Abstract
Previous work from our laboratory has established bovine hippocampal membranes as a convenient natural source for studying neuronal receptors such as the G-protein coupled serotonin1A receptor. In this paper, we have explored the organization and dynamics of bovine hippocampal membranes using environment-sensitive and differentially localized fluorescent probes NBD-PE and NBD-cholesterol, utilizing wavelength-selective and time-resolved fluorescence measurements. The NBD group in NBD-PE is localized at the membrane interface while in NBD-cholesterol it is localized deeper in the membrane. Our results show that native hippocampal membranes offer considerable motional restriction as evidenced from red edge excitation shift of NBD probes. However, this effect progressively decreases with increasing cholesterol depletion in the case of NBD-cholesterol, possibly indicating a reduction in membrane heterogeneity. In contrast, REES of NBD-PE in hippocampal membranes does not show any significant change upon cholesterol depletion indicating relative lack of sensitivity of the membrane interface to cholesterol depletion. These observations are supported by changes in fluorescence polarization with cholesterol depletion. Taken together, these results imply that the deeper hydrocarbon region of the hippocampal membrane is more sensitive to changes in membrane organization and dynamics due to cholesterol depletion than the interfacial region. The motional restriction in native membranes is maintained even in the absence of proteins. The fluorescence lifetimes of both the NBD probes show slight reduction upon cholesterol depletion indicating a change in micro-environmental polarity possibly due to water penetration. These results are relevant in understanding the complex organization of hippocampal membranes and could have possible functional implications.
| Acronyms | ||
| NBD | = | 7-nitrobenz-2-oxa-1,3-diazol-4-yl |
| NBD-cholesterol | = | 25-[N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-methyl]amino]27-norcholestorol |
| NBD-PE | = | N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine |
| BCA | = | bicinchoninic acid |
| MβCD | = | methyl-β-cyclodextrin |
| DMPC | = | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DPPC | = | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DPH | = | 1,6-diphenyl-1,3,5-hexatriene |
| PMSF | = | phenylmethylsulfonyl fluoride |
Introduction
The nervous system characteristically contains a very high concentration of lipids, and displays remarkable lipid diversity Citation[1]. The lipid composition of cells that make up the nervous system is unique and has been correlated with increased complexity in the organization of the nervous system during evolution Citation[2]. Organization and dynamics of cellular membranes, particularly membrane lipids, in the nervous system play a crucial role in the function of neuronal membrane receptors. Cholesterol is an important lipid in this context since it is known to regulate the function of neuronal receptors Citation[3], Citation[4] thereby affecting neurotransmission and giving rise to mood and anxiety disorders Citation[5]. The central nervous system accounts for only 2% of the body mass yet contains ∼25% of free cholesterol present in the whole body Citation[6]. Although the brain is highly enriched in cholesterol, the organization and dynamics of brain cholesterol is still poorly understood Citation[7]. Brain cholesterol is synthesized in situ Citation[8] and is developmentally regulated Citation[9]. The organization, traffic, and dynamics of brain cholesterol are stringently controlled since the input of cholesterol into the central nervous system is almost exclusively from in situ synthesis as there is no evidence for the transfer of cholesterol from blood plasma to brain Citation[6]. As a result, a number of neurological diseases share a common etiology of defective cholesterol metabolism in the brain Citation[10]. Several epidemiological studies indicate a possible role of lipids in a variety of neurological disorders which have been shown to involve deregulated lipid metabolism Citation[1], Citation[10]. Yet, the organization and dynamics of neuronal membranes as a consequence of alterations in membrane lipid composition (specifically cholesterol) is poorly understood Citation[7]. In view of the importance of cholesterol in relation to membrane domains, the effect of alteration in the cholesterol content of neuronal membranes on membrane dynamics and protein/receptor function represents an important determinant in the analysis of neurogenesis and several neuropathologies.
Previous work from our laboratory has established bovine hippocampal membranes as a useful natural source for studying the serotonin1A (5-HT1A) receptor which is an important member of the seven transmembrane domain G-protein coupled receptor family Citation[11]. We have partially purified and solubilized the 5-HT1A receptor from bovine hippocampus in a functionally active form Citation[12]. Interestingly, we have recently shown the requirement of membrane cholesterol in modulating ligand binding activity of the bovine hippocampal 5-HT1A receptor using a number of approaches Citation[4], Citation[13]. In order to correlate these cholesterol-dependent functional changes with changes in membrane organization and dynamics, we have examined the phase properties of bovine hippocampal membranes and their modulation with cholesterol and protein content utilizing Laurdan generalized polarization (GP) Citation[14].
Since the membrane is considered as a two-dimensional anisotropic fluid, any possible change in membrane order may not be uniform and restricted to a unique location in the membrane. It is therefore prudent to monitor the change in membrane order at more than one site in order to obtain a comprehensive understanding of any change in membrane dynamics. In this paper, we have monitored the organization and dynamics of bovine hippocampal membranes using environment-sensitive and differentially localized fluorescent probes NBD-PE and NBD-cholesterol (see ) utilizing the wavelength-selective fluorescence approach. Wavelength-selective fluorescence comprises a set of approaches based on the red edge effect in fluorescence spectroscopy and can be used to directly monitor the environment and dynamics around a fluorophore in an organized molecular assembly. The wavelength-selective fluorescence approach represents a powerful and sensitive tool to study membrane organization and dynamics Citation[15–17]. NBD-labeled lipids are particularly suitable for such studies due to the relatively large change in dipole moment of the NBD group upon excitation Citation[18] and are extensively used as fluorescent analogues of native lipids in biological and model membranes to study a variety of processes Citation[17], Citation[19].
Figure 1. The top panel (a) shows the chemical structures of NBD-cholesterol and NBD-PE. The lower panel (b) is a schematic representation of half of the membrane bilayer showing the localizations of the NBD groups of NBD-PE and NBD-cholesterol in phosphatidylcholine membranes. The horizontal line at the bottom indicates the center of the bilayer. Adapted and modified from reference 17.
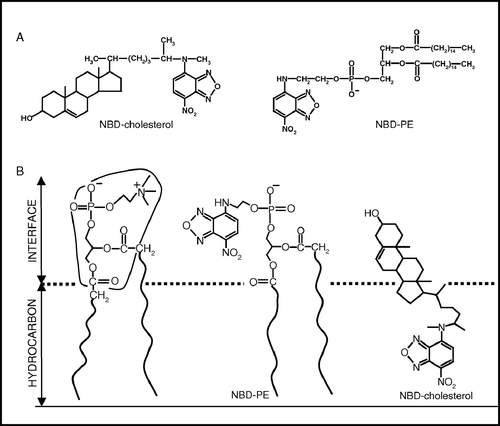
We have previously used the differentially localized NBD-labeled probes, NBD-PE and NBD-cholesterol, to monitor organization and dynamics in different regions in model membranes where the NBD group of the respective probes localize Citation[17]. In NBD-PE, the NBD group is covalently attached to the headgroup of a phosphatidylethanolamine molecule (a). The NBD group in NBD-PE has earlier been shown to be localized in the interfacial region of the membrane (see b) Citation[20–22]. In contrast to this, the NBD group in NBD-cholesterol is attached to the flexible acyl chain of the cholesterol molecule (a). The NBD group of this molecule has been found to be localized deep in the hydrocarbon region of the membrane (b) Citation[17], Citation[20], Citation[21]. The NBD group is polar and has been previously shown by electrophoretic measurements to be uncharged at neutral pH while incorporated in membranes Citation[21]. It has a tendency to loop back to the membrane interface while being attached to acyl chains, as monitored by spectroscopic and ionization properties of the NBD group Citation[21], and measurement of NBD depths by the parallax method Citation[20]. Interestingly, the NBD group of NBD-cholesterol has been shown not to loop back (in a time-averaged sense) Citation[20], Citation[21] either due to the rigidity of the sterol ring and/or the reduction of hydrophilicity due to the methyl group attached to NBD Citation[19]. Use of NBD-PE and NBD-cholesterol would therefore allow us to monitor the changes in membrane organization in different regions of the membrane. We report here changes in hippocampal membrane organization and dynamics due to alterations in cholesterol and protein contents using a combination of wavelength-selective and time-resolved fluorescence measurements.
Materials and methods
Materials
Cholesterol, MβCD, DMPC, DPPC, EDTA, EGTA, iodoacetamide, PMSF, sucrose, sodium azide, Tris and MOPS were obtained from Sigma Chemical Co. (St Louis, MO, USA). BCA reagent kit for protein estimation was from Pierce (Rockford, IL, USA). Amplex Red cholesterol assay kit was from Molecular Probes (Eugene, OR, USA). NBD-cholesterol and NBD-PE were obtained from Avanti Polar Lipids (Alabaster, AL, USA). Concentrations of stock solutions of NBD-cholesterol and NBD-PE in methanol were estimated using their molar extinction coefficients (ε) of 22,000 and 21,000 M−1cm−1 at 484 and 463 nm, respectively Citation[17]. The purity of NBD-cholesterol and NBD-PE were checked by thin layer chromatography on silica gel precoated plates (Sigma) in chloroform/methanol/water (65:35:4 and 65:35:5, v/v/v, for NBD-cholesterol and NBD-PE, respectively), and were found to be pure when detected by color or fluorescence Citation[17]. All other chemicals used were of the highest purity available. Solvents used were of spectroscopic grade. Water was purified through a Millipore (Bedford, MA) Milli-Q system and used throughout. Fresh bovine brains were obtained from a local slaughterhouse within 10 min of death and the hippocampal region was carefully dissected out. The hippocampi were immediately flash frozen in liquid nitrogen and stored at −70°C till further use.
Methods
Preparation of native hippocampal membranes
Native hippocampal membranes were prepared as described previously Citation[4]. Briefly, bovine hippocampal tissue (∼100 g) was homogenized as 10% (w/v) in a polytron homogenizer in buffer A (2.5 mM Tris, 0.32 M sucrose, 5 mM EDTA, 5 mM EGTA, 0.02% sodium azide, 0.24 mM PMSF, 10 mM iodoacetamide, pH 7.4). The homogenate was centrifuged at 900 g for 10 min at 4°C. The resultant supernatant was filtered through four layers of cheesecloth and centrifuged at 50,000 g for 20 min at 4°C. The pellet obtained was suspended in 10 vol. of buffer B (50 mM Tris, 1 mM EDTA, 0.24 mM PMSF, 10 mM iodoacetamide, pH 7.4) using a hand-held Dounce homogenizer and centrifuged at 50,000 g for 20 min at 4°C. This procedure was repeated until the supernatant was clear. The final pellet (native membranes) was suspended in a minimum volume of buffer C (50 mM Tris, pH 7.4), homogenized using a hand-held Dounce homogenizer, flash frozen in liquid nitrogen and stored at −70°C. Protein concentration was assayed using the BCA assay kit with bovine serum albumin as standard Citation[23]. The phospholipid content of these membranes is typically ∼960 nmol/mg of total protein Citation[24].
Cholesterol depletion of native membranes
Native hippocampal membranes were depleted of cholesterol using MβCD as described previously Citation[4]. Briefly, membranes with a total protein concentration of 2 mg/ml were treated with different concentrations of MβCD in 10 mM MOPS buffer (pH 7.4) at 25°C in a temperature controlled water bath with constant shaking for 1 h. Membranes were then spun down at 50,000 g for 5 min, washed once with MOPS buffer, and resuspended in the same buffer. Cholesterol was estimated using the Amplex Red cholesterol assay kit Citation[25].
Lipid extraction from native and cholesterol-depleted membranes
Lipid extraction was carried out according to the method of Bligh and Dyer Citation[26] from native hippocampal membranes. The lipid extract was finally resuspended in a mixture of chloroform-methanol (1:1, v/v).
Estimation of inorganic phosphate
Concentration of lipid phosphate was determined subsequent to total digestion by perchloric acid Citation[27] using Na2HPO4 as standard. DMPC was used as an internal standard to assess lipid digestion. Samples without perchloric acid digestion produced negligible readings.
Sample preparation
Membranes (native and cholesterol-depleted) containing 100 nmol of total phospholipid were suspended in 2 ml of 10 mM MOPS buffer (pH 7.4). The NBD probes were added from a methanolic stock solution in such a way that the final fluorophore concentration was 1 mol% with respect to the total phospholipid content. The resultant probe concentration was 0.5 µM in all cases and the methanol content was always low (0.06%, v/v). This ensures optimal fluorescence intensity with negligible membrane perturbation. NBD probes were added to membranes while being vortexed for 1 min at room temperature and then kept in the dark for 1 h before measurements. Background samples were prepared the same way except that NBD probes were omitted. The protein concentration was ∼0.04 mg/ml.
Lipid extracts containing 100 nmol of total phospholipid in chloroform-methanol (1:1, v/v) were mixed well with 1 nmol of NBD-cholesterol (or NBD-PE) in methanol. The sample was mixed well and dried under a stream of nitrogen while being warmed gently (∼45°C). After further drying under a high vacuum for at least 6 h, 2 ml of 10 mM MOPS, pH 7.4 buffer was added and lipid samples were hydrated (swelled) at ∼70°C while being intermittently vortexed for 3 min to disperse the lipid and form homogeneous multilamellar vesicles (MLVs). The MLVs were kept at ∼70°C for an additional hour to ensure proper swelling as the vesicles were made. Such high temperatures were necessary for hydrating the samples due to the presence of lipids with high melting temperature in neuronal tissues Citation[14]. Samples were kept in the dark at room temperature (25°C) overnight before taking fluorescence measurements.
Steady state fluorescence measurements
Steady state fluorescence measurements with NBD probes were performed with a Hitachi F-4010 steady state spectrofluorometer equipped with a stirring accessory, and using 1 cm path length quartz cuvettes. Excitation and emission slits with nominal bandpass of 5 nm were used for all measurements. Background intensities of samples in which the fluorophore was omitted were negligible in most cases and were subtracted from each sample spectrum to cancel out any contribution due to the solvent Raman peak and other scattering artifacts. The spectral shifts obtained with different sets of samples were identical in most cases. In other cases, the values were within±1 nm of the ones reported (We have used the term maximum of fluorescence emission in a somewhat wider sense here. In every case, we have monitored the wavelength corresponding to maximum fluorescence intensity, as well as the center of mass of the fluorescence emission. In most cases, both these methods yielded the same wavelength. In cases where minor discrepancies were observed, the center of mass of emission has been reported as the fluorescence maximum). Fluorescence polarization measurements were performed using a Hitachi polarization accessory. Polarization values were calculated from the equation Citation[28]:1 where IVV and IVH are the measured fluorescence intensities (after appropriate background subtraction) with the excitation polarizer vertically oriented and emission polarizer vertically and horizontally oriented, respectively. G is the grating correction factor and is the ratio of the efficiencies of the detection system for vertically and horizontally polarized light, and is equal to IHV/IHH. All experiments were carried out with multiple sets of samples and average values of polarization are shown in and . The optical densities of the samples measured at the respective excitation wavelengths were low.
Time-resolved fluorescence measurements
Fluorescence lifetimes were calculated from time-resolved fluorescence intensity decays using a Photon Technology International (London, Western Ontario, Canada) LS-100 luminescence spectrophotometer in the time-correlated single photon counting mode, using 1 cm path length quartz cuvettes. This instrument uses a thyratron-gated nanosecond flash lamp filled with nitrogen as the plasma gas (16±1 inches of mercury vacuum) and is run at 17–22 kHz. Lamp profiles were measured at the excitation wavelength using Ludox (colloidal silica) as the scatterer. To optimize the signal to noise ratio, 10,000 photon counts were collected in the peak channel. The excitation wavelengths used were 460 nm (for NBD-cholesterol) and 465 nm (for NBD-PE), and emission was collected at 512 and 531 nm for NBD-cholesterol and NBD-PE, respectively. All experiments were performed using excitation and emission slits with a bandpass of 8 nm or less. The sample and the scatterer were alternated after every 5% acquisition (i.e., after 500 counts are collected in the peak channel each time) to ensure compensation for shape and timing drifts occurring during the period of data collection. This arrangement also prevents any prolonged exposure of the sample to the excitation beam thereby avoiding any possible photodamage of the fluorophore. The data stored in a multichannel analyzer was routinely transferred to an IBM PC for analysis. Fluorescence intensity decay curves so obtained were deconvoluted with the instrument response function and analysed as a sum of exponential terms:2 where F(t) is the fluorescence intensity at time t and αi is a preexponential factor representing the fractional contribution to the time-resolved decay of the component with a lifetime τi such that Σi αi=1. The decay parameters were recovered as described previously Citation[14]. Mean (average) lifetimes <τ> for biexponential decays of fluorescence were calculated from the decay times and preexponential factors using the following equation Citation[28]:
3
Results
Red edge excitation shifts of membrane-bound NBD probes
In general, for a fluorophore in a bulk non-viscous solvent, the fluorescence decay rates and the wavelength of maximum emission are independent of the excitation wavelength. This is because of Kasha's rule which states that fluorescence normally occurs from the zero vibrational level of the first excited electronic state of a molecule Citation[15]. However, this generalization breaks down in case of polar fluorophores in motionally restricted media such as very viscous solutions or condensed phases, that is, when the mobility of the surrounding matrix relative to the fluorophore is considerably reduced. Under such conditions, when the excitation wavelength is gradually shifted to the red edge of the absorption band, the maximum of fluorescence emission exhibits a concomitant shift toward higher wavelengths. Such a shift in the wavelength of maximum emission toward higher wavelengths, caused by a corresponding shift in the excitation wavelength toward the red edge of the absorption band, is termed red edge excitation shift or REES Citation[15]. This effect is mostly observed with polar fluorophores in motionally restricted media such as viscous solutions or condensed phases where the dipolar relaxation time for the solvent shell around a fluorophore is comparable to or longer than its fluorescence lifetime. REES arises from slow rates of solvent relaxation (reorientation) around an excited state fluorophore which depends on the motional restriction imposed on the solvent molecules in the immediate vicinity of the fluorophore. Utilizing this approach, it becomes possible to probe the mobility parameters of the environment itself (which is represented by the relaxing solvent molecules) using the fluorophore merely as a reporter group. Further, since the ubiquitous solvent for biological systems is water, the information obtained in such cases will come from the otherwise ‘optically silent’ water molecules. Since REES is observed only under conditions of restricted mobility, it serves as a faithful indicator of the dynamics of the fluorophore environment. REES represents a powerful approach which can be used to directly monitor the environment and dynamics around a fluorophore in a complex biological system Citation[15]. The unique feature about REES is that while all other fluorescence techniques (such as fluorescence quenching, energy transfer, and polarization measurements) yield information about the fluorophore (either intrinsic or extrinsic) itself, REES provides information about the relative rates of solvent (water in biological systems) relaxation dynamics which is not possible to obtain by other techniques. This makes REES extremely useful since hydration plays a crucial modulatory role in a large number of important cellular events including protein folding, lipid-protein interactions and ion transport.
We have recently shown that membrane cholesterol is essential for ligand binding and G-protein coupling of the hippocampal 5-HT1A receptor Citation[4], Citation[13]. In order to monitor the effect of membrane cholesterol on the organization and dynamics of hippocampal membranes, we carried out cholesterol depletion in native hippocampal membranes using MβCD. MβCD is a water-soluble compound and has previously been shown to selectively and efficiently extract cholesterol from membranes by including it in a central nonpolar cavity Citation[4]. shows that upon treatment with increasing concentrations of MβCD, the cholesterol content of bovine hippocampal membranes shows progressive reduction. Thus, when native membranes are treated with 10 mM MβCD, the cholesterol content is reduced to ∼62% of the initial value. This effect levels off with increasing concentrations of MβCD, with the cholesterol content of hippocampal membranes being reduced to ∼10% of the original value when the membranes are treated with 40 mM MβCD (see ). Importantly, shows that the change in phospholipid content under these conditions was found to be negligible (< 9%) in all cases. This shows that the depletion of cholesterol by MβCD is predominantly specific (see also Citation[4]).
Table I. Lipid contents in native hippocampal membranes as a function of increasing cholesterol depletion.
The emission maxima of NBD-labeled lipids are sensitive to the polarity of the probe microenvironment Citation[19], Citation[21]. The fluorescence emission maxima of NBD-cholesterol and NBD-PE in native hippocampal membranes are at 509 and 533 nm, respectively (see ). The blue shift of the emission maximum for NBD-cholesterol (compared to NBD-PE) is indicative of its deeper location in the non-polar region of the membrane, as reported earlier by one of us Citation[20], Citation[21]. The shifts in the maxima of fluorescence emission of NBD-cholesterol and NBD-PE as a function of excitation wavelength in native and cholesterol-depleted membranes and in liposomes of lipid extracts from native membranes are shown in . For NBD-cholesterol, as the excitation wavelength is changed from 460–500 nm, the emission maximum shifts from 509–528 nm which corresponds to a REES of 19 nm in case of native membranes (see a). In contrast, the emission maximum shifts from 512–524 nm, which corresponds to a REES of 12 nm in cholesterol-depleted membranes (also see a). Such a shift in the wavelength of emission maximum with change in the excitation wavelength is characteristic of the red edge effect and indicates that the NBD moiety in NBD-cholesterol is localized in a motionally restricted region of the membrane that offers considerable resistance to solvent reorientation in the excited state. Interestingly, the emission maximum displays a shift from 510–532 nm (corresponding to a REES of 22 nm) as the excitation wavelength is changed from 460–500 nm in the liposomes of lipid extract from native membranes (see b). Such a high degree of REES obtained in liposomes from lipid extracts of native membranes (i.e., even in the absence of proteins) indicates that the motional restriction in native membranes is maintained even in the absence of proteins. It should be noted here that the fluorescence of NBD-cholesterol is relatively weak Citation[21], and we found it difficult to work in excitation wavelengths longer than 500 nm because of the very low signal-to-noise ratio and artifacts due to the Raman peak that remained even after background subtraction. When NBD-PE is used as a probe, the emission maximum shifts from 533–538 nm in case of native membranes, and from 532–537 nm in cholesterol-depleted membranes as the excitation wavelength is changed from 460–507 nm (see b), thereby resulting in a REES of 5 nm in both cases. The corresponding shift in emission maximum is from 527–539 nm (corresponding to a REES of 12 nm) in liposomes of lipid extract from native membranes (see b).
Figure 2. Effect of changing excitation wavelength on the wavelength of maximum emission of (a) NBD-cholesterol and (b) NBD-PE in native membranes (○), cholesterol-depleted (with 40 mM MβCD) membranes (▴) and liposomes of lipid extract from native membranes (□). The ratio of fluorophore to total phospholipid was maintained at 1:100 (mol/mol). The protein concentration is ∼0.04 mg/ml. See Materials and methods for other details.
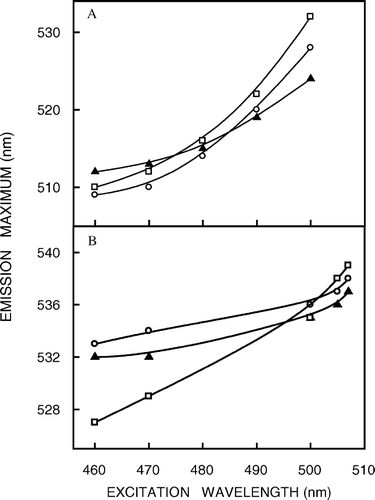
Figure 3. (a) Effect of cholesterol depletion (by treatment with increasing concentrations of MβCD) on the magnitude of red edge excitation shift (REES) of NBD-cholesterol (○) and NBD-PE (•) in native membranes. All other conditions are as in . See Materials and methods for other details. (b) A comprehensive representation of the magnitude of red edge excitation shift (REES) obtained with NBD-cholesterol (shaded bars) and NBD-PE (white bars) in native membranes as a function of increasing cholesterol depletion (when treated with increasing concentrations of MβCD) and in liposomes of lipid extract from native membranes (data for native and cholesterol-depleted membranes taken from a). All other conditions are as in . See Materials and methods for other details.
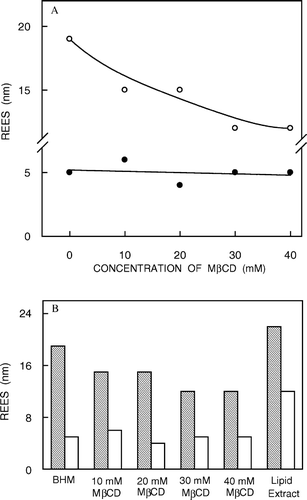
The effect of increasing cholesterol depletion on the magnitude of REES is shown in a. When NBD-cholesterol is used as a probe, REES exhibits a progressive reduction with increasing cholesterol depletion. While native hippocampal membranes display a REES of 19 nm, the corresponding value for membranes with mild cholesterol depletion is 15 nm. With increasing cholesterol depletion, the magnitude of REES further reduces to 12 nm. The reduction in the magnitude of REES with increasing cholesterol depletion could be correlated to a reduction in membrane heterogeneity. Such a reduction in membrane heterogeneity has previously been correlated to removal of membrane cholesterol Citation[14], Citation[29], Citation[30]. In contrast, REES of NBD-PE shows no significant change over the entire range of cholesterol depletion. Taken together, and show that REES of NBD-cholesterol (where the NBD group is localized at the deeper hydrophobic region of the membrane) is more sensitive to changes in membrane organization and dynamics due to cholesterol depletion than that of NBD-PE (in which the NBD group is localized at the membrane interface). This is consistent with the previous observation that changes in membrane organization brought about by cholesterol are largely restricted to the fatty acyl region of the membrane Citation[31]. This is further supported by the recent observation that cholesterol depletion disrupts the orientation of a fluorophore located within the hydrophobic region of the membrane but not of an interfacial probe Citation[32].
Fluorescence polarization of membrane-bound NBD probes
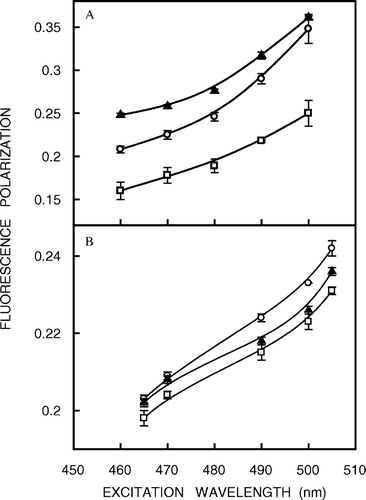
In addition to the dependence of fluorescence emission maxima on the excitation wavelength, fluorescence polarization is also known to depend on the excitation wavelength in motionally restricted media Citation[16], Citation[17]. Due to strong dipolar interactions with the surrounding solvent molecules, there is a decreased rotational rate of the fluorophore in the solvent-relaxed state. On red edge excitation, a selective excitation of this subclass of fluorophore occurs. Because of strong interaction with the polar solvent molecules in the excited state, the ‘solvent relaxed’ fluorophores rotate more slowly, thereby increasing the polarization. The excitation polarization spectra (i.e., a plot of steady-state polarization vs. excitation wavelength, keeping emission wavelength constant) of NBD probes in native and cholesterol-depleted membranes as well as in liposomes of lipid extracts from native membranes are shown in . The considerable increase in fluorescence polarization in all cases as the excitation wavelength is shifted toward the red edge reinforces our earlier conclusion that the NBD group in these probes is in a motionally restricted environment.
Figure 4. Fluorescence polarization of (a) NBD-cholesterol and (b) NBD-PE in native membranes (○), cholesterol-depleted (with 40 mM MβCD) membranes (▴) and liposomes of lipid extract from native membranes (□) as a function of excitation wavelength. The emission wavelength was 512 nm for NBD-cholesterol and 531 nm for NBD-PE. All other conditions are as in . The data points shown are the means±SE of multiple measurements. See Materials and methods for other details.
The fluorescence polarization of the NBD probes incorporated in hippocampal membranes as a function of increasing cholesterol depletion is shown in a. Interestingly, the fluorescence polarization of NBD-PE remains largely invariant with cholesterol depletion. This is consistent with our earlier result of no significant change in REES of NBD-PE accompanying cholesterol depletion (see a). However, there is a significant increase in fluorescence polarization of NBD-cholesterol (∼19%) with progressive depletion of cholesterol. This further reinforces our earlier observation that cholesterol depletion affects the membrane order in the deeper hydrophobic region of the membrane. In addition, the increase in fluorescence polarization for NBD-cholesterol with depletion of cholesterol suggests reduction in rotational mobility in the deeper hydrocarbon region of the membrane. Importantly, analysis of apparent rotational correlation times from Perrin's equation Citation[28] showed that the observed change in fluorescence polarization of the NBD-cholesterol is free from fluorescence lifetime-induced artifacts. This increase in fluorescence polarization could be attributed to an increase in the attractive forces between the lipid hydrocarbon chains with reduction in cholesterol content in the deeper hydrophobic region of these membranes Citation[31]. It has been shown by NMR, ESR and diffraction techniques that in fluid bilayers the stiff fused ring of cholesterol extends beyond carbon 9 position in the adjacent lipid. Importantly, the interactions of the sterol ring with the lipid acyl chain produce a fairly constant ordering at the upper half of the chain. Interestingly, the ability of cholesterol to reduce the disorder in the acyl chains of phospholipids displays a pronounced depth dependence. In the presence of cholesterol, the acyl chain region of phospholipids from C2 (the carbon next to the ester moiety) to about C7 to C10 into the bilayer (which constitute the upper half of the acyl chain, i.e., the portion nearest to the glycerol backbone) exists in a relatively ordered state because of van der Waals attractive interactions between the rigid sterol ring system and the polymethylene groups of the phospholipid Citation[31]. From the middle of the chain to the bilayer center (the bottom half of the chain where the NBD group of NBD-cholesterol is localized), the conformational freedom of the phospholipid acyl chain segments increases because these –CH2 groups come into contact with the relatively flexible isooctyl side chain attached to C17 of cholesterol molecule.
Figure 5. (a) Effect of cholesterol depletion on the fluorescence polarization of NBD-cholesterol (○) and NBD-PE (•) in native membranes. Excitation was at 460 nm and emission was monitored at 512 nm for NBD-cholesterol while excitation was at 465 nm and emission was monitored at 531 nm for NBD-PE. The polarization data shown are the means±standard errors of multiple measurements. (b) A comprehensive representation of fluorescence polarization of NBD-cholesterol (shaded bars) and NBD-PE (white bars) in native membranes as a function of increasing cholesterol depletion and in liposomes of lipid extract from native membranes (data for native and cholesterol-depleted membranes taken from a). The data points shown are the means±SE of multiple measurements. All other conditions are as in . See Materials and methods for other details.
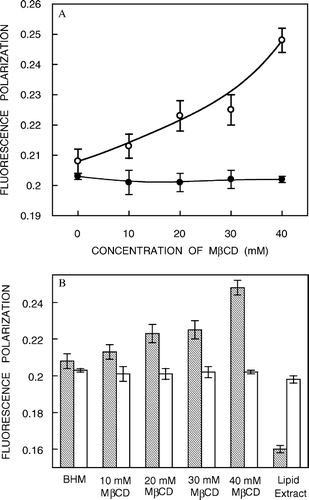
b shows a comprehensive representation of fluorescence polarization of NBD probes in native and cholesterol-depleted membranes and in liposomes of lipid extract from native membranes. It is apparent that while the fluorescence polarization of NBD-PE remains more or less invariant in lipid extracts, that of NBD-cholesterol shows a reduction (∼23%) indicating that removal of proteins from native membranes affects the organization of the membrane significantly in the deeper hydrocarbon region (see b).
Fluorescence lifetime of membrane-bound NBD probes
Fluorescence lifetime serves as a sensitive indicator of the local environment in which a given fluorophore is placed and is sensitive to excited state reactions. Differential extents of solvent relaxation around a given fluorophore in the excited state could be expected to give rise to differences in its fluorescence lifetime. In addition, it is well known that fluorescence lifetime of the NBD group is sensitive to the environment in which it is placed and is reduced in the presence of water Citation[33]. The fluorescence lifetimes of NBD probes in native and cholesterol-depleted membranes and in liposomes of lipid extracts from native membranes are shown in . As can be seen from the table, all fluorescence decays could be fitted well with a biexponential function. We chose to use the mean fluorescence lifetime as an important parameter for describing the behaviour of NBD probes in these membranes since it is independent of the number of exponentials used to fit the time-resolved fluorescence decay. The mean fluorescence lifetimes of NBD probes were calculated using Equation 3 and shown in . shows that the mean fluorescence lifetimes of the NBD probes in hippocampal membranes show a modest decrease with increasing extents of cholesterol depletion possibly due to changes in micro-environmental polarity upon cholesterol depletion. This increase in micro-environmental polarity is also evident from the red shift of 3 nm (from 509–512 nm) of the emission maximum of NBD-cholesterol upon cholesterol depletion (see a).
Figure 6. Effect of cholesterol depletion on the mean fluorescence lifetime of NBD-cholesterol (○) and NBD-PE (•) in native membranes. Mean fluorescence lifetimes were calculated from using Equation 3. The data points shown are the means±SE of multiple measurements. All other conditions are as in . See Materials and methods for other details.
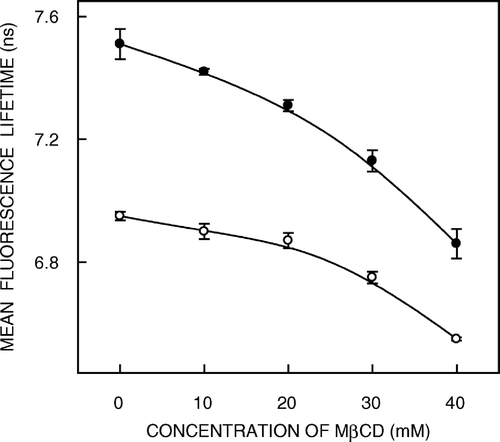
Table II. Mean fluorescence lifetimes of NBD-labeled lipids in native membranes with increasing cholesterol depletion and in lipid extractsa.
Discussion
Although the membrane lipid composition of the bovine hippocampus is not known, the phospholipid composition of rat hippocampus has recently been reported Citation[34–36]. Analysis of the phospholipid composition of the rat hippocampus shows phosphatidylethanolamine, phosphatidylcholine, and phosphatidylserine as the predominant headgroups, while the fatty acid composition is enriched with 16:0, 18:0, 18:1, 18:2, 20:4, and 22:6 fatty acids. In addition, plasmalogens have been reported to be present in rat hippocampus.
In this paper, we have monitored the influence of cholesterol and proteins on the organization and dynamics of hippocampal membranes using environment-sensitive differentially localized fluorescent (NBD) probes. Knowledge of dynamics would help in analyzing functional data generated by modulation of membrane lipid composition Citation[4], Citation[13]. We have earlier used REES of NBD-labeled lipids as an efficient tool to explore the organization and dynamics of membranes Citation[17]. The choice of a suitable probe is of considerable importance in REES measurements. NBD-labeled probes are appropriate for such studies since the dipole moment of the NBD group changes by ∼4 D upon excitation Citation[18], an important criterion for a fluorophore to exhibit REES effects Citation[15]. Further, the NBD group possesses some of the most desirable properties for serving as an excellent probe for spectroscopic applications. It is very weakly fluorescent in water and upon transfer to hydrophobic media, it fluoresces brightly in the visible range and shows a large degree of environmental sensitivity Citation[18], Citation[21], Citation[33].
A common concern in studies using probes is any possible perturbation induced by the probe and its effect, if any, on the measured parameters. Since the concentrations of the NBD probes used by us are low (1 mol% with respect to the total phospholipid content), any such perturbation would be minimized. More importantly, control experiments using 1 mol% NBD probes (NBD-PE and NBD-cholesterol) in model membranes of DPPC did not display any variation in either the phase transition temperature or the enthalpy change associated with it (Kamlekar RK, Mukherjee S, Swamy MJ, Chattopadhyay A, unpublished observation). This rules out any probe-induced perturbation in these membranes.
Effect of cholesterol on the organization and dynamics of hippocampal membranes
Our results show that native hippocampal membranes offer considerable motional restriction as evidenced from REES of NBD-cholesterol. However, this effect progressively decreases with increasing cholesterol depletion possibly indicating a reduction in membrane heterogeneity. REES therefore appears to be an indicator of membrane heterogeneity. REES of NBD-PE in hippocampal membranes, on the other hand, remains invariant upon cholesterol depletion indicating relative lack of sensitivity of the membrane interfacial region to cholesterol depletion Citation[31], Citation[32]. The motional restriction experienced in hippocampal membranes is supported by changes in fluorescence polarization with excitation wavelength. Taken together, these results imply that the deeper hydrocarbon region of the membrane is more sensitive to changes in membrane organization and dynamics due to cholesterol depletion than the interfacial region. In addition, fluorescence lifetimes of both the NBD probes show slight reduction upon cholesterol depletion possibly indicating water penetration leading to a change in micro-environmental polarity.
Interestingly, cholesterol depletion of native membranes is accompanied by an increase in the polarization of NBD-cholesterol. This brings out the essential difference in the information content obtained using these two approaches, i.e., REES and fluorescence polarization. Fluorescence polarization is an indicator of the rotational diffusion of the membrane probe and represents the local environment of the probe Citation[28]. REES, on the other hand, provides information about the relative reorientation (relaxation) rate of the surrounding dipoles (water molecules) and does not directly report probe dynamics Citation[15]. It is important to mention here that the measured fluorescence parameters were stable and did not exhibit any variation over time following cholesterol depletion.
Effect of proteins on the organization and dynamics of hippocampal membranes
In order to examine whether proteins influence the observed change in dynamics, we made measurements in liposomes of lipid extracts from native membranes which are devoid of protein but whose lipid composition reflects that of native membranes. Our results indicate that the motional restriction observed in the case of native membranes is maintained in liposomes from lipid extracts of native membranes, i.e., even in the absence of proteins, as evidenced from high degree of REES obtained in these membranes (b). Interestingly, it is apparent that while the fluorescence polarization of NBD-PE in lipid extracts is comparable to that obtained in native membranes, that of NBD-cholesterol is lower indicating that removal of proteins from native membranes affects the organization of the membrane significantly in the hydrocarbon region (b). This implies higher rotational mobility of NBD-cholesterol in lipid extracts compared to that in native membranes, which possibly could be attributed to reduced packing between the lipid acyl chains due to the presence of voids or defects created by bulky proteins, giving rise to lower polarization of NBD-cholesterol.
Taken together, our results constitute one of the first reports on depth-dependent changes in the organization and dynamics of hippocampal membranes and its modulation by cholesterol depletion using NBD fluorescence. These results could be relevant in understanding the complex organization of hippocampal membranes and may have possible functional implications. Our future efforts will focus on exploring the relationship of these changes in dynamics with function of the hippocampal 5-HT1A receptor.
This work was supported by the Council of Scientific and Industrial Research, Government of India. A.C. is an Honorary Professor of the Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore (India). S. M., S. K., and T. J. P. thank the Council of Scientific and Industrial Research for the award of Senior Research Fellowships. We gratefully acknowledge Md. Jafurulla and Sandeep Shrivastava for help with the preparation of native membranes and cholesterol depletion from native membranes. Special thanks are due to H. Raghuraman for helpful discussions. We thank S. Rajanna for help with the tissue collection and members of our laboratory for critically reading the manuscript.
References
- Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov 2005; 4: 594–610
- Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res 1985; 24: 69–176
- Burger K, Gimpl G, Fahrenholz F. Regulation of receptor function by cholesterol. Cell Mol Life Sci 2000; 57: 1577–1592
- Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim Biophys Acta 2004; 1663: 188–200
- Papakostas GI, Öngür D, Iosifescu DV, Mischoulon D, Fava M. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol 2004; 14: 135–142
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol 2001; 12: 105–112
- Wood WG, Schroeder F, Avdulov NA, Chochina SV, Igbavboa U. Recent advances in brain cholesterol dynamics: transport, domains, and Alzheimer's disease. Lipids 1999; 34: 225–234
- Kabara JJ. A critical review of brain cholesterol metabolism. Prog Brain Res 1973; 40: 363–382
- Turley SD, Bruns DK, Dietschy JM. Preferential utilization of newly synthesized cholesterol for brain growth in neonatal lambs. Am J Physiol 1998; 274: E1099–E1105
- Porter FD. Malformation syndromes due to inborn errors of cholesterol synthesis. J Clin Invest 2002; 110: 715–724
- Pucadyil TJ, Kalipatnapu S, Chattopadhyay A. The serotonin1A receptor: a representative member of the serotonin receptor family. Cell Mol Neurobiol 2005; 25: 553–580
- Kalipatnapu S, Chattopadhyay A. Membrane protein solubilization: recent advances and challenges in solubilization of serotonin1A receptors. IUBMB Life 2005; 57:505–512.
- Pucadyil TJ, Chattopadhyay A.. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res 2006. DOI: 10.1016/j.plipres.2006.02.002.
- Mukherjee S, Chattopadhyay A. Monitoring the organization and dynamics of bovine hippocampal membranes utilizing Laurdan generalized polarization. Biochim Biophys Acta 2005; 1714: 43–55
- Chattopadhyay A. Exploring membrane organization and dynamics by the wavelength-selective fluorescence approach. Chem Phys Lipids 2003; 122: 3–17
- Chattopadhyay A, Mukherjee S. Depth-dependent solvent relaxation in membranes: wavelength-selective fluorescence as membrane dipstick. Langmuir 1999; 15: 2142–2148
- Chattopadhyay A, Mukherjee S. Red edge excitation shift of a deeply embedded membrane probe: implications in water penetration in the bilayer. J Phys Chem B 1999; 103: 8180–8185
- Mukherjee S, Chattopadhyay A, Samanta A, Soujanya T. Dipole moment change of NBD group upon excitation studied using solvatochromic and quantum chemical approaches: implications in membrane research. J Phys Chem 1994; 98: 2809–2812
- Chattopadhyay A. Chemistry and biology of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: fluorescent probes of biological and model membranes. Chem Phys Lipids 1990; 53: 1–15
- Chattopadhyay A, London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry 1987; 26: 39–45
- Chattopadhyay A, London E. Spectroscopic and ionization properties of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids in model membranes. Biochim Biophys Acta 1988; 938: 24–34
- Abrams FS, London E. Extension of the parallax analysis of membrane penetration depth to the polar region of model membranes: use of fluorescence quenching by a spin-label attached to the phospholipid polar headgroup. Biochemistry 1993; 32: 10826–10831
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 1985; 150: 76–85
- Pucadyil TJ, Chattopadhyay A. Exploring detergent insolubility in bovine hippocampal membranes: a critical assessment of the requirement for cholesterol. Biochim Biophys Acta 2004; 1661: 9–17
- Amundson DM, Zhou M. Fluorometric method for the enzymatic determination of cholesterol. J Biochem Biophys Methods 1999; 38: 43–52
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917
- McClare CWF. An accurate and convenient organic phosphorus assay. Anal Biochem 1971; 39: 527–530
- Lakowicz JR. Principles of fluorescence spectroscopy. Kluwer-Plenum Press, New York 1999
- Krishnamoorthy G, Ira. Fluorescence lifetime distribution in characterizing membrane heterogeneity. J Fluoresc 2001; 11: 247–253
- Fiorini RM, Valentino M, Glaser M, Gratton E, Curatola G. Fluorescence lifetime distributions of 1,6-diphenyl-1,3,5-hexatriene reveal the effect of cholesterol in microheterogeneity of erythrocyte membrane. Biochim Biophys Acta 1988; 939: 485–492
- Bittman R. Has nature designed the cholesterol side chain for optimal interaction with phospholipids?. Subcell Biochem 1997; 28: 145–171
- Benninger RKP, Önfelt B, Neil MAA, Davis DM, French PMW. Fluorescence imaging of two-photon linear dichroism: cholesterol depletion disrupts molecular orientation in cell membranes. Biophys J 2005; 88: 609–622
- Lin S, Struve WS. Time-resolved fluorescence of nitrobenzoxadiazole-aminohexanoic acid: effect of intermolecular hydrogen-bonding on non-radiative decay. Photochem Photobiol 1991; 54: 361–365
- Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Kim H-Y. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. J Lipid Res 2002; 43: 611–617
- Ulmann L, Mimouni V, Roux S, Porsolt R, Poisson J-P. Brain and hippocampus fatty acid composition in phospholipid classes of aged-relative cognitive deficit rats. Prostaglandins Leukot Essent Fatty Acids 2001; 64: 189–195
- Wen Z, Kim H-Y. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem 2004; 89: 1368–1377