Abstract
The ligand binding pocket of Cys-loop receptors consists of a number of binding loops termed A–F. Here we examine the 5-HT3 receptor loop A residues Asn-128, Glu-129 and Phe-130 using modelling, mutagenesis, radioligand binding and functional studies on HEK 293 cells. Replacement of Asn-128 results in receptors that have wild type [3H]granisetron binding characteristics but large changes (ranging from a five-fold decrease to a 1500-fold increase) in the 5-HT EC50 when compared to wild type receptors. Phe-130 mutant receptors show both increases and decreases in Kd and EC50 values, depending on the amino acid substituted. The most critical of these residues appears to be Glu-129; its replacement with a range of other amino acids results in non-binding and non-functional receptors. Lack of binding and function in some, but not all, of these receptors is due to poor membrane expression. These data suggest that Glu-129 is important primarily for receptor expression, although it may also play a role in ligand binding; Phe-130 is important for both ligand binding and receptor function, and Asn-128 plays a larger role in receptor function than ligand binding. In light of these results, we have created two new homology models of the 5-HT3 receptor, with alternative positions of loop A. In our preferred model Glu-129 and Phe-130 contribute to the binding site, while the location of Asn-128 immediately behind the binding pocket could contribute to the conformation changes that result in receptor gating. This study provides a new model of the 5-HT3 receptor binding pocket, and also highlights the importance of experimental data to support modelling studies.
| Acronyms | ||
| 5-HT | = | 5-hydroxytryptamine |
| nACh receptor | = | nicotinic acetylcholine receptor |
| HEK | = | human embryonic kidney |
| WT | = | wild type |
Introduction
5-HT3 receptors are members of the Cys-loop family of ligand-gated ion channels (LGIC), which includes nicotinic acetylcholine (nACh), GABAA and glycine receptors. The receptors function as a pentameric arrangement of subunits, with each subunit having a large extracellular N-terminal region and four transmembrane domains (M1–M4). The extracellular domain contains the ligand binding site, and our understanding of this region has been significantly advanced by the publication of the high resolution structure of the acetylcholine binding protein (AChBP), a soluble protein with homology to the extracellular domain of LGICs (Brejc et al. [Citation2001]). This structure has confirmed that ligand binding is coordinated by six regions of the sequence (loops A–F) that were previously implicated in ligand binding using biochemical techniques.
The availability of the high resolution structure of AChBP has led to a series of homology models of the extracellular domain of several Cys-loop receptors including nACh, GABAA and 5-HT3 receptors (Cromer et al. [Citation2002], Le Novere et al. [Citation2002], Maksay et al. [Citation2003], Reeves et al. [Citation2003], Schapira et al. [Citation2002], Thompson et al. [Citation2005]). Ligands have been docked into the binding pockets of these homology models and have revealed a number of potential interactions with amino acid residues in the binding loops. However it is not yet clear if molecular details can be accurately extrapolated from AChBP to Cys-loop receptors. For example, comparison of the AChBP crystal structure and cryo-electron microscopy images of nACh receptors has shown differences between the location of binding loops B and C (Unwin [Citation2005]). These discrepancies may reflect the bound and unbound states of the receptor, but it is important to appreciate that the models only provide a working hypothesis of the interactions between the ligand and receptor, and must be tested experimentally before conclusions can be drawn about their accuracy. Here we investigate the accuracy of a homology model of the 5-HT3 receptor (Reeves et al. [Citation2003], Thompson et al. [Citation2005]). According to this model, the only loop A residue within 5 Å of the docked agonist 5-HT is Asn-128. Adjacent residues Glu-129 and Phe-130 are more distant, and are presumed not to interact with the ligand. However, previous mutagenesis studies have revealed that Glu-129 and Phe-130 can modify the binding and functional characteristics of the receptor, suggesting that the homology model may need revision (Boess et al. [Citation1997], Steward et al. [Citation2000]). To explore the roles of these residues, we generated a series of mutations of Asn-128, Glu-129 and Phe-130, and examined the binding and functional characteristics of the resulting receptors.
Figure 1. Adjacent subunits (principal and complementary) showing the positions of Asn-128, Glu-129 and Phe-130 in the original homology model of the 5-HT3 receptor (Reeves et al. [Citation2003], Thompson et al. [Citation2005]). (A) Only two of the five subunits have been shown for ease of viewing. In the right hand panel the complementary subunit has been removed and the binding site rotated to view it from the side. (B) An alignment of the 5-HT3A, nAChR α1 and AChBP receptor subunit sequences. The binding loops of the receptors are indicated by black lines above the alignment and their location can be seen in the structure above. The positions of the β-sheets are shown by grey lines beneath the text and are taken from the AChBP protein crystal structure (Brejc et al. [Citation2001]). Asn-128, Glu-129 and Phe-130 are highlighted as white text in a black box. Accession numbers for the 5-HT3A, nACh α1 and AChBP subunit sequences are Q6J1J7, P02710 and P58154 respectively.
![Figure 1. Adjacent subunits (principal and complementary) showing the positions of Asn-128, Glu-129 and Phe-130 in the original homology model of the 5-HT3 receptor (Reeves et al. [Citation2003], Thompson et al. [Citation2005]). (A) Only two of the five subunits have been shown for ease of viewing. In the right hand panel the complementary subunit has been removed and the binding site rotated to view it from the side. (B) An alignment of the 5-HT3A, nAChR α1 and AChBP receptor subunit sequences. The binding loops of the receptors are indicated by black lines above the alignment and their location can be seen in the structure above. The positions of the β-sheets are shown by grey lines beneath the text and are taken from the AChBP protein crystal structure (Brejc et al. [Citation2001]). Asn-128, Glu-129 and Phe-130 are highlighted as white text in a black box. Accession numbers for the 5-HT3A, nACh α1 and AChBP subunit sequences are Q6J1J7, P02710 and P58154 respectively.](/cms/asset/4984cf30-da45-4a9c-a7a7-0385d1838a44/imbc_a_183090_f0001_b.gif)
Materials and methods
Materials
All cell culture reagents were obtained from Gibco BRL (Paisley, UK), except foetal calf serum which was from Labtech International (Ringmer, UK). [3H]-granisetron (81 Ci/mmol) was from PerkinElmer (Boston, MA, USA). FlexStation membrane potential (FMP) dye was from Molecular Devices Ltd (Wokingham, UK). Biotinylated anti-rabbit IgG, fluorescein isothiocyanate avidin D and Vectashield mounting medium were from Vector Laboratories (California, USA). All other reagents were of the highest obtainable grade.
Cell culture
Human embryonic kidney (HEK) 293 cells were maintained on 90 mm tissue culture plates at 37°C and 7% CO2 in a humidified atmosphere. They were cultured in DMEM:F12 (Dulbecco's Modified Eagle Medium/Nutrient Mix F12 (1:1)) with GLUTAMAX I™ containing 10% foetal calf serum and passaged when confluent. Cells were stably transfected with pcDNA3.1 containing the complete coding sequences for the 5-HT3A(b) receptor subunit (Accession Number; AY605711) or mutant receptors as previously described (Hargreaves et al. [Citation1996]). For FlexStation assays, stably transfected cells were plated (approx 3×104 cells/well) onto black sided, clear bottomed, 96-well plates (Greiner Bio-One, Stonehouse, UK) and incubated 1–2 days before assay. For radioligand binding studies, cells were transiently transfected using calcium phosphate precipitation at 80–90% confluency (Chen & Okayama [Citation1987]) and incubated for 3–4 days before assay.
Site-directed mutagenesis
Mutagenesis reactions were performed using the method developed by Kunkel ([Citation1985]) using the 5-HT3A(b) subunit DNA. Oligonucleotide primers were designed according to the recommendations of Sambrook et al. ([Citation1989]), using some suggestions of the Primer Generator (Turchin & Lawler [Citation1999]; http://www.med.jhu.edu/medcenter/primer/primer.cgi). A silent restriction site was incorporated in each to assist rapid identification.
Radioligand binding
This was as described previously with minor modifications (Lummis et al. [Citation1993]). Briefly, transfected cells were washed twice with phosphate buffered saline and then scraped into ice-cold HEPES buffer (10 mM, pH 7.4) containing the following proteinase inhibitors: 1 mM EDTA, 50 µg/ml soybean trypsin inhibitor, 50 µg/ml bacitracin and 0.1 mM PMSF, and frozen at −20°C. After thawing, they were washed twice with HEPES buffer, resuspended, and 50 µg of cell membranes incubated in 0.5 ml HEPES buffer containing 0.02–20 nM [3H]-granisetron. Non-specific binding was determined by the addition of 1 µM quipazine. Reactions were incubated for 1 h at 4°C and terminated by rapid vacuum filtration using a Brandel cell harvester onto GF/B filters presoaked for 3 h in 0.3% polyethyleneimine followed by two rapid washes with 4 ml ice cold HEPES buffer. Radioactivity was determined by scintillation counting (Beckman LS6000sc). Protein concentration was estimated using the Bio-Rad Protein Assay with BSA standards. Data were analysed using Prism software (GraphPad, San Diego, CA, USA).
FlexStation analysis
This was as previously described (Price & Lummis [Citation2005]). Briefly, cells grown on a 96-well plate were gently rinsed twice with Flex buffer (10 mM HEPES, 115 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, pH 7.4). 50 µl Flex buffer and 50 µl FMP (diluted 2:5 in Flex buffer) were added to each well and the cells were incubated at room temperature for 45 min before being put in the FlexStation (Molecular Devices Ltd., Wokingham, UK). Fluorescence was measured every 2 sec for 200 sec. Control (Flex buffer) or 5-HT (0.001 µM to 1.0 mM) was added to each well after 20 sec. Softmax Pro (Molecular Devices Ltd, Wokingham, UK) or Prism was used for data analysis. Fluorescence responses were normalized to the maximal fluorescence response and concentration-response curves generated using , where EC50 is the concentration required for the half-maximal response, and nH is the Hill coefficient.
Immunofluorescent localization
This was performed as described previously (Spier et al. [Citation1999]). Briefly, transfected cells were washed with three changes of Tris-buffered saline (TBS: 0.1 M Tris/HCl pH 7.4, 0.9% NaCl) and fixed using ice cold 4% paraformaldehyde in phosphate buffer (66 mM Na2HPO4, 38 mM NaH2PO4 pH 7.2). After 2 TBS washes, the cells were incubated in pAb120 antisera (Spier et al. [Citation1999]) at 1:1000 dilution in TBS to determine cell surface expression. Intracellular receptor expression was determined by inclusion of 0.3% Triton X-100. Primary antibody incubation was overnight at 4°C. Biotinylated anti-rabbit IgG (Vector Laboratories, California, USA) and fluorescein isothiocyanate (FITC) avidin D (Vector Laboratories, California, USA) were used to detect bound antibody. Coverslips were mounted in Vectashield mounting medium (Vector Laboratories, California, USA) and immunofluorescence observed using a confocal microscope.
Modelling
New models of the 5-HT3 receptor subunit extracellular domain were built using MODELLER 6v2 (Sali & Blundell [Citation1993]) as described previously (Reeves et al. [Citation2003]) using modified alignments with AChBP. For new model 1, a single amino acid gap was inserted in the AChBP sequence following D85 and a single amino acid gap was inserted in the 5-HT3 receptor subunit sequence following V131. For new model 2, two amino acid-spaced gaps were inserted at each of these positions in the AChBP and 5-HT3 receptor subunit sequences. The new models were superimposed on the original model with 5-HT and granisetron docked into the binding site using the Magic Fit function of Swiss-PdbViewer (Reeves et al. [Citation2003], Thompson et al. [Citation2005]).
Results
Radioligand binding properties of wild type and mutant receptors
Specific, saturable binding was detected for 10 of the 17 mutants. F130A and F130W had Kd values significantly larger than wild type, while the Kd values for N128A, N128E, N128D, N128L, N128Q, N128R, N128V and F130Y showed no significant difference to wild type (). Repeatable, specific, saturable binding could not be found for any of the Glu-129 mutants.
Table I. [3H]-Granisetron binding affinities.
Immunofluorescent localization of wild type and mutant receptors
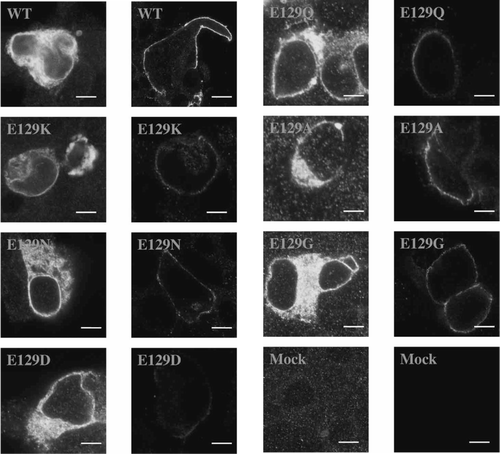
Intracellular fluorescence and cell surface fluorescence, visualized as a well-defined ring on the cell exterior, was observed for all cells successfully transfected with wild type or Asn-128 and Phe-130 mutant receptors (20–40% of cells), but not mock transfected cells (, LHS). In contrast, while the number of cells displaying intracellular fluorescence was similar to wild type for all Glu-129 mutant receptors, high levels (20–40%) of robust cell surface fluorescence were only observed for E129G and E129A mutant receptors. Surface expression was poor in cells transfected with E129D, E129N, E129Q, E129K and E129H mutant receptor DNA, although a ring of fluorescence was observed in 0.5–2% of cells, indicating that some receptors in some cells could reach the plasma membrane. In those cells that were labelled, however, fluorescence was usually weak (, RHS).
Figure 2. Non-binding Glu-129 mutant 5-HT3 receptors expressed in HEK 293 cells. Levels of expression in permeabilized cells (left hand column) were similar to wild type for all mutants (20–40% of cells were fluorescently labelled), but only reached these levels in non–permeabilized cells (right hand column) for E129G and E129A receptors. Fluorescent cell surface labelling was present in < 2% cells for all other mutants, although, as shown, some receptors did appear to reach the plasma membrane in some cells. Scale bars indicate 10 µm.
Functional characterization of wild type and mutant receptors
Binding of 5-HT to the 5-HT3 receptor triggers the opening of a cation-selective pore resulting in a change in the membrane potential of the stimulated cell. A dye sensitive to membrane potential changes can therefore be used to measure changes in membrane potential resulting from channel opening (Price & Lummis [Citation2005]). B (inset) shows a typical FlexStation response to HEK 293 cells expressing mutant F130Y receptors stimulated with 5-HT. Data revealed cells expressing wild type receptors have an EC50 of 0.20 µM compared to values of 1–3 µM reported in electrophysiological studies (Spier & Lummis [Citation2000], Price & Lummis [Citation2004]). This is not unexpected as the EC50s are measured using different techniques. For electrophysiological studies EC50s represent the agonist concentration required to open 50% of channels, while in fluorescent studies they represent the agonist concentration required to depolarize the membrane potential to 50% of its original value. As cellular events are triggered by changes in membrane potential, the latter may be a more accurate indication of ligand potency. However, while this technique may yield good EC50s, the data are not suitable for determining kinetic measurements of channel opening and closing rates.
Figure 3. Dose-response curves derived from FlexStation responses to 5-HT stimulation. Data have been normalized to the maximum fluorescence absorbance (Fmax) and plotted as the mean±SEM. (A) Asn-128 mutants. (B) Phe-130 mutants. Inset: Typical Flexstation responses to 5-HT (0, 0.1, 0.5 or 1.0 µM). 5-HT was added at 20 sec to HEK 293 cells expressing F130Y-5-HT3 receptors. F = fluorescence in arbitrary units; t=time in seconds.
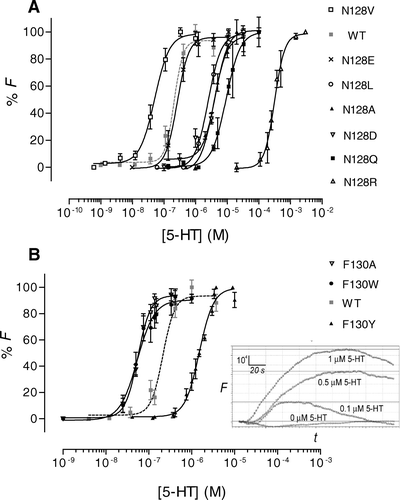
All Asn-128 mutants responded to stimulation by 5-HT (). Changing Asn-128 to Ala, Asp, Leu or Gln increased EC50s by factors of ∼10–45, while Arg substitution increased the EC50 by a factor of ∼1500. N128E mutation did not significantly affect the EC50. Conversely, N128V increased the potency of 5-HT at the receptor with a four-fold decrease in EC50.
Glu-129 mutants (E129A, E129D, E129Q, E129G, E129H, E129K and E129N) showed no response to 5-HT up to 1 mM (). Concentrations above this showed a non-specific stimulation of untransfected HEK 293 cells and could not be used (n=7–10).
Table II. Functional parameters.
All Phe-130 mutants responded to 5-HT with EC50 values significantly different to wild type (). F130Y mutants had an EC50 ∼7 times greater than wild type, whilst F130A and F130W mutants displayed a four-fold decrease in EC50. The change in EC50 for F130Y mutant receptors was very similar to that reported previously (8-fold increase; Steward et al. [Citation2000]), demonstrating that this parameter obtained using the FlexStation is comparable to whole cell electrophysiological data.
Modelling
In new model 1 the NEF sequence is moved towards the N terminus, such that Asn-128 effectively replaces Ile-127, Glu-129 replaces Asn-128 and Phe-130 replaces Glu-129. Glu-129 is now prominently located facing into the binding pocket, Asn-128 faces away from the binding pocket and Phe-130 is located on the edge of the binding pocket. In new model 2 the NEF sequence is moved even further towards the N terminus so that Asn-128 effectively replaces Leu-126, Glu-129 replaces Ile-127 and Phe-130 replaces Asn-128. In this model Asn-128 and Glu-129 face away from the binding pocket and Phe-130 is now prominently located facing into the binding pocket. The models are shown in .
Figure 4. Original and new models of the 5-HT3 receptor extracellular domain with docked 5-HT and granisetron. (A) Subunit dimer showing the ligand binding site (circled) at the subunit interface. (B) Close-up of the ligand binding site of the original model showing residues Asn-128, Glu-129 and Phe-130 of binding loop A in relation to docked 5-HT (from Reeves et al. [Citation2003]) and granisetron (from Thompson et al. [Citation2005]). (C) New model 1 with residues moved closer to the N-terminus so that Glu-129 now points towards the binding site. (D) New model 2 with residues moved further towards the N-terminus so that Phe-130 now points towards the binding site.
![Figure 4. Original and new models of the 5-HT3 receptor extracellular domain with docked 5-HT and granisetron. (A) Subunit dimer showing the ligand binding site (circled) at the subunit interface. (B) Close-up of the ligand binding site of the original model showing residues Asn-128, Glu-129 and Phe-130 of binding loop A in relation to docked 5-HT (from Reeves et al. [Citation2003]) and granisetron (from Thompson et al. [Citation2005]). (C) New model 1 with residues moved closer to the N-terminus so that Glu-129 now points towards the binding site. (D) New model 2 with residues moved further towards the N-terminus so that Phe-130 now points towards the binding site.](/cms/asset/ad135f41-72b8-4adc-a900-e5117f7bf875/imbc_a_183090_f0004_b.jpg)
Discussion
In this study we have examined the roles of the loop A residues Asn-128, Glu-129 and Phe-130, residues which are located in, or close to, the binding pocket in a homology model of the 5-HT3 receptor (Reeves et al. [Citation2003]). Our data suggest that Glu-129 is the most critical of these three residues as its replacement with a range of other amino acid residues results in non-binding and non-functional receptors. Substituting alternative residues at positions 128 and 130 modifies, but does not ablate, the function of the receptor, suggesting that these residues play a less important role than Glu-129 in the structure and/or function of the receptor. The roles of each of the residues are discussed in more detail below.
The role of Asn-128
Modification of Asn-128 alters the EC50. This parameter combines binding affinity and functional efficacy, and it is not possible to tell from these data which is being affected. However, modification of Asn-128 does not change the [3H]granisetron binding affinity. As granisetron is a competitive antagonist, (i.e., a ligand that does not produce a conformational change that results in channel opening) it can provide information specifically about the binding site. Thus, these data suggest that this residue is involved in receptor function subsequent to ligand binding (i.e., it plays a role in the conformational change that ultimately generates the functional response). An alternative explanation is that Asn-128 is involved specifically in agonist (but not antagonist) binding. If this is the case then we would have anticipated a direct interaction with 5-HT, and indeed the original model of 5-HT docked into the binding pocket showed a potential hydrogen bond between the side chain of Asn-128 and the hydroxyl of 5-HT (Reeves et al. [Citation2003]). However, our results show no relationship between hydrogen bonding ability and change in EC50. For example, changing Asn-128 to Val, whose side chain cannot hydrogen bond, resulted in a receptor with a decreased EC50, while replacing Asn-128 with Gln, whose side chain can form hydrogen bonds, resulted in a 45-fold increase in EC50. Thus a hydrogen bond is unlikely to play a role here. In nACh receptors there is evidence that the equivalent residue is involved in ligand binding; a Tyr to Phe (Y93F) mutation results in receptors that have a lower affinity for ACh (Sine et al. [Citation1994]), and the equivalent residue in AChBP (Tyr-89) has been shown to make van der Waals contacts with ligands in the binding pocket (Celie et al. [Citation2004]). In contrast, our data shows little evidence of a direct interaction of Asn-128 with ligands, suggesting our alignment of this residue with Tyr-89, and its resultant prominent position in the binding pocket, may be inaccurate.
The difference in EC50 values for the different Asn-128 mutants may give some indication of important properties of the residue at this position, and suggest that the size and hydrophobicity of the residue at this position may be important, with smaller and more hydrophobic residues (such as Val) being favoured. This supports a location of Asn-128 facing away from the binding pocket and into the protein interior, where it could readily contribute to a conformational change. More data is required to confirm this, and definitive evidence would require high resolution structural data and/or the determination of channel opening and closing rate constants, neither of which have so far have been obtained for 5-HT3 receptors.
The role of Glu-129
We could not obtain any reproducible binding or functional data for any of the Glu-129 mutants we examined. Our immunofluorescence results provide a reason for at least some of these data: E129D, E129H, E129Q, E129K and E129N mutant receptors express poorly at the plasma membrane. Nevertheless E129G and E129A mutant receptors, which similarly do not bind radioligand or respond to 5-HT application, do express at the plasma membrane at similar levels to wild type receptors. These observations suggest that there are at least two critical roles of Glu-129, one in receptor assembly and/or targeting and the other in ligand binding and/or receptor function. Boess et al. ([Citation1997]) have also examined the role of this residue. They obtained data for E129D mutant receptors, which had reduced antagonist binding affinity but an EC50 for 5-HT similar to wild type receptors, indicating a role in ligand binding, but not in the conformational change leading to the functional response. They also obtained some results for E129Q and E129A receptors, but could not fully characterize these mutants as they expressed too poorly. Thus our results are consistent with Glu-129 being intimately involved in assembly and/or targeting, and suggest that only a small neutral residue (Gly or Ala) can substitute for Glu in this role. However, if these mutant receptors do reach the cell surface they are non-functional. Our data cannot distinguish whether this is an indirect effect (i.e., the result of modifying the structure of the binding pocket in some way), or a direct effect on the binding of the ligand, but, as described above, there is strong support for the latter from the Boess et al. ([Citation1997]) data. We therefore propose that a second role of Glu-129 is to interact directly with 5-HT in the binding pocket. It is noteworthy that previous studies have shown that the binding site of the 5–HT3 receptor is acidic, and have proposed that it probably contains at least 2 acidic residues (Tairi et al. [Citation1998]). Our original model only has one acidic residue (Glu-236) in the binding pocket, and realigning the loop A region by a single amino acid would also place Glu-129 into this pocket.
The role of Phe-130
Phe-130 has previously been proposed to be an important determinant of agonist binding in the 5-HT3 receptor (Steward et al. [Citation2000]). Our current studies extend this work by examining two new mutant receptors, F130A and F130W, which both decrease the affinity of [3H]granisetron binding, but increase the sensitivity of the receptor to 5-HT. Conversely F130Y mutants do not affect [3H]granisetron binding but increase the EC50. This suggests that this residue plays a role in the ligand binding site and may also be involved in the conformational change leading to the functional response, although it is possible that the mutations exert their effects via structural changes to the binding site, or even global changes to the receptor. In the original model Phe-130 faces away from the binding site, where it could play a role in conformational changes and/or structural effects. However, in our revised model (see below) it is too far from the binding pocket to be directly involved in ligand binding.
Interestingly, Steward et al. ([Citation2000]) generated a mutant receptor (F130N) that displayed a functional response when activated with acetylcholine (EC50=256 µM; Steward et al. [Citation2000]). To test whether this response to acetylcholine was the effect of a mutating Phe-130 to its nACh receptor counterpart or was a side effect of an increase in receptor sensitivity to agonists, we tested the response of the F130A mutant to acetylcholine. Preliminary results, using atropine as an inhibitor of the endogenous muscarinic receptors present on HEK 293 cells showed a small, but significant response to 1 mM ACh stimulation when compared to wild type receptors (data not shown). As we observed non-specific responses at acetylcholine concentrations greater than 1 mM, it was not possible to obtain an EC50 value. Nevertheless, as F130A and F130N both respond to acetylcholine stimulation, it suggests that these mutations may have converted the binding site of the 5-HT3 receptor so that it is more sensitive to less selective ligands, and can be activated at lower concentrations.
Implications for the role of binding loop A
The binding loop A residues Asn-128, Glu-129, and Phe-130 are conserved in all known 5-HT3A and 5-HT3B receptor subunits and consequently it is likely that all these residues are critical for receptor binding and/or function (Reeves & Lummis [Citation2002]). The original model of the 5-HT3 receptor binding pocket, based on the structure of AChBP, predicts that the side chain of Asn-128 points into the binding pocket and interacts with 5-HT via a hydrogen bond. The results of this study do not support this hypothesis. The low levels of sequence homology between AChBP and Cys-loop receptors in the loop A region means that generating an accurate sequence alignment here is difficult. The GABAA/AChBP alignment performed by Cromer et al. ([Citation2002]) was edited by hand to optimize the accuracy of the model, but they state that the most questionable region of alignment was in this binding loop A region. In light of this questionable alignment for the 5-HT3 receptor, and the binding and function results for the NEF region of binding loop A, it is possible that our previous 5-HT3 receptor subunit alignment with AChBP, and therefore the resulting homology model, are not completely accurate in this region.
With our new data in mind, we have generated new models, showing alternative forms of the binding site (). B depicts the ligand binding site with 5-HT and granisetron docked as predicted by the original model. Here, Asn-128 points into the binding site and could form a hydrogen bond with ligands, while Glu-129 and Phe-130 are directed away from the ligand.
New model 1, shown in C, shows the amino acids within binding loop A moved towards the N-terminus, so that Glu-129 effectively replaces Asn-128 and points directly into the binding pocket. In this model Glu-129 could stabilize the ligand-receptor interaction by hydrogen bonding and/or charge interactions with 5-HT. Asn-128 is directed away from the binding pocket and is most closely associated with Val-131. As a consequence, a change in size or charge of Asn-128 might interfere with this interaction, and a small hydrophobic residue would be favoured. Phe-130 points towards the binding site, where it could have a role in both binding and function.
New model 2, in D, shows binding loop A residues moved even further towards the N-terminus so that Phe-130 effectively replaces Asn-128 and points directly into the binding site. This model places Phe-130 near the aromatic group of 5-HT and in close proximity to granisetron. From this model it seems likely that a large increase in the size of the residue at Phe-130 (i.e., to Trp) would cause steric clashes in the binding site and adversely affect ligand binding. Glu-129 points away from the ligand and is close to Asp-132, which would not be favourable. Asn-128 is quite distant, and it is not clear how changes here would modify the binding of function of the receptor.
The results of this modelling suggest that the experimental evidence best supports new model 1 (C). In this model the presence of Glu-129 in the binding site would be consistent with our hypothesis that is a critical binding residue, and supports the results showing that the micro-environment of the binding site is acidic (Tairi et al. [Citation1998]). Phe-130 points towards the binding site and could have a role in both binding and function. It may also assist in creating an aromatic region here. Finally, Asn-128 faces way from the binding pocket, where it could play a role in function but would have little effect on ligand binding.
In conclusion, we have examined the roles of the loop A residues Asn-128, Glu-129 and Phe-130, and our data show that Glu-129 has a greater effect on receptor function than Asn-128 and Phe-130. In addition to its role in receptor assembly and/or targeting, we propose that it is the most likely candidate to contribute to interactions between the ligand and the receptor, and suggest that a realignment of the 5-HT3 receptor subunit sequence relative to AChBP may generate a more accurate model of the binding pocket. We await high resolution crystallographic images of the receptor to test this hypothesis.
We gratefully acknowledge the support of the Wellcome Trust. SCRL is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science. NLS was in receipt of a Gates scholarship.
References
- Boess FG, Steward LJ, Steele JA, Liu D, Reid J, Glencorse TA, Martin IL. Analysis of the ligand binding site of the 5-HT3 receptor using site directed mutagenesis: importance of glutamate 106. Neuropharmacology 1997; 36: 637–647
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001; 411: 269–276
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 2004; 41: 907–914
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 1987; 7: 2745–2752
- Cromer BA, Morton CJ, Parker MW. Anxiety over GABAA receptor structure relieved by AChBP. Trends Biochem Sci 2002; 27: 280–287
- Hargreaves AC, Gunthorpe MJ, Taylor CW, Lummis SC. Direct inhibition of 5-hydroxytryptamine3 receptors by antagonists of L- type Ca2 + channels. Mol Pharmacol 1996; 50: 1284–1294
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 1985; 82: 488–492
- Le Novere N, Grutter T, Changeux JP. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2 + -binding sites. Proc Natl Acad Sci USA 2002; 99: 3210–3215
- Lummis SC, Sepulveda MI, Kilpatrick GJ, Baker J. Characterization of [3H]meta-chlorophenylbiguanide binding to 5-HT3 receptors in N1E-115 neuroblastoma cells. Eur J Pharmacol 1993; 243: 7–11
- Maksay G, Bikadi Z, Simonyi M. Binding interactions of antagonists with 5-hydroxytryptamine3A receptor models. J Recept Signal Transduct Res 2003; 23: 255–270
- Price KL, Lummis SC. The role of tyrosine residues in the extracellular domain of the 5-hydroxytryptamine3 receptor. J Biol Chem 2004; 279: 23294–23301
- Price KL, Lummis SC. FlexStation examination of 5-HT3 receptor function using Ca2 + - and membrane potential-sensitive dyes: advantages and potential problems. J Neurosci Methods 2005; 149: 172–177
- Reeves DC, Lummis SC. The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review). Mol Membr Biol 2002; 19: 11–26
- Reeves DC, Sayed MF, Chau PL, Price KL, Lummis SC. Prediction of 5-HT3 receptor agonist-binding residues using homology modeling. Biophys J 2003; 84: 2338–2344
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 1993; 234: 779–815
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, New York 1989; 15,54–15,56
- Schapira M, Abagyan R, Totrov M. Structural model of nicotinic acetylcholine receptor isotypes bound to acetylcholine and nicotine. BMC Struct Biol 2002; 2: 1
- Sine SM, Quiram P, Papanikolaou F, Kreienkamp HJ, Taylor P. Conserved tyrosines in the alpha subunit of the nicotinic acetylcholine receptor stabilize quaternary ammonium groups of agonists and curariform antagonists. J Biol Chem 1994; 269: 8808–8816
- Spier AD, Lummis SC. The role of tryptophan residues in the 5-Hydroxytryptamine3 receptor ligand binding domain. J Biol Chem 2000; 275: 5620–5625
- Spier AD, Wotherspoon G, Nayak SV, Nichols RA, Priestley JV, Lummis SC. Antibodies against the extracellular domain of the 5-HT3 receptor label both native and recombinant receptors. Mol Brain Res 1999; 67: 221–230
- Steward LJ, Boess FG, Steele JA, Liu D, Wong N, Martin IL. Importance of phenylalanine 107 in agonist recognition by the 5-hydroxytryptamine3A receptor. Mol Pharmacol 2000; 57: 1249–1255
- Tairi AP, Hovius R, Pick H, Blasey H, Bernard A, Surprenant A, Lundstrom K, Vogel H. Ligand binding to the serotonin 5HT3 receptor studied with a novel fluorescent ligand. Biochemistry 1998; 37: 15850–15864
- Thompson AJ, Price KL, Reeves DC, Chan SL, Chau PL, Lummis SC. Locating an antagonist in the 5-HT3 receptor binding site using modeling and radioligand binding. J Biol Chem 2005; 280: 20476–20482
- Turchin A, Lawler JF, Jr. The primer generator: a program that facilitates the selection of oligonucleotides for site-directed mutagenesis. Biotechniques 1999; 26: 672–676
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol 2005; 346: 967–989