). B analyzes the time-dependent change in the number of pores, as well as the total and average changes in pore area. The increase in average pore area and the decrease in their number are indicative of pores fusing with each other (percolation). To obtain a straightforward, quantitative readout of the increase in pore size, we utilized the time-dependent change in the PFIVar. The proportionality of directly measured pore area to PFIVar is described in C.
Figure 3. Quantitative analysis of membrane pore expansion and percolation during TX-100 extraction. (A) Images of a region of interest of the membrane of a typical COS-7 cell expressing the raft-associated protein GPI-GFP after addition of 1% (V/V) TX-100 in PBS at 17°C. Bar = 2 µm (see also QuickTime movie S2A in the online supplementary information). (B) Analysis on a semi-logarithmic plot of the changes in total and average pore areas as well as number of pores during differential extraction. Analysis was carried out on the region of interest marked in A. (C) Analysis on a logarithmic scale of the proportionality of variance of pixel fluorescence intensity with directly measured pore area based on the data from B.
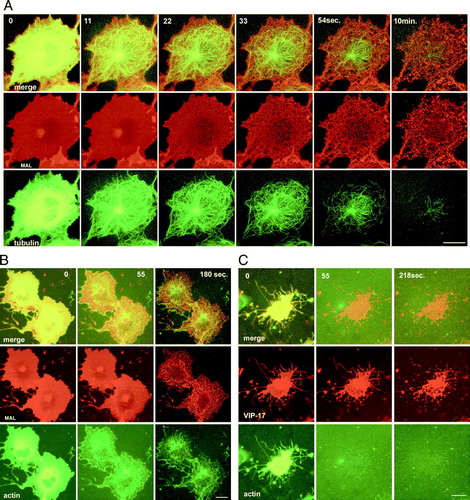
Together, these data demonstrate that TX-100 generates submicroscopic pores in the membrane that expand, presumably as the solubilization proceeds, due to the influx of partitioning detergent into the membrane. We next analyzed whether cytoskeletal elements remaining after the extraction play a role in holding the detergent-resistant membranes (DRMs) in place. In cells co-expressing tubulin-GFP and MAL-DiHcRED, polymerized microtubules (MTs) disintegrated and disappeared during the extraction process (Supplementary A and QuickTime movie S6A in the online supplementary information). However, the actin cytoskeleton remained at least partially intact, suggesting that it might be holding the DRMs bound to the substrate. Nevertheless, the remaining actin fibers only moderately co-localized with the DRMs. To further test whether actin filaments play a role in supporting the DRMs, cells were pretreated with latrunculin B to induce actin depolymerization. The DRM morphology did not change, and remained within the general cell outline that had rounded up due to the lack of actin filaments. Interestingly, in both cases, upon perforation of the PM and leakage of the tubulin or actin monomers, the microtubules and the actin fibers could be clearly visualized prior to their disintegration. As in Supplementary in the online supplementary material, the early elimination of soluble cytoskeletal proteins indicated that PM perforation by submicroscopic pores constitutes the initial stage of the solubilization process.
We next compared the rate and extent of pore expansion and percolation during differential extraction of the membrane from two different cell lines. As opposed to COS-7 cells, MDCK cells preserve the capacity to form polarized monolayers with defined apical and basolateral membrane domains. Formation and maintenance of the apical membrane involve lipid-raft-mediated traffic mechanisms. The apical membrane is thus enriched with cholesterol, sphingomyelin, glycosphingolipids and numerous proteins (van Meer & Simons [Citation1986]).
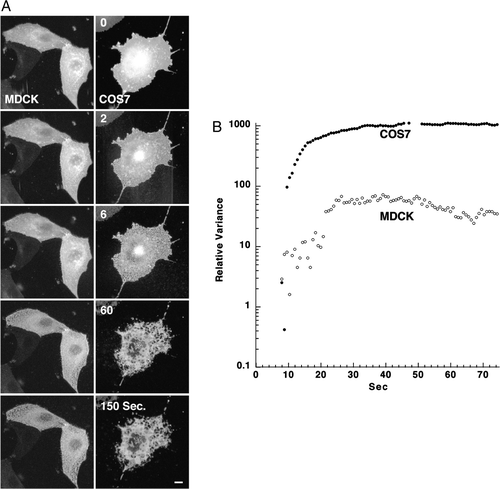
In A, a typical differential extraction is illustrated, carried out on COS-7 and non-polarized MDCK cells expressing GPI-YFP. The extent of pore expansion was compared using PFIVar analysis (B). demonstrates that non-polarized MDCK cell membranes are more resistant to TX-100-mediated pore expansion and percolation. The final PFIVar values were 50-fold higher than those of intact membranes for non-polarized MDCK, compared to the 900-fold difference found for COS-7 cells. We hypothesized that this resistance is related to the fact that MDCK membranes are enriched in lipid-raft-associated lipids and proteins, which are sorted to and comprise the apical membrane domain when a polarized monolayer is formed.
Figure 4. Analysis of membrane pore expansion in non-polarized MDCK and COS-7 cells. (A) Representative time-lapse images of non-polarized MDCK (left panel) and COS-7 (right panel) cells expressing GPI-GFP after addition of 1% (V/V) TX-100 in PBS at 17°C (experiment repeated 7 times for each cell type). Bar = 10 µM (see also QuickTime movie S4A in the online supplementary information). (B) Quantitative analysis on a semi-logarithmic scale of time-dependent membrane pore expansion of a single typical non-polarized MDCK (empty dots) and COS-7 (filled dots) cells using PFIVar.
The effect of MAL on the extent of pore expansion
Next, we analyzed the effect of introducing a single protein on the process of TX-100-mediated pore expansion and percolation. MAL is a tetra-spanning, small (17 kD), highly hydrophobic protein that is involved in lipid-raft-associated apical sorting in epithelial cells. Additionally, MAL is exclusively expressed in epithelial cells and in other cells with specialized membrane domains, for example: activated T cells (Alonso & Weissman [Citation1987]) and myelinating oligodendrocytes (Decker & ffrench-Constant [Citation2004]). Endogenous MAL is constitutively expressed in non-polarized MDCK cells but is absent from COS-7 cells. We hypothesized that if modulation of the lipid membrane underlies MAL's functional mechanism, then the effect of its de-novo introduction into membranes of COS-7 cells can be detected by PFIVar analysis of TX-100-mediated pore expansion.
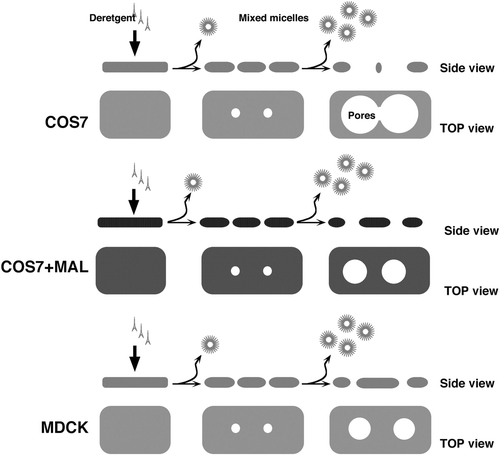
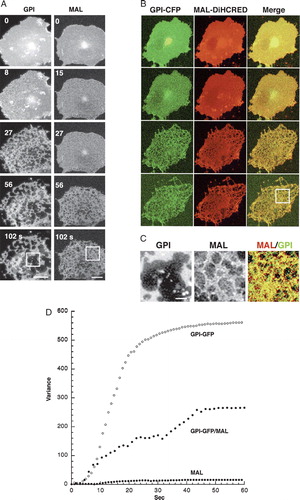
demonstrates that the PM of MAL-DiHcRED-expressing COS-7 cells was more resistant to pore expansion and percolation than that of GPI-FP-expressing COS-7 cells. In membranes containing MAL-DiHcRED, pore expansion and percolation were slower, and proceeded to a lesser extent (A). This effect was also observed in cells co-expressing GPI-FP and MAL-DiHcRED (B). We next analyzed differential extraction in cells co-expressing both GPI-FP and MAL-DiHcRED. Although the PFIVar values were higher for dual expression of GPI-FP and MAL-DiHcRED than for MAL-DiHcRED alone, they were still significantly lower than for GPI-FP alone. In addition, the rate of pore expansion was much slower for cells expressing both GPI-FP and MAL-DiHcRED than for their GPI-FP-expressing counterparts. Thus, the effect of MAL on membrane resistance to pore expansion is dominant. These results are also consistent with the difference between COS-7 and MDCK cells, the latter having potentially higher levels of membrane components that comprise the apical membrane domain. The effect of MAL and the difference between MDCK and COS-7 cells in solubilization morphology are schematically summarized in Supplementary in the online supplementary information.
Figure 5. Comparison of membrane pore expansion among COS-7 cells expressing MAL-FP, GPI-GFP, or both. (A) Representative time-lapse images of COS-7 cells expressing GPI-FP (left panel, experiment repeated 20 times) or MAL-FP (right panel, experiment repeated 30 times), after addition of 1% (V/V) TX-100 in PBS at 17°C. Bar = 20 µM. (see also QuickTime movies S5GPI and S5MAL in the online supplementary information). (B) Dual-color analysis of a typical COS-7 cell co-expressing GPI-CFP (green) and MAL-DiHcRED (red) after addition of 1% (V/V) TX-100 in PBS at 17°C (experiment repeated 11 times). Bar = 20 µM. (C) Comparison of the magnified inserts of the cells shown in A and B. Bar = 5 µM. (D) Quantitative analysis of time-dependent pore expansion during extraction of single cells each expressing GPI-FP, MAL-FP or both. This Figure is reproduced in color in Molecular Membrane Biology online.
Discussion
During cold 1% TX-100 extraction, the solubility of a given set of membrane proteins is consistently the sole parameter being monitored. Cells are washed or incubated with the detergent solution and are then analyzed on a western blot, sometimes after flotation on a gradient (Plant et al. [Citation2000]). In this study, real-time confocal microscopy was used to follow the differential extraction with cold TX-100 of cell membranes containing fluorescently-tagged membrane proteins. The extraction was performed on adherent cultured cells expressing fluorescently-tagged proteins and was continuously followed by CLSM. Using this method, we established that the extraction is initiated with the formation of evenly distributed submicroscopic pores that expand and percolate within 1–2 min, until they reach an apparent steady state. Solubilization of non-raft membranes was visualized, as was the rapid disappearance of membranes labeled with the non-raft marker VSVG-CFP (C and QuickTime movie S1A in the online supplementary information). We investigated several other detergents (4% w/v CHAPS, 0.5% Luberol, and 1% w/v Tween 20) that have been reported for use in differential solubilizations (Schuck et al. [Citation2003]). However, only with Triton-X 100 did we get results compatible with the literature and with biochemical analysis such as the western blot in . This is probably due to the fact that our working temperature is restricted to 15–18°C and not 4°C as required. It has also been claimed that the cell type is at least as important as the type of detergent used for solubilization.
The rapidly expanding and percolating pores most likely correspond to the stage at which partitioning of the detergent into the membrane drives membrane solubilization. Hence, previously published images demonstrating large pores in cells expressing GPI-anchored proteins describe an end-point steady state of the cell membranes in the presence of 1% TX-100 (Mayor & Maxfield [Citation1995]). The mechanism of submicroscopic pore formation is not clear, but it may provide information on the organization of the membrane before treatment with the detergent. Possibly, the evenly distributed membrane domains provide the nuclei for pore formation. However, it should be noted that this possibility is challenged by the report that this process is a direct consequence of introducing the detergent into the system (Heerklotz [Citation2002]). Nevertheless, this study provides, for the first time, a morphological analysis of the transitional state of a biological membrane during solubilization resulting from treatment with a non-ionic detergent. The established ‘three-stage hypothesis’ of membrane solubilization (Jackson et al. [Citation1982]; Lichtenberg [Citation1985]) proposes that initially (stage I), the detergent partitions into the membrane. Stage II is the transitional stage, in which the detergent-saturated membrane co-exists with mixed micelles, and at stage III, the membrane is fully solubilized. Here, membrane pore expansion and percolation () can be associated with stages I and II. The rims at the membrane pore perimeter are comprised of a lipid-detergent mixture that favors the curvature of lipid monolayer that links the inner and outer leaflets of the membrane (Lichtenberg et al. [Citation2005]). The growing pores are the outcome of membrane solubilization, namely, lipid-detergent mixed micelles emerging from the highly curved rims. However, after an apparent steady state is reached, when pores cease to grow and to percolate, a significant amount of detergent-saturated, but still visually intact membranes are observed, corresponding to the DRMs. These observations confirm previous work summarized in a recent review (Lichtenberg et al. [Citation2005]), in which mixed micelle formation was suggested to be driven by ‘curvophilic’ detergents.
presents a quantitative analysis of the pore expansion and percolation process in DRMs labeled with the DRM-associated GPI-FP. Changes in PFIVar were used as a straightforward readout for the time-dependent changes in pore area. Based on this analysis, the GPI-FP-labeled surface membranes of non-polarized MDCK cells were consistently found to be more resistant to TX-100 solubilization than COS-7 cell membranes tagged with the same protein (). Detergent-mediated pores expanded at a slower rate and were significantly smaller after the system arrived at an apparent steady state. The difference in solubilization between COS-7 and non-polarized MDCK cells is most likely derived from the overall difference in the lipid and protein contents of their PMs. This difference lies in both the levels and types of lipid and protein species. Interestingly, the presence of MAL in COS-7 surface membranes was sufficient to significantly slow down and restrict pore expansion and percolation. This does not signify that the lack of MAL in COS-7 cells is the main cause for their increased solubility, but rather that MAL, presumably through interaction with distinct lipids, increases membrane resistance to detergent-mediated solubilization. This effect of MAL can be either direct, via interaction with a distinct lipid, or indirect, by sequestration of a lipid leading to an increase in its biosynthesis. Thus, along with the hypothesis that DRMs are associated with an ordered liquid phase, the MAL-mediated increase in the COS-7 surface membrane's resistance to detergent can be facilitated by promoting the transformation of the membrane to an ordered liquid phase. Such an interaction is conceivable since MAL is a relatively small (17 kD), highly hydrophobic protein, mostly embedded in the membrane bilayer. This hypothesis is also supported by biochemical information that MAL forms oligomers (Puertollano et al. [Citation1997]). MAL has been shown to be an essential component of the apical sorting machinery of proteins such as GPI-anchored ones (Puertollano & Alonso [Citation1999]). Moreover, it has been recently demonstrated that one of the requirements of GPI-GFP sorting to the apical domain in MDCK cells is its oligomerization (Paladino et al. [Citation2004]). It is therefore attractive to hypothesize that this oligomerization of the raft-associated GPI-anchored protein is facilitated by MAL. This hypothesis is supported by our ability to co-immunoprecipitate GPI-GFP and MAL (data not shown). Together with the results presented here, we suggest that MAL is involved in promoting the formation of a domain with characteristics reminiscent of an ordered liquid DRM that serves as the platform for apical sorting. A testable prediction of this hypothesis is that cholesterol might play a role in the MAL-facilitated domains, either indirectly or similar to caveolin (Murata et al. [Citation1995]). The effects shown here were unique to MAL and were not observed with other DRM-associated proteins, such as the farnesylated-palmitoylated fluorescent protein, Influenza hemagglutinin (HA), or cystic fibrosis transmembrane regulator (CFTR).
Finally, MAL contains the MARVEL domain (Sanchez-Pulido et al. [Citation2002]). This domain is comprised of a four-transmembrane-helix organization and is identified in proteins of the MAL, physin, gyrin and occludin families, many of which have been associated with localization to specialized membrane domains. The function of many of the MARVEL-domain containing proteins is related to localization within cholesterol-rich membranes. Thus, the effect of de-novo introduction of these proteins on membrane-solubilization dynamics and morphology might shed light on their function in the initiation and stabilization of specialized membrane domains.
For Supplementary Online Material
Movie captions:
Movie S1C. Complete solubilization of VSV-GFP-associated membrane. Cold TX-100 solubilization of the membrane of a COS7 cell expressing the non-raft membrane glycoprotein VSVG-GFP.
Movie S2A. Membrane perforation by TX-100. Cold TX-100 solubilization of the membrane of a COS7 cell coexpressing the raft associated MAL-DiHcRED and cytosolic soluble free CFP.
Movie S3A. Membrane pore formation and percolation. High resolution time lapse imaging of Cold TX-100 solubilization of the membrane of a COS7 cell expressing the raft marker GPI-GFP. Images were captures at 1 sec intervals.
Movie S4A. Comparison of cold TX-100 solubilization of the membrane of a COS7 and MDCK cells expressing the raft associated GPI-GFP. See A.
Movie S5AGPI. Cold TX-100 solubilization of the membrane of COS7 cell expressing the raft associated GPI-GFP. See A.
Movie S5AMAL. Cold TX-100 solubilization of the membrane of COS7 cell expressing the raft associated MAL-DiHcRED. See A.
Movie S7A. Visualization of the MT cytoskeleton during TX-100 extraction. Cold TX-100 solubilization of the membrane of a COS7 cell coexpressing the raft associated (MAL-DiHcRED) and cytosolic tubulin-GFP.
Online Supplementary Figure legends
Supplementary Figure 1. MAL-CFP in intracellular membrane is detergent sensitive. Images of a COS7 cell coexpressing MAL-CFP after addition of TX-100 at 17°.
Supplementary Figure 2. Visualization of the cytoskeleton during TX-100 extraction. (A) Images of a COS7 cell coexpressing MAL-DHcRED (red) and tubulin-GFP (green)after addition of TX-100 at 17°; (see also Quicktime movie S4A in supplementary information). (B) Images of COS7 cells coexpressing the raft proteins MAL-DHcRED (red) and the actin-GFP (green) after addition of TX-100 at 17°C. (C) Images of a COS7 cell coexpressing the raft proteins VIP17-diHcRED (red) and actin-GFP (green) pretreated with latrunculin B 0.5 µg/ml prior to addition of TX-100 at 17°C. Bars = 20 µm.
We thank Jan Ellenberg (EMBL, Hedelberg) for the kind gift of DiHcRED, Ben Nichols (MRC, Cambridge) for GPI-YFP and GPI-CFP, Dov Lichtenberg, Michael Kozlov (Tel-Aviv University) and Dganit Danino (Technion, Haifa) for fruitful discussions, and Jeanne Schepshelovitch for technical expertise. This work was supported by German-Israeli Foundation for Scientific Research and Development (GIF) Young Scientist program grant number 2017-1024.13/2000 and partially supported by the Israel Science Foundation (ISF).
References
- Alonso MA, Weissman SM. cDNA cloning and sequence of MAL, a hydrophobic protein associated with human T-cell differentiation. Proc Natl Acad Sci USA 1987; 84: 1997–2001
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 1998; 14: 111–136
- Brown DA, Rose JK. Sorting of Gpi-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell-surface. Cell 1992; 68: 533–544
- Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA 1999; 96: 6241–6248
- Decker L, ffrench-Constant C. Lipid rafts and integrin activation regulate oligodendrocyte survival. J Neurosci 2004; 24: 3816–3825
- Gerlich D, Beaudouin J, Kalbfuss B, Daigle N, Eils R, Ellenberg J. Global chromosome positions are transmitted through mitosis in mammalian cells. Cell 2003; 112: 751–764
- Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol 2004; 6: 238–243
- Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J 2002; 83: 2693–701
- Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 2001; 13: 470–477
- Jackson ML, Schmidt CF, Lichtenberg D, Litman BJ, Albert AD. Solubilization of phosphatidylcholine bilayers by octyl glucoside. Biochemistry 1982; 21: 4576–4582
- Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J Cell Biol 1998; 142: 69–84
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta 1985; 821: 470–478
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 2005; 30: 430–436
- Martin-Belmonte F, Puertollano R, Millan J, Alonso MA. The MAL proteolipid is necessary for the overall apical delivery of membrane proteins in the polarized epithelial Madin-Darby canine kidney and fischer rat thyroid cell lines. Mol Biol Cell 2000; 11: 2033–2045
- Mayor S, Maxfield FR. Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol Biol Cell 1995; 6: 929–944
- Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic 2004; 5: 231–240
- Munro S. Lipid rafts: elusive or illusive?. Cell 2003; 115: 377–388
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA 1995; 92: 10339–10343
- Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, Phair RD, Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J Cell Biol 2001; 153: 529–541
- Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, Zurzolo C. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J Cell Biol 2004; 167: 699–709
- Plant PJ, Lafont F, Lecat S, Verkade P, Simons K, Rotin D. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J Cell Biol 2000; 149: 1473–1484
- Puertollano R, Alonso MA. MAL, an integral element of the apical sorting machinery, is an itinerant protein that cycles between the trans-Golgi network and the plasma membrane. Mol Biol Cell 1999; 10: 3435–3447
- Puertollano R, Li S, Lisanti MP, Alonso MA. Recombinant expression of the MAL proteolipid, a component of glycolipid-enriched membrane microdomains, induces the formation of vesicular structures in insect cells. J Biol Chem 1997; 272: 18311–18315
- Sanchez-Pulido L, Martin-Belmonte F, Valencia A, Alonso MA. MARVEL: a conserved domain involved in membrane apposition events. Trends Biochem Sci 2002; 27: 599–601
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 2003; 100: 5795–5800
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 2004; 116: 577–589
- van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. Embo J 1986; 5: 1455–1464
- Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol 2001; 155: 557–570