Abstract
Peroxisomes are organelles found in all eukaryotic cells. Peroxisomes import integral membrane proteins post-translationally, and PEX19 is a predominantly cytosolic, farnesylated protein of mammalian and yeast cells that binds multiple peroxisome membrane proteins and is required for their correct targeting/insertion to the peroxisome membrane. We report the characterisation of the Arabidopsis thaliana homologue of PEX19 which is a predominantly cytosolic protein. AtPEX19 is encoded by two genes (designated AtPEX19-1 and AtPEX19-2) that are expressed in all tissues and at all developmental stages of the plant. Quantitative real time PCR shows that AtPEX19-1 and AtPEX19-2 have distinct expression profiles. Using in vitro translation and co-immunoprecipitation AtPEX19-1 was shown to bind to the Arabidopsis peroxisomal membrane protein PEX10. Additionally, bacterially expressed recombinant AtPEX19-1 was able to bind a fusion protein consisting of the C-terminus of PEX10 and glutathione S-transferase in pull-down assays, thereby demonstrating that non-farnesylated AtPEX19 can interact with the C-terminus of AtPEX10. Purified recombinant AtPEX19-1 was analysed by gel filtration chromatography and was found to have a molecular weight consistent with it forming a dimer and a dimer was detected in Arabidopsis cell extracts that was slightly destabilised in the presence of DTT. Moreover, cross-linking studies of native AtPEX19 suggest that in vivo it is the dimeric species of the protein that preferentially forms complexes with other proteins.
Introduction
Peroxisomes are single-membrane bound metabolic organelles, found in virtually all eukaryotes. There are two conserved functions of peroxisomes found amongst evolutionary diverse organisms, namely β-oxidation of fatty acids and hydrogen peroxide metabolism. Other specialized roles depend on the organism and cell type.
Plant peroxisomes are involved in a range of important functions such as the mobilisation of storage reserves in germinating seedlings via β-oxidation and the glyoxylate cycle Citation[1], the salvage of carbon through photorespiration Citation[2] and defence against oxidative stresses Citation[3]. Plant peroxisomes are also implicated in the generation of a variety of significant hormones and other signalling molecules, including indole acetic acid (from indole butyric acid) Citation[4], jasmonate Citation[5], Citation[6], nitric oxide and several reactive oxygen species Citation[3]. It has also emerged that plant peroxisomes are involved in light regulated gene expression Citation[7], embryogenesis Citation[8], Citation[9] and the breaking of seed dormancy Citation[10].
Although peroxisome function varies between species, the biogenesis of peroxisomes relies on a common class of conserved genes, referred to as PEX genes, which encode proteins termed peroxins. In yeasts the nomenclature is PEXN (gene) PexNp (protein), in plants and mammals it is PEXN (gene) PEXN (protein). To date, over 30 peroxins have been identified. They are involved in peroxisomal matrix protein import, membrane biogenesis and organelle proliferation, although their exact mechanistic functions are still only partially understood. The mechanisms used for importing peroxisomal membrane proteins (PMPs) are distinct from those that translocate proteins into the peroxisomal matrix. Only three peroxins potentially play a role in PMP targeting: PEX3 Citation[11], Citation[12], PEX16 Citation[13], Citation[14]; and PEX19 Citation[15–18]. Recent observations in Saccharomyces cerevisiae have shown that an interaction between Pex3p and Pex19p is necessary for peroxisome biogenesis at the endoplasmic reticulum Citation[19]. In cells deficient in any of these peroxins, PMPs are either degraded or mistargeted to other subcellular locations Citation[11], Citation[20], Citation[21].
PEX19 has been identified as a candidate for both a chaperone Citation[22] and a receptor Citation[21] for PMPs. Many pex19 mutants lack any detectable peroxisome membranes, or have low levels of PMPs which are mislocalised to the cytosol or mitochondria Citation[11], Citation[21]. However in pex19 mutants of Y. lipolytica and P. pastoris, small vesicular structures similar in density to wild type peroxisomes and containing some matrix proteins, were found Citation[17], Citation[23], Citation[24]. There is controversy regarding whether PEX19 preferentially binds newly synthesized Citation[25] or existing Citation[22] PMPs, or whether it may even play a role in the assembly of protein complexes in the peroxisome membrane Citation[26]. There is also disagreement as to whether PEX19 binds a region that contains peroxisomal targeting information (mPTS) and therefore functions as a PMP receptor Citation[21], Citation[25], or if PEX19 binding and PMP targeting are separable events Citation[26], Citation[27]. Recently, the Pex19p binding sites on several Saccharomyces PMPs have been experimentally determined and shown to be essential for correct targeting, providing strong evidence for a receptor function for Pex19p Citation[28]. Targeting of PMPs also requires at least one transmembrane domain (TMD) in addition to the Pex19p binding site, in order to bring about correct localization to peroxisomes Citation[28]. Thus an mPTS has two functionally distinct domains, a Pex19p binding site and a TMD.
The cellular distribution of PEX19 (predominantly cytosolic with a small amount associated with peroxisomes) is consistent with a function in shuttling PMPs from the cytosol to the peroxisome membrane. The integral peroxisome membrane protein PEX3 has been shown to act as a receptor for PEX19 Citation[29]. Membrane association of PEX19 has been suggested to be through farnesylation of the C-terminus at a conserved prenylation motif (CaaX box). However, there are conflicting reports as to whether farnesylation of PEX19 is important for function Citation[16], Citation[17], Citation[21], Citation[27]. PEX19 has a broad specificity, binding to many PMPs and domain mapping of the protein Citation[30–32] has led to the suggestion that it may bind PMPs in multiple places for multiple functions Citation[30].
Arabidopsis thaliana contains two PEX19 homologues. We have characterized one of these and show that AtPEX19 is predominately cytosolic. In vitro translated and recombinant AtPEX19-1 binds to the PMP, AtPEX10. We also provide evidence that AtPEX19 is a dimer in vitro and that the dimeric form is preferentially cross-linked to other proteins in vivo: a novel feature not yet demonstrated in yeast or mammalian PEX19.
Materials and methods
Constructs for expression of recombinant PEX19 and GST-PEX10267-381
Molecular biology methods were carried out according to Sambrook and Russell Citation[33]. EST clone ATTS4952 encoding AtPEX19-1 was obtained from Dr Thierry Desprez, INRA, Versailles, France. The PEX19-1 (At3g03490) reading frame was amplified by PCR using primers 5′-GCGGAATTCGGCGAACAGTCACACCGAT-3′ and 5′-CGCGCCTCGAGTCACATGATACAGCAATT-3′ that introduced an EcoRI site adjacent to the second amino acid (Ala) and an XhoI site down stream of the termination codon, and recloned into pET28(b) (Novagen) cut with the same restriction enzymes. The encoded protein is referred to as His6T7PEX19-1 as it contains both the His and T7 tags N-terminal to PEX19 and has a theoretical pI of 5.00 and Mw of 31,842. A similar construct where PEX19-1 was cloned into the NheI-SacI sites of pET28(a) was obtained from Dr Susanna Cristobal, Uppsala University, Sweden. This encodes a protein we refer to as His6PEX19-1 as it lacks the T7 tag and has a theoretical pI of 5.00 and Mw of 30,482. When expressed in E. coli His6T7PEX19-1 is insoluble but can be solubilized and refolded whereas His6PEX19-1 is expressed in a soluble form. His6T7PEX19-1 was used for antibody production and the co-immunoprecipitation experiment. His6PEX19-1 was used in all other experiments.
The carboxyl terminal region of AtPEX10 (At2g26350) cDNA was amplified using Gateway™ primers (forward strand 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGCGTCGGAGTAATTTGTCA-3′ and reverse strand 3′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCTAAAAATCAGAATGATACAA-5′) with PfuTurbo proofreading DNA polymerase (Stratagene). The PCR product was inserted in the Gateway™ donor vector pDONR201 (Invitrogen) via the Gateway™ BP reaction and then transferred into pDEST15 (Invitrogen) via the Gateway™ LR reaction.
Bacterial culture for protein expression
His6PEX19-1 and GST-PEX10267-381 were expressed in E. coli BL21 (DE3) pLysS cells (Novagen) and His6T7PEX19-1 in BL21 (DE3) Codon Plus RIL cells (Invitrogen), as described (Novagen pET hand book. 10th ed. (www.novagen.com). The majority of the His6T7PEX19-1 protein was insoluble, and so the inclusion bodies were solubilized in 20 mM Na phosphate buffer pH 7.5, 10 mM imidazole, 0.5M NaCl, 8M urea (50 µl/ml culture) and solubilized protein was purified by passage over a Ni2 + column (GE Healthcare) according to the manufacturer's instructions. The purified protein was dialysed overnight at 4°C against 20 mM Na phosphate buffer pH 7.5 and used to raise polyclonal antibodies in rabbits (Scottish Antibody Production Unit, Carluke, Scotland, UK). The pellet of cells expressing His6PEX19-1 was resuspended in 50 ml 50 mM NaCl, 20 mM Tris pH 8.0, containing 20 mg lysozyme and a Complete™ protease inhibitor tablet (Roche). The suspensions were incubated for 20 min on ice, sonicated 6 times for 30 sec on/30 sec off, and centrifuged at 20,000 g for 30 min at 5°C. Soluble His6PEX19-1 was purified on a 5 ml Hi-Trap nickel affinity column (GE Healthcare) according to the manufacturer's instructions. For molecular weight estimation using gel filtration chromatography, 1.5 ml of purified His6PEX19-1 (10 mg/ml) was applied in 50 mM NaCl, 20 mM Tris pH 8.0, to a Superdex 75 26/60 column (GE Healthcare), pre-equilibrated in the same buffer. For the production of soluble GST-PEX10267-381, when absorbance of the culture at 600 nm reached 0.4, it was heat-shocked at 45°C for 20 min then cooled rapidly to 18°C. IPTG was added to 0.5 mM and the culture incubated at 18°C, 200 rpm for a further 4 h.
Isolation of His6PEX19-1GST-PEX10267-381 complex
Cell pellets from 400 ml cultures of BL21(DE3)pLysS expressing His6PEX19-1 and BL21(DE3)pLysS expressing GST-PEX10267-381 were individually resuspended in 10 ml PBS, 1 mM DTT containing 5 mg lysozyme and a Complete™ protease inhibitor tablet (Roche). The suspensions were incubated for 20 min on ice then sonicated 6 times for 30 sec on/30 sec off, before centrifugation at 20,000 g for 30 min at 5°C. The supernatants (3 ml) were applied to a 1 ml glutathione agarose column (Sigma) either singly or as a mixture. The column was washed with 20 ml PBS and bound proteins eluted stepwise with PBS containing 10 mM reduced glutathione in 2 ml fractions. The fractions were examined by SDS-PAGE and Western blot. His6PEX19-1 was visualized using antisera raised to His6T7PEX19-1 at a 1:10,000 dilution followed by goat anti-rabbit IgG-horse radish peroxidase (Sigma, 1:10,000 dilution) with enhanced chemiluminescent detection. GST-PEX10267-381 was visualised using anti-GST antibodies (Sigma G7781).
Cross-linking Arabidopsis cells in vivo with formaldehyde
Arabidosis cells suspension Citation[34] were grown in 100 ml MS medium (4.43g/l, sucrose 30g/l, NAA 0.5mg/l, and Kinetin 0.05mg/l, pH, 5.8) at 24°C under dim light with a 16-h photoperiod in a rotary shaker at 110 rpm. The cells were split every seven days at a 1:10 dilution into the same media. A 100 ml culture was centrifuged at 1000 rpm, the cell pellet was resuspended in PBS containing 1% formaldehyde and incubated at room temperature for 1 h. To quench the reaction glycine was added to a final concentration of 300 mM in PBS and incubated for a further 30 min. The cells were centrifuged at 1000 rpm and washed by re-suspension in PBS before further centrifugation and flash freezing in liquid nitrogen. 5g of frozen cells were ground to powder under liquid nitrogen and added to 15 ml 50 mM Tris, pH 7.5, 1% SDS containing 1 µM E-64 (Sigma), 1 Complete™ protease inhibitor tablet (Roche) and 100 µl Plant Protease inhibitor cocktail (Sigma). The suspension was agitated for 30 min at 4°C before centrifugation at 10,000 g for 20 min at 4°C. The supernatant was removed and heated in SDS-PAGE sample buffer at 65°C for 3 min before immediate analysis by Western blotting as previously described.
Isolation of membrane and organelle fractions from cell cultures
Isolation and sucrose gradient isolation of organelles, in the presence of 3 mM DTT, was carried out as in Citation[35], except the gradients were centrifuged in a Beckman SW28 rotor for 5 h at 26,000 rpm (4°C). Protein and sucrose concentrations were estimated as in Citation[35] except the protein standard was BSA. To separate soluble and membrane fractions, cell cultures were ground in liquid nitrogen and extracted as for the sucrose gradients, in the presence and absence of 3 mM DTT. The extracts were centrifuged in a Beckman TLS 55 rotor at 55,000 rpm (259,000 g) for 30 min (4°C), and the membranes were resuspended in the starting volume of extraction buffer. These fractions were run on Tris/Glycine PAGE with SDS (denaturing gels) and without SDS (native gels). Western blotting was carried out as previously described. Anti-His6T7PEX19 serum was used at 1:5,000–1:10,000 dilution. Anti-thiolase serum was used at 1:50,000 dilution.
Co-immunoprecipitation
His6T7PEX19-1 and PEX10 (in pGEM-T easy) were transcribed and translated in a coupled wheatgerm lysate system (TNT) in the presence of [35S] L-methionine according to the manufacturers (Promega) protocol. After 90 min at 30°C, equal volumes (25 µl) of His6T7PEX19-1 and PEX10 translation reactions were mixed and incubated for a further 90 min at 30°C then 22 microlitres of each reaction were diluted into 0.55 ml IP buffer and the immunoprecipitation carried out according to Citation[36] using either non-immune antiserum or anti poly-His antiserum (Sigma H1029). Siliconized tubes were used to avoid non-specific binding of radio-labelled proteins.
Quantitative real-time PCR
Arabidopsis thaliana adult plants were grown in soil (3:3:1 compost:sand:perlite, Sinclair Horticulture Ltd. containing Intercept™, 0.28 g/l) in a glass-house at 22°C without supplementary light. Flowers consisted of open and closed flowers with their pedicels and younger stages to the floral meristem. Siliques comprised young stages with the outer floral whorls drying to more mature siliques starting to yellow. Leaf tissue consisted of a mixture of cauline and rosette leaves with their pedicels. Tissues were collected mid-morning and immediately placed in liquid nitrogen. For light and dark-grown seedlings seeds were plated onto 1/2MS media (Duchefa) containing 1% (w/v) sucrose and 0.9% (w/v) plant agar (Duchefa), stratified at 5°C for four days then grown at 22°C with a 16 h: 8 h L: D cycle. Plates for dark-grown seedlings were wrapped in two layers of aluminium foil and placed alongside plates for light-grown seedlings. After 7 days, seedlings were harvested under green-filtered, dim light into containers in liquid nitrogen. Tissue was ground in liquid nitrogen, dispersed in extraction buffer (Tris.HCl, pH 7.9, NaCl, 150mM, EDTA 1mM and SDS 1% (w/v)) and purified by phenol:chloroform extraction, ethanol precipitation and LiCl precipitation Citation[33]. Genomic DNA was removed and RNA further purified on Qiagen columns by two on-column DNase I digestions according to manufacturers instructions. cDNA was synthesized from RNA (2 µg) according to manufacturers protocols (Superscript II, Invitrogen) except a 40 µl reaction volume was used. For quantitative PCR, reaction products were diluted 5-fold before use.
Gene-specific primers PEX19-1F 5′TCATGCAAAAGATGCAGGAA-3, PEX19-1R 5′GAAGCTGTTTGGCCCCAT-3′, PEX19-2F 5′AACTGTTTGGCCTTGTCCAG-3′ and PEX19-2 R 5′GAACAAGAACCGAAACCCAAT-3′ were used at concentrations of 400 nM and 300 nM respectively with 2 µl of cDNA, water or standard in a 25 µl reaction containing 12.5 µl 2x SybrMix (Bio-Rad, Hemel Hempstead, UK). Actin-2 primers were as described Citation[37]. Amplifications were carried out on an Icycler instrument (Bio-Rad, Hemel Hempstead, UK). The reactions produced threshold values which were converted to starting quantities using a standard curve constructed from a pool of cDNAs from the various tissues serially diluted five-fold and amplified using PEX19-1, PEX19-2 and Actin-2 gene specific primers.
Results
Arabidopsis thaliana has two PEX19-like genes
The Arabidopsis thaliana genome contains two reading frames At5g17550 and At3g03490 with significant homology to human PEX19 (28.2% amino acid identity) and Saccharomyces cerevisiae Pex19p (24.5% amino acid identity) (). An EST (ATTS4952 GI:773437) corresponding to At3g03490, which we have designated AtPEX19-1 was fully sequenced (accession number AJ564199). AtPEX19-1 and AtPEX19-2 are 82% identical to one another at the nucleotide level and 79% identical at the amino acid level. All four proteins share conserved CAAX box motifs at the carboxyl terminus. In addition there are two regions that are particularly well conserved. The first is a block of 13 alternating acidic and hydrophobic residues near the amino terminus that are predicted to form a perfect acidic amphipathic helix (http://www.site.uottawa.ca/∼turcotte/resources/HelixWheel/) and a second region in the C-terminal half of the protein that also shares alternating polar and hydrophobic residues and a predicted helical structure (). Using the secondary structure prediction program GOR4, Arabidopsis PEX19-1 is estimated to be predominantly α-helical (58%) with a substantial proportion of random coil (36.7%) Citation[38].
Figure 1. Arabidopsis contains two PEX19 genes. Alignment of Arabidopsis PEX19-1 and 19-2 with human PEX19 and Saccharomyces cerevisiae PEX19. Amino acid sequences were aligned with the programme T-coffee Citation[43]. Grey shading indicates hydrophobic residues in conserved regions that are predicted to form amphipathic helices. The CAAX motif directing farnesylation is underlined and cysteine residues are indicated in bold.
![Figure 1. Arabidopsis contains two PEX19 genes. Alignment of Arabidopsis PEX19-1 and 19-2 with human PEX19 and Saccharomyces cerevisiae PEX19. Amino acid sequences were aligned with the programme T-coffee Citation[43]. Grey shading indicates hydrophobic residues in conserved regions that are predicted to form amphipathic helices. The CAAX motif directing farnesylation is underlined and cysteine residues are indicated in bold.](/cms/asset/cb793e71-a5cf-47f1-bf67-ba41f77e8165/imbc_a_173785_f0001_b.gif)
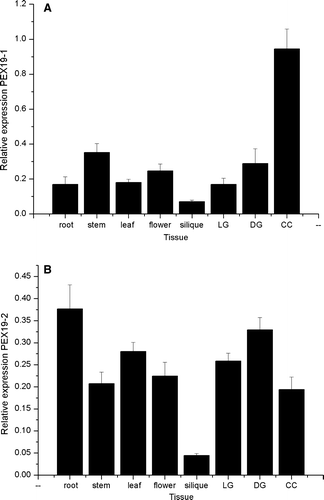
To determine whether both genes were expressed, gene specific primers were designed and used in a reverse-transcription real time quantitative PCR reaction with cDNA derived from different Arabidopsis tissues. Both genes are expressed in all tissues including roots, leaves, flowers, siliques and stems, light and dark grown seedlings and cell culture, but have distinctive expression patterns (A and 2B). For example PEX19-1 is expressed at a five-fold higher level in cell culture compared to root where as the expression of PEX19-2 is almost twice as high in root as in cell culture. On-line supplementary A shows a more detailed expression pattern by tissue for PEX19-1 based on public microarray data. On-line supplementary B shows expression of PEX19-1 as a function of plant age. PEX19-2 is not present on the affymetrix ATH_1 array.
Figure 2. Expression profiles of Arabidopsis PEX19-1 and PEX19-2 in different tissues. Starting quantity values as determined from a standard curve derived from a serial dilution of a pool of cDNA comprising equal volumes of each cDNA are plotted for each tissue. Each value is the mean±SE of six values (2 biological replicates with 3 technical replicates of each sample), except PEX19-1 in silique which is the mean±SE of 5 values (2 biological replicates with 2 and 3 technical replicates respectively). DG, LG stands for dark grown and light grown seedlings respectively; CC stands for cell culture.
Arabidopsis PEX19 is a predominantly cytosolic protein
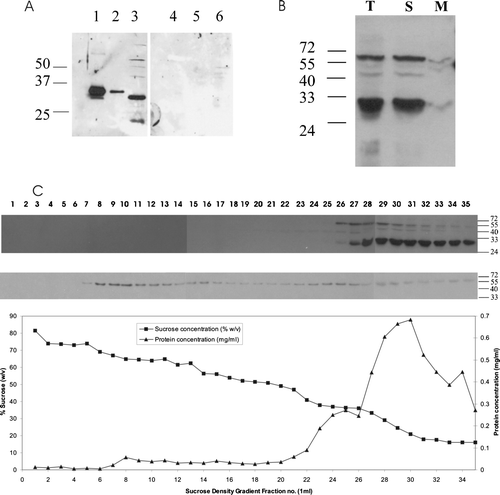
Antibodies were raised against recombinant His6T7PEX19-1, which detected a protein of about 30 kDa in 2-day Arabidopsis seedlings (A, lane 3 left panel). This protein was absent when the antibodies were preincubated with the recombinant His6T7PEX19-1 (A, right panel). The antibody readily detected 10 and even 1 ng of recombinant His6T7PEX19-1 which has a molecular weight of 31,842 (A, left panel lanes 1 and 2). Due to the high degree of similarity between AtPEX19-1 and AtPEX19-2 we assume the antibody detects both isoforms in plant samples and therefore refer to the cross-reacting band as AtPEX19. To determine the localization of AtPEX19 in Arabidopsis cells we used a suspension culture cell line. These suspension culture cells have abundant peroxisomes as shown by electron microscopy (data not shown). Suspension culture cells were fractionated into a total membrane and soluble fraction and probed with anti-AtPEX19 antibodies. AtPEX19 was recovered predominantly in the soluble fraction (B, lane S), with only a small proportion associated with the membrane fraction. (B, lane M). Sucrose density gradient centrifugation was used to prepare organelle fractions from tissue culture cells. The position of peroxisomes was determined by immunoblotting with the marker protein thiolase (C, bottom panel). This shows that intact peroxisomes are found predominantly in fractions 8–13 with a substantial amount of thiolase found at the top of the gradient, which represents protein that has been released from damaged organelles. A duplicate blot was probed with anti-AtPEX19 antibodies, where native AtPEX19 was found at the top of the gradient. On very long exposures a trace of PEX19 was found associated with the peroxisome fraction (data not shown). Thus AtPEX19 is predominantly cytosolic.
Figure 3. Arabidopsis PEX19 is predominantly a cytosolic protein. (A) Anti-PEX19 antibodies recognize native PEX19, a 30 kDa protein in Arabidopsis seedlings. Left panel, immunoblot probed with anti-PEX19 anti-serum 1:10,000 dilution; lane 1, 10 ng recombinant His6T7PEX19, lane 2, 1 ng His6T7PEX19, lane 3, total protein extract equivalent to 20 2-day-old Arabidopsis seedlings. Right panel, as for left panel except that the anti-PEX19 antiserum was preincubated with 10 µg His6T7PEX19 prior to immunodecoration of the blot. (B) PEX19 is mainly cytosolic. Lysate of tissue culture cells (T) was separated into a soluble fraction (259,000 g supernatant; S), and a membrane associated fraction (259,000 g pellet; M). Equivalent volumes were separated by SDS-PAGE and probed with anti-PEX19 serum. (C) A post nuclear supernatant prepared from tissue culture cells was separated by sucrose density gradient centrifugation and equal volumes of each fraction blotted and detected with anti-PEX19 (top panel) and anti-thiolase (lower panel). The graph shows protein concentration (mg/ml) and sucrose concentration (% w/v) in the various fractions.
Arabidopsis PEX19-1 forms a dimmer
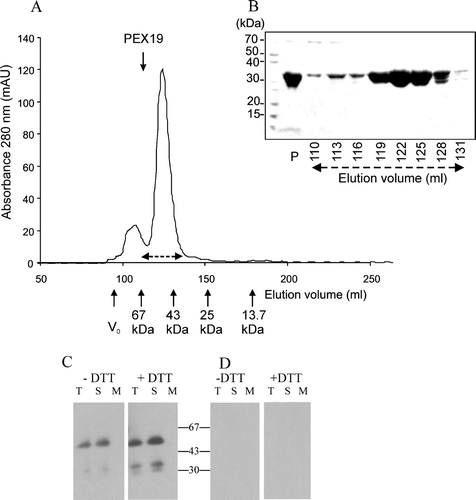
His6PEX19-1 was over expressed as a soluble protein in E. coli. The recombinant protein was purified to near homogeneity using nickel affinity chromatography (B, lane ‘P’). The molecular mass as determined by matrix assisted laser desorption/ionization mass spectrometry, was 30,479 (not shown), which was consistent with the calculated mass. However, when analysed by gel filtration chromatography in the absence of reducing agents, the purified protein began to elute at 110 ml, just after the 67 kDa calibration marker, corresponding to a protein of approximately 60 kDa (A). The data suggests that His6PEX19-1 forms a dimer which is extremely stable, since little monomeric protein was observed. When analysed by reducing SDS-PAGE, His6PEX19-1 migrated with an apparent molecular mass of slightly greater than 30 kDa consistent with denatured, monomeric polypeptide chains (B).
Figure 4. Recombinant His6PEX19-1 forms a dimer. (A) His6PEX19-1 that had been purified by nickel affinity chromatography was applied to a Superdex 75 20/60 gel filtration column and eluted with a retention volume of 115 ml (shown by downward pointing arrow) indicating it has a mass of 60 kDa, consistent with being a dimer. The column was calibrated using a gel filtration low molecular weight calibration kit (GE Health Care) with retention volumes and masses of the protein standards indicated on the chromatogram (upward pointing arrows). For both standards and sample, the retention volume where each protein begins to elute from the column is indicated. V0 is the void volume. (B) Coomassie stained SDS-PAGE showing His6PEX19-1 in fractions eluted from the gel filtration column. His6PEX19-1 in fractions collected between 110 and 134 ml (indicated by broken double arrows on A) show that it migrates with an apparent mass of approximately 30 kDa in SDS-PAGE, despite elution from the column with a mass of approximately 60 kDa. The lane indicated as P shows nickel affinity column purified His6PEX19 before gel filtration chromatography. (C) Immunoblot of 12% native Tris/glycine gels, showing equivalent volumes of total (T), soluble (S) and membrane (M) fractions, (see B), extracted in the presence (+) or absence (-) of DTT and probed with anti-AtPEX19 antibody and (D) anti-AtPEX19 antibody, preincubated with recombinant PEX19 as in B.
The anti-PEX19 antibody detects a species of around 60 kDa in fractions derived from seedlings and cell cultures (A, lane 3; B all lanes), which caused us to consider if PEX19 might be dimeric in vivo. To address this, samples were extracted from cell cultures in the presence and absence of DTT and run on native gels (C). Two immuno reactive species were seen with the faster migrating species increased slightly in abundance when samples were extracted in the presence of DTT. Cross-reaction of both species with the antibody was eliminated when the antibody was pre-incubated with recombinant PEX19 (D). Thus PEX19 most likely forms a disulphide-bonded dimer.
In vitro translated and recombinant PEX19-1 binds PEX10
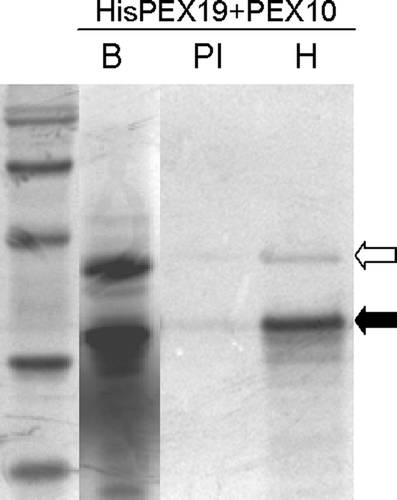
In other organisms PEX19 binds to a range of PMPs including PEX10 Citation[17]. The ability of AtPEX19-1 to bind to AtPEX10 was tested via co-immunoprecipitation (). In vitro translations of the individual full length proteins were mixed, incubated together for 90 min at 30°C then were subjected to immunoprecipitation under non-denaturing conditions with either non-immune serum (PI) or anti His antibody (H). AtPEX10 (open arrow) was clearly co-immunoprecipitated with His6T7PEX19-1 (solid arrow) when anti-His tag antibody was used, but not when a non-immune serum was substituted for the primary antibody. AtPEX10 was equally efficiently precipitated with His6T7PEX19-1 regardless of whether the proteins were translated separately and mixed or translated together (data not shown).
Figure 5. In vitro translated AtPEX19-1 interacts with PEX10. His6T7PEX19-1 and PEX10 were in vitro translated and mixed (lane B) prior to immunoprecipitation by non-immune serum (PI) or anti-His antibodies (H) followed by SDS-PAGE and phosphoimaging. Solid arrow indicates the position of His6T7PEX19-1; open arrow indicates the position of PEX10. 14C Molecular weight standards are, 97 kDa (doublet), 66 kDa, 45 kDa, 30 kDa, 20.1 kDa..
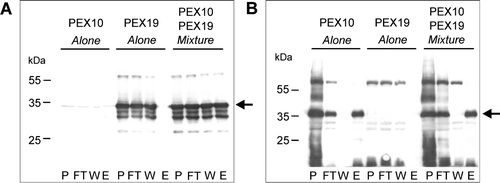
To establish whether the recombinant His6PEX19-1 was able to bind AtPEX10, pull down experiments were performed. As full length AtPEX10 is unlikely to be soluble when expressed in E. coli, a fusion protein, GST–PEX10267-381 was constructed. This corresponds to the region of PEX10, C-terminal to the predicted second transmembrane domain and includes the entire RING finger. Lysates from E. coli cells expressing GST-PEX10267-381 or His6PEX19-1 were applied singly, or as a mixture, to glutathione columns. The unbound material was collected and the column washed with 20 column volumes of washing buffer prior to elution with glutathione. The fractions were analysed by Western blotting using antibodies against His6T7PEX19-1 and GST (). Where lysate containing His6PEX19-1 was applied to the GST column on its own, (A, middle panel) His6PEX19-1 was recovered in the flow through (FT) and wash (W) fractions but not in the eluate fraction (E). However, when lysate containing His6PEX19-1 was mixed with lysate containing GST-PEX10267-381, a portion of His6PEX19-1 was recovered in the glutathione eluate (A, right most panel, lane E). When GST-PEX10267-381 alone was applied to the column, as expected there was no PEX19 immunoreactivity in any fraction (A, left most panel). A duplicate blot to that shown in A was probed with anti-GST antibodies (B). GST-PEX10267-381 binds to the column and is eluted with glutathione (B, left and right most panels). When His6PEX19-1 alone was applied to the column, no immunoreactivity was detected at the molecular weight corresponding to His6PEX19-1 (compare A) showing that the anti-GST antibodies do not cross-react with His6PEX19-1. These results demonstrate that His6PEX19-1 is only retained on the glutathione column in the presence of GST-PEX10267-381. Therefore both in vitro translated and recombinant AtPEX19-1 bind to AtPEX10.
Figure 6. Recombinant His6PEX19-1 binds to the C-terminus of PEX10. Pull down experiments using lysates from E. coli expressing His6PEX19-1 (PEX19) or GST-PEX10267-381 (PEX10) were performed; The lysates were applied alone or as a mixture of both, to a glutathione column, the flow through collected, column washed and specifically bound material was eluted with 10 mM reduced glutathione. The fractions (P, sample prior to column application, FT, flow through, W, wash and E, eluate) were analysed by Western blot, immuno-stained with anti-PEX19 (panel A) or anti-GST (panel B) antibodies. Positions of His6PEX19-1 (panel A) and GST-PEX10267-381 (panel B) are indicated by arrows
PEX19 binds to other proteins as a dimer in vivo
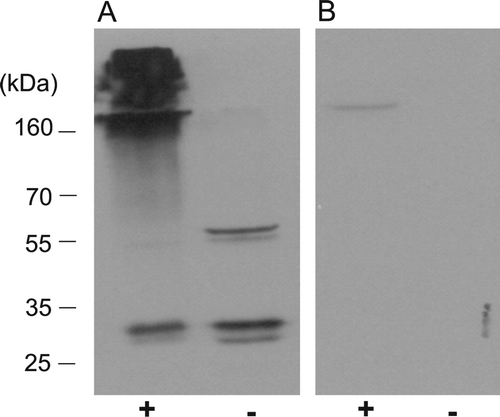
To examine the binding characteristics of Arabidopsis PEX19 in vivo, whole suspension cell culture cells were cross-linked using 1% formaldehyde and the cell lysate analysed by Western blotting (). Evidence that AtPEX19 binds to other proteins was apparent, since when subjected to the cross-linking procedure, the majority of the protein migrated down the gel with apparent masses of greater than 160 kDa, suggesting that binding complexes made with other proteins were rendered permanent by the procedure (A). Native AtPEX19 from the non-cross-linked cells migrated with molecular masses of approximately 30 and 60 kDa consistent with monomeric and dimeric protein. Interestingly, the band corresponding to the dimer was not observed in the cross-linked sample (A, left lane), indicating that the dimer may be preferentially involved in the formation of complexes with other proteins. To eliminate the possibility that the band thought to be a dimer in the non-cross-linked sample, is a result of non-specific binding by the antisera, samples were also analysed by Western blot using antisera incubated with 10 µg recombinant His6PEX19-1 prior to immunodecoration of the blot (B).
Figure 7. The dimeric form of PEX19 is preferentially cross-linked to other proteins in vivo. Arabidopsis suspension cells were cross-linked in vivo with 1% formaldehyde. (A) The cell lysates, cross-linked (lane +), or not cross-linked (lane -), were examined by immunoblotting using antiserum raised against recombinant His6PEX19-1 at 1:10,000 dilution. (B) As panel A, but the antisera was incubated with rHis6PEX19 (10? µg) for 30 min at 37°C before immunodecoration of the blot.
Discussion
In this study we have characterized PEX19 from Arabidopsis thaliana. There are two genes encoding very similar PEX19 isoforms, both of which are expressed. Quantitative real time PCR experiments show expression in a range of cell types consistent with a requirement for maintenance of peroxisomes in a wide range of tissues. The level of expression of the two genes differs between tissues. The functional significance of this observation is not known, but as PEX19 forms dimers, differential expression of the two genes potentially allows differing proportions of both homodimers and the heterodimer to be formed. It will be interesting to establish whether these different molecular species are formed in vivo and whether they have different properties, perhaps reflecting the functional diversity of plant peroxisomes Citation[39]. Like its counterparts in mammals and yeasts, AtPEX19 is a predominantly cytosolic protein, suggesting that the interaction with the peroxisome membrane is transient or quite labile.
On gel filtration we found that His6PEX19-1 elutes with a retention volume corresponding to a molecular size of 60 kDa, although SDS-PAGE and MALDI-MS indicate only a single polypeptide chain, consistent with the calculated mass of His6PEX19-1. The elution profile is very symmetric indicating a uniform population of molecules. On SDS-PAGE gels of plant cell fractions a varying amount of a molecular species that reacts with anti-PEX19-1 antibody and is approximately twice the molecular weight of the monomeric PEX19 is often seen, supporting the notion that AtPEX19 forms a stable dimer which is incompletely reduced in SDS-PAGE gels. Native gel electrophoresis also shows two forms of PEX19. When extracts are prepared in the absence of DTT the slower migrating form predominates, whilst in the presence of DTT the amount of the faster migrating form increases. Thus, the dimer appears to be held together by disulphide linkages. AtPEX19-1 and AtPEX19-2 both contain 3 cysteines, two within the CAAX box motif and one thirty four amino acids amino terminal to the CAAX box. Only the first cysteine of the CAAX box is conserved in PEX19 from all species (). In we show that the dimeric species is preferentially cross-linked in vivo and forms large heterogeneous complexes. As PEX19 is a relatively small protein it may function as a homo or hetero oligomer to keep large hydrophobic PMPs in solution. Interestingly Otzen et al. Citation[24] showed by fluorescence correlation spectroscopy and native gels that Hansenula polymorpha Pex19p-GFP is detected in complexes of approximately 250 kDa. This is consistent with a homotetramer of 60 kDa HpPex19p-GFP molecules, although this interpretation is complicated by the fact that GFP itself has a tendency to dimerise at high concentrations Citation[40].
Like its mammalian and yeast counterparts AtPEX19-1 binds the peroxisome membrane protein PEX10. The GST pull down experiments demonstrate that there is a binding site for PEX19 within the C terminus of AtPEX10. This is in line with previous results that indicated that the region between amino acids 217 and 380 of Pichia pastoris Pex10p was important for the interaction with Pex19p in the yeast 2 hybrid system Citation[17]. As the recombinant AtPEX19 is not farnesylated, the pull down experiments demonstrate that farnesylation is not required for PEX10 binding and that this interaction can take place post translationally. However this does not exclude the possibility that PEX19 can interact with nascent PMPs or that farnesylation could enhance or regulate PMP binding, or interaction of PEX19 with the peroxisome membrane. The function of PEX19 as a dimer makes sense as many PMPs have two or more mPTS sequences that function co-operatively to bring about efficient targeting.
Recently Pex19p binding sites were identified and characterized in S. cerevisiae for Pex13p and ScPex11p and their importance for correct targeting in vivo demonstrated Citation[28]. The essential features of these sites are that they are short 11mer linear peptides, most likely α-helical in nature and containing both hydrophobic and basic residues. How might these be recognized by PEX19? The alignment in reveals two strikingly conserved regions between human, S. cerevisiae and Arabidopsis PEX19s. These two regions consist of alternating hydrophobic (shaded gray) and polar, often acidic, residues. For the Arabidopsis proteins both regions are predicted to lie within regions of alpha helix. Thus they would make attractive possibilities to bind a positively charged helical peptide. A similar molecular recognition event occurs between Tom20, a mitochondrial import receptor and the positively charged amphipathic α-helical mitochondrial-targeting signal. Tom20 binds the hydrophobic face of the peptide Citation[41] and Tom22 is presumed to bind the charged face due to the presence of negatively charged domains within this protein Citation[42]. Interestingly, when PEX19 is deleted or mutated several PMPs are mistargeted to mitochondria Citation[21], Citation[24], suggesting that in the absence of PEX19, the PEX19 binding motif could be recognized by the mitochondrial targeting machinery.
This paper was first published online on prEview on 26 June 2006.
Supplementary Figure 1. Expression profiles of Arabidopsis PEX19-1. Expression profiles were constructed from public microarray data using tools available on the GENEVESTIGATOR website Citation[44]. (A) Expression in various plant organs generated by the Gene Atlas tool. (B) Expression at different developmental stages, defined by Citation[45]. Stage 0, seed germination; stage 1, leaf development; stage 3, rosette growth; stage 5, inflorescence emergence; stage 6, flower production; stage 8, silique development generated by the gene chronologer tool.
![Supplementary Figure 1. Expression profiles of Arabidopsis PEX19-1. Expression profiles were constructed from public microarray data using tools available on the GENEVESTIGATOR website Citation[44]. (A) Expression in various plant organs generated by the Gene Atlas tool. (B) Expression at different developmental stages, defined by Citation[45]. Stage 0, seed germination; stage 1, leaf development; stage 3, rosette growth; stage 5, inflorescence emergence; stage 6, flower production; stage 8, silique development generated by the gene chronologer tool.](/cms/asset/add4f68d-ea47-451c-9517-1a40be0a4739/imbc_a_173785_f0008_b.gif)
We thank Dr Thierry Desprez, INRA, Versailles, for the EST clone ATTS4952 and Susanna Cristobal, University of Uppsala, Sweden, for the His6PEX19-1 expression clone, Alison Ashcroft, Mass spectrometry Facility, Faculty of Biological sciences, University of Leeds, for MALDI-MS, James Williams for constructing His6T7PEX19-1, Barbara Johnson for excellent technical assistance, Dr Carine De Marcos Lousa for helpful comments on the manuscript and the Biotechnology and Biological Sciences Research Council (BBSRC) for financial support.
References
- Graham IA, Eastmond PJ. Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 2002; 41: 156–181
- Reumann S. The photorespiratory pathway of leaf peroxisomes. Plant peroxisomes biochemistry, cell biology and biotechnological applications, A Baker, IA Graham. Kluwer Academic Publishers, Dordrecht 2002; 141–190
- Corpas FJ, Barroso JB, del Rio LA. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 2001; 6: 145–150
- Zolman BK, Yoder A, Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 2000; 156: 1323–1337
- Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 2000; 97: 10625–10630
- Theodoulou FL, Job K, Slocombe SP, Footitt S, Holdsworth M, Baker A, Larson TR, Graham IA. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol 2005; 137: 835–840
- Hu JP, Aguirre M, Peto C, Alonso J, Ecker J, Chory J. A role for peroxisomes in photomorphogenesis and development of Arabidopsis. Science 2002; 297: 405–409
- Schumann U, Wanner G, Veenhuis M, Schmid M, Gietl C. AthPEX10, a nuclear gene essential for peroxisome and storage organelle formation during Arabidopsis embryogenesis. Proc Natl Acad Sci USA 2003; 100: 9626–9631
- Sparkes IA, Brandizzi F, Slocombe SP, El-Shami M, Hawes C, Baker A. An Arabidopsis pex10 null mutant is embryo lethal, implicating peroxisomes in an essential role during plant embryogenesis. Plant Physiol 2003; 133: 1809–1819
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson TR, Graham IA, Baker A, Holdsworth M. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. Embo J 2002; 21: 2912–2922
- Hettema E. H., Girzalsky W., van den Berg M., Erdmann R., Distel B. Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. Embo J 2000; 19: 223–233
- South ST, Sacksteder KA, Li XL, Liu YF, Gould SJ. Inhibitors of COPI and COPII do not block PEX3-mediated peroxisome synthesis. J Cell Biol 2000; 149: 1345–1359
- South ST, Gould SJ. Peroxisome synthesis in the absence of preexisting peroxisomes. J Cell Biol 1999; 144: 255–266
- Honsho M, Hiroshige T, Fujiki Y. The membrane biogenesis peroxin Pex16p-Topogenesis and functional roles in peroxisomal membrane assembly. J Biol Chem 2002; 277: 44513–44524
- Gotte K, Girzalsky W, Linkert M, Baumgart E, Kammerer S, Kunau WH, Erdmann R. Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol Cell Biol 1998; 18: 616–628
- Matsuzono Y, Kinoshita N, Tamura S, Shimozawa N, Hamasaki M, Ghaed K, Wanders RJA, Suzuki Y, Kondo N, Fujiki Y. Human PEX19: cDNA cloning by functional complementation, mutation analysis in a patient with Zellweger syndrome, and potential role in peroxisomal membrane assembly. Proc Natl Acad Sci USA 1999; 96: 2116–2121
- Snyder WB, Faber KN, Wenzel TJ, Koller A, Luers GH, Rangell L, Keller GA, Subramani S. Pex19p interacts with Pex3p and Pex10p and is essential for peroxisome biogenesis in Pichia pastoris. Mol Biol Cell 1999; 10: 1745–1761
- Soukupova M, Sprenger C, Gorgas K, Kunau WH, Dodt G. Identification and characterization of the human peroxin PEX3. Eur J Cell Biol 1999; 78: 357–374
- Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell 2005; 122: 85–95
- Ghaedi K, Tamura S, Okumoto K, Matsuzono Y, Fujiki Y. The peroxin Pex3p initiates membrane assembly in peroxisome biogenesis. Mol Biol Cell 2000; 11: 2085–2102
- Sacksteder KA, Jones JM, South ST, Li XL, Liu YF, Gould SJ. PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J Cell Biol 2000; 148: 931–944
- Snyder WB, Koller A, Choy AJ, Subramani S. The peroxin Pex19p interacts with multiple, integral membrane proteins at the peroxisomal membrane. J Cell Biol 2000; 149: 1171–1177
- Lambkin GR, Rachubinski RA. Yarrowia lipolytica cells mutant for the peroxisomal peroxin Pex19p contain structures resembling wild-type peroxisomes. Mol Biol Cell 2001; 12: 3353–3364
- Otzen M, Perband U, Wang D, Baerends RJS, Kunau WH, Veenhuis M, Van der Klei IJ. Hansenula polymorpha Pex19p is essential for the formation of functional peroxisomal membranes. J Biol Chem 2004; 279: 19181–19190
- Jones JM, Morrell JC, Gould SJ. PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. J Cell Biol 2004; 164: 57–67
- Fransen M, Vastiau I, Brees C, Brys V, Mannaerts GP, Van Veldhoven PP. Potential role for Pex19p in assembly of PTS-receptor docking complexes. J Biol Chem 2004; 279: 12615–12624
- Fransen M, Wylin T, Brees C, Mannaerts GP, Van Veldhoven PP. Human Pex19p binds peroxisomal integral membrane proteins at regions distinct from their sorting sequences. Mol Cell Biol 2001; 21: 4413–4424
- Rottensteiner H, Kramer A, Lorenzen S, Stein K, Christiane LF, Volkmer-Engert R, Erdmann R. Peroxisomal membrane proteins contain common Pex19p-binding sites that are an integral part of their targeting signals. Mol Biol Cell 2004; 15: 3406–3417
- Fang Y, Morrell JC, Jones JM, Gould SJ. PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J Cell Biol 2004; 164: 863–875
- Fransen M, Vastiau I, Brees C, Brys V, Mannaerts GP, Van Veldhoven PP. Analysis of human Pex19p's domain structure by pentapeptide scanning mutagenesis. J Mol Biol 2005; 346: 1275–1286
- Shibata H, Kashiwayama Y, Imanaka T, Kato H. Domain architecture and activity of human Pex19p, a chaperone-like protein for intracellular trafficking of peroxisomal membrane proteins. J Biol Chem 2004; 279: 38486–38494
- Mayerhofer PU, Kattenfeld T, Roscher AA, Muntau AC. Two splice variants of human PEX19 exhibit distinct functions in peroxisomal assembly. Biochem Biophys Res Commun 2002; 291: 1180–1186
- Sambrook J, Russell DW. Molecular cloning, a laboratory manual. 3rd ed. New York: Cold Spring Harbour Laboratory Press; 2001.
- Tugal HB, Pool M, Baker A. Arabidopsis 22-kilodalton peroxisomal membrane protein. Nucleotide sequence analysis and biochemical characterization. Plant Physiol 1999; 120: 309–320
- Lisenbee CS, Heinze M, Trelease RN. Peroxisomal ascorbate peroxidase resides within a subdomain of rough endoplasmic reticulum in wild-type Arabidopsis cells. Plant Physiol 2003; 132: 870–882
- Lopez-Huertas E, Oh JS, Baker A. Antibodies against Pex14p block ATP-independent binding of matrix proteins to peroxisomes in vitro. FEBS Lett 1999; 459: 227–229
- Charlton W, Matsui K, Johnson B, Graham IA, Ohme-Takagi M, Baker A. Salt-induced expression of peroxisome-associated genes requires components of the ethylene, jasmonate and abscisic acid signalling pathways. Plant Cell Environ 2005; 28: 513–524
- Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: Network Protein Sequence Analysis. Trends Biochem Sci 2000; 25: 147–150
- Kamada T, Nito K, Hayashi H, Mano S, Hayashi M, Nishimura M. Functional differentiation of peroxisomes revealed by expression profiles of peroxisomal genes in Arabidopsis thaliana. Plant Cell Physiol 2003; 44: 1275–1289
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 2002; 296: 913–916
- Abe Y, Shodai T, Muto T, Mihara K, Torii H, Nishikawa S-i, Endo T, Kohda D. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell 2000; 100: 551–560
- Brix J, Rudiger S, Bukau B, Schneider-Mergener J, Pfanner N. Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J Biol Chem 1999; 274: 16522–16530
- Notredame C, Higgins DG, Heringa J. T-coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 2000; 302: 205–217
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 2004; 136: 2621–2632
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001; 13: 1499–1510