Abstract
In hair cells of the inner ear, phosphatidylserine (PS), detected with fluorescent annexin V labeling, was rapidly exposed on the external leaflet of apical plasma membranes upon dissection of the organ of Corti. PS externalization was unchanged by caspase inhibition, suggesting that externalization did not portend apoptosis or necrosis. Consistent with that conclusion, mitochondrial membrane potential and hair-cell nuclear structure remained normal during externalization. PS externalization was triggered by forskolin, which raises cAMP, and blocked by inhibitors of adenylyl cyclase. Blocking Na+ influx by inhibiting the mechanoelectrical transduction channels and P2X ATP channels also inhibited external PS externalization. Diminished PS externalization was also seen in cells exposed to LY 294002, which blocks membrane recycling in hair cells by inhibiting phosphatidylinositol 3-kinase. These results indicate that PS exposure on the external leaflet, presumably requiring vesicular transport, results from elevation of intracellular cAMP, which can be triggered by Na+ entry into hair cells.
Introduction
Hair cells of the inner ear, the sensory cells responsible for hearing and balance, are epithelial cells with specialized microvilli, called stereocilia, located on their apical surfaces. Sound moves stereocilia and gates an ionic current that affects the hair cell membrane potential. Hair cells have two major domains: an apical domain, bathed in high K+, low Na+, and low Ca2 + endolymph, and a basolateral domain, bathed by plasma-like perilymph (). Apical tight junctions not only precludes endolymph-perilymph exchange, but prevent proteins and lipids from readily diffusing between domains Citation[1]
Figure 1. Organization of the apical pole of an auditory hair cell. Stereocilia are located at the top of a hair cell and insert into the cuticular plate, a meshwork of actin filaments located between the stereocilia and the soma. Tight junctions connecting hair cells and supporting cells divide hair cells into apical and basolateral domains. This Figure is reproduced in colour in Molecular Membrane Biology online.
Biological membranes are highly asymmetric, with phospholipids non-randomly distributed between the two leaflets Citation[2]. The outer leaflet primarily contains phosphatidylcholine and sphingomyelin, while the inner leaflet contains the aminophospholipids, including phosphatidylethanolamine and phosphatidylserine (PS). Phosphatidic acid and the phosphatidylinositols are also concentrated on the cytoplasmic side of the membrane. Mechanisms for maintaining transmembrane lipid distribution are only partially understood. An ATP-dependent aminophospholipid translocase (flippase) moves PS to the inner (cytoplasmic) leaflet of the plasma membrane. Another ATP-dependent translocase (floppase) moves lipids in the opposite direction. In addition, a nonspecific scramblase allows all types of phospholipids, regardless of headgroup, to rapidly equilibrate across the bilayer Citation[3]. Exposure of PS on the external cell surface occurs in both normal and pathologic conditions Citation[4], Citation[5]. For example, PS exposure on platelet surfaces is fundamental to blood coagulation Citation[5]. PS surface exposure in a hepatocytic cell line has been implicated in biliary lipid secretion Citation[6]. Regional PS exposure in the acrosomal region of the spermatozoa is critical for binding to the zona pellucida, oocyte penetration, and signaling Citation[7]. A subset of T lymphocytes with low levels of the tyrosine phosphatase CD45 externalize PS in response to P2X7 activation and other signals Citation[8]; such cells are functionally normal. In a final example, massive degranulation of mast cells in response to IgE stimulation leads to reversible PS externalization Citation[9]. Of course, PS exposure that follows a rapid rise in cytosolic Ca2 + concentration and ATP depletion is also well known to precede apoptosis and necrosis Citation[10], Citation[11].
PS is partially exposed to the extracellular environment when bullfrog hair cells are isolated Citation[12]. We used annexin V to examine PS exposure in hair cells of the mammalian cochlea. Annexin V is a ∼35 kD, Ca2 + -dependent phospholipid-binding protein that has a high affinity for PS; it is often used as a probe for apoptosis Citation[13]. Here we show that non-apoptotic PS exposure occurs in the apical plasma membrane of hair cells, and, like in other cell types Citation[9], this PS externalization occurs by a mechanism related to vesicular trafficking.
Materials and methods
Tissue preparation
Guinea pigs (both female and male albino guinea pigs, 250–350 g) were anesthetized with an intramuscular injection of 1 ml/kg of 60 mg/ml ketamine, 2.4 mg/ml xylazine, and 1.2 mg/ml acepromazine in H2O, then killed by decapitation. Cochleae were rapidly removed from the bulla, opened, and dissected in a Petri dish filled with a standard artificial perilymph composed of (in mM): NaCl 125, KCl 3.5, glucose 5, HEPES 10, CaCl2 1.3, and MgCl2 1.5. NaH2PO4 0.51. The osmolarity of the solution was adjusted to 310 mOsm with NaCl and the pH was adjusted to 7.4 with NaOH. All experiments were performed at room temperature. A segment of the organ of Corti from the third turn of the cochlea was carefully removed and used as the experimental tissue. The organ of Corti was then transferred to a perfusion bath that was coated with silicon rubber (Sylgard 184, Dow Corning). The tissue was pinned to the silicone rubber layer in the bottom of a chamber (4 ml) that was equipped with a perfusion system. The procedures of this study were approved by the Animal Institutional Care and Use Committee of the Oregon Health & Sciences University.
Isolation of hair cells
The isolation procedure was essentially the same as described in our earlier study Citation[14]. The anesthetized animal was decapitated, and the bulla and the otic capsule were opened. The apical and third turns of the cochlea were removed and transferred into a drop of perilymph solution containing collagenase (1 mg/ml Sigma Type IV) in a Petri dish for 10 min. After enzyme treatment, the tissues were washed three times in fresh perilymph. Hair cells were manually dissociated with a fine needle (WPI, Inc) and gently transferred into small Petri dishes for experiments. After dissociation, these cells are relatively depolarized (−13 to −15 mV Citation[15]). We have not measured transduction currents from these cells, although in the experience of others with this preparation (e.g., ref. Citation[16]), such currents will be at best extremely small.
Annexin V staining
In order to detect exposure of PS on the external layer of the plasma membrane, tissues and isolated hair cells were labeled with either annexin V-Alexa Fluor 488 or annexin V-Alexa Fluor 568 (Molecular Probes, Eugene, Oregon USA), by incubating tissues with 1 µg/ml fluorescent annexin V in the medium for 10 min. Tissues were washed and mounted. Labeling was visualized by fluorescence microscopy using 488 nm or 568 excitation and at 519 nm or 618 emission filters using a Nikon E 600 fluorescence microscope equipped with a CCD camera controlled by Metamorph software or with a Bio-Rad MRC 1024 confocal laser microscope system using a 40X, NA = 1.0 objective lens. To co-localize PS and the whole external leaflet of plasma membranes, isolated hair cells were placed in artificial perilymph solution containing 1 µg/ml annexin V and 10 µM N-(4-sulfobutyl)-4-(4-(4-(dipentylamino)phenyl)butadienyl)pyridinium (RH421; Molecular Probes, Eugene Oregon USA) for 10 min, then were washed with dye free solution. To detect mitochondrial activity, tissues and isolated hair cells were placed in an artificial perilymph solution containing annexin V and 100 nM of the membrane potential dye tetramethyl rhodamine methyl ester (TMRM+; Molecular Probes, Eugene, Oregon USA) for 10 min, and then were washed with dye-free solution.
Co-localization of F-actin and PS externalization
To detect whether PS externalization is correlated with cytoskeleton disassembly, tissues were labeled with fluorescent annexin V, fixed with 4% paraformaldehyde for 2 h, washed with phosphate-buffered saline (PBS) for 30 min, and permeabilized in 0.5% Triton-X 100 for 1 h. Tissues were incubated with either rhodamine-phalloidin or FITC-phalloidin (Molecular Probes, Eugene, Oregon USA) at 4 units/ml for 1 h, then rinsed with PBS for 10 min. Imaging was performed using confocal microscopy.
Fluorescent imaging of intracellular Na+
Intracellular Na+ concentrations were estimated by incubation with Sodium Green (10 µM) (Molecular Probes, Eugene, Oregon USA) for 15 min, followed by a wash with fresh standard perilymph solution. Sodium Green fluorescence signals were imaged with 488 nm excitation and 519 nm emission filters using confocal microscopy.
Cell viability measurements
Tissues were dissected and placed in a flow bath. After 10 min incubation, propidium iodide (PI, 1 µg/ml) was added to the solution for 10 min, following washout with fresh perilymph solution. Images were acquired with 568 nm excitation and 618 nm emission filters.
FM 4-64 fluorescence
To monitor the effect of LY 294002 on cell endocytosis and exocytosis, 1 µM FM 4-64 (Molecular Probes, Eugene, Oregon USA) was added into the artificial perilymph solution. Cells were observed on a Nikon microscope with a 40X, NA = 0.8 water-immersion lens. FM 4-64 was excited with band pass filters (peak 568 nm) and collected with an emission filter (peak 635 nm). The mean fluorescence intensity of FM 4-64 in the control and LY 294002 treated tissues, was determined by the software in a window approximately covering the entire inner hair cells in the four control tissue images (20 cells were selected in each sample picture and one sample picture from each animal) and four LY 294002 treated tissue images. A mean background was determined in a small window located away from fluorescence tissue, and was subtracted from the mean fluorescence intensity value. The data were averaged within the total four control images and the four LY 294002 treated tissue images.
Scala media perfusion
The pipette (5–7 mm tip diameter) was held in a hydraulic microdrive/micromanipulator and advanced into scala media by penetration through a slit in the round window and through the basilar membrane. The 3rd turn lateral wall of the cochlea was opened to allow the endolymph to escape. Perfusion used either artificial endolymph, composed of (in mM) KCl 126, NaCl 1, KHCO3 25, MgCl2 0.025 CaCl2 0.025, K2HPO4 1.4, mannitol 25, or artificial perilymph, composed of (in mM): NaCl 125, KCl 3.5, glucose 5, HEPES 10, CaCl2 1.3, and MgCl2 1.5. NaH2PO4 0.51; each solution contained annexin V-Alexa Fluor 568 (1 µg/ml). Perfusion was accomplished using a precision syringe pump at the rate of 200 nl/min for 40 min. After perfusion, animals were sacrificed and the organ of Corti was isolated; fluorescence signals from tissues were observed as above under a Bio-Rad MRC 1024 confocal laser microscope system using 40X and 60X objective lenses.
Reagent applications
To modulate PS externalization in hair cells of isolated organ of Corti, reagents were applied in the artificial perilymph solution. Unless indicated, the reagent remained in artificial perilymph throughout the entire experiment ().
Table I. Reagents employed.
Statistical analysis
Data are presented as mean±SD; values for n refer to either the numbers of animals or the numbers of hair cells. Differences between data sets were assessed with the Student's t-test. Differences were considered significant at p<0.05.
Results
Apical PS externalization in hair cells
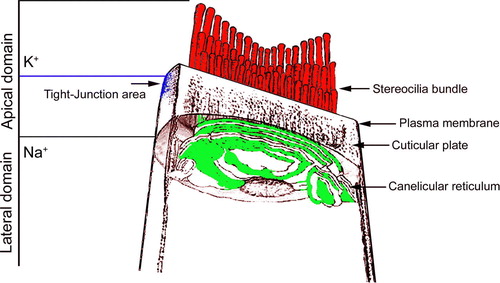
When we labeled external plasma membranes of isolated hair cells with RH 421 (A) and fluorescent annexin V, we found that PS externalization – detected by annexin V – only occurred in apical membranes (B). In a whole-mount preparation of the organ of Corti labeled with fluorescent annexin V, PS externalization was seen in apical domains of both inner hair cells (IHCs) and outer hair cells (OHCs) (D). The intensity of fluorescence annexin V labeling of IHCs was substantially higher than of OHCs (E).
Figure 2. Confocal images of hair cells labeled with annexin V-Alexa fluor 488 and RH 421 in regular perilymph solution. (A–C) RH 421 labels the entire cell membrane, while annexin V labeling is restricted to the apical domain, including the membranes of the stereocilia and in the cuticular plate region. (D) annexin V labeling of a whole-mount organ of Corti. Annexin V labeled PS labels IHCs more intensely than OHCs. (E) quantification of annexin V labeling of 30 IHC and 114 OHCs. Labeling was significantly different (p<0.05). Bar: 10 µm.
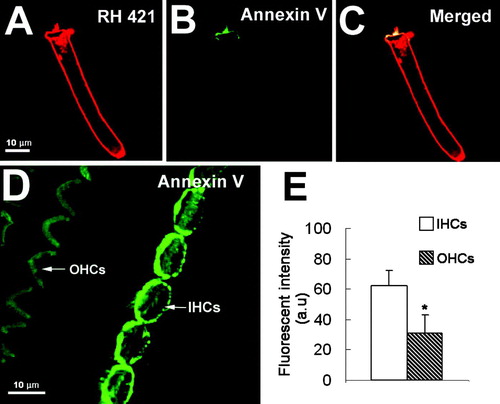
When tissues were fixed and permeabilized prior to labeling, annexin V fluorescence was observed throughout cell membranes in all cells of the organ of Corti (). The strongest fluorescence signals were still in the apical regions of IHCs and OHCs, however, particularly in IHCs (). This result suggests either than the concentration of PS is higher in apical membranes or that PS was more readily extracted from basal membranes during permeabilization.
Figure 3. Annexin V in fixed and permeabilized organ of Corti. (A) annexin V fluorescence was strong in OHC stereocilia (SC/OHC, stereocilia of outer hair cell). (B) Annexin V fluorescence in IHC stereocilia and cuticular plate region (SC/IHC, stereocilia of inner hair cell). (C) Annexin V labeling was present throughout the IHC cell membrane but was particularly strong at the apical pole; there was less annexin labeling in the basal poles (AP/IHC, apical pole of inner hair cell; BP/IHC, basal pole of inner hair cell).
Apical PS externalization of PS is not a sign of necrosis or apoptosis
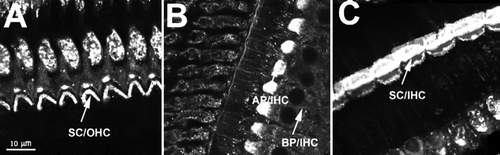
PS externalization occurs rapidly in many cell types exposed to high calcium or ATP. Under such conditions, a cell may commit to cell death by either apoptotic or necrotic processes Citation[10], Citation[11]. To test if PS externalization foreshadows apoptosis or necrosis, we labeled cells with fluorescent annexin V and the DNA-binding dye propidium iodide (PI) (), which detects the integrity of cell plasma membranes Citation[17]. We never observed PI nuclear labeling of hair cells during the 0.5–3 h incubation in artificial perilymph, despite high levels of PS externalization (D). By contrast, when we exposed the organ of Corti or isolated OHCs with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG), a DNA-alkylating agent that potently activates cell apoptosis for 30 min Citation[18], we observed apoptotic cells with PI staining in their nuclei and PS externalization throughout the plasma membrane. E shows an example of a control isolated OHC where annexin fluorescence was only seen in the apical membrane. In contrast, F shows an isolated OHC exposed to MNNG for 30 min; annexin V fluorescence was seen throughout the membrane and PI labeled nucleus.
Figure 4. PS exposure is not linked to apoptosis or necrosis. (A–D) Organ of Corti bathed for 30 min in perilymph solution. (A) DIC image. (B) annexin V labeling. (C) annexin V, focusing on the IHC layer. (D) PI staining. No PI staining is observed in the level of the nucleus of either IHCs or OHCs. (E) PS externalization in the apical cellular membrane in an isolated control OHC. Representative of results from ten animals (∼50 imaged cells). (F) After treatment with MNNG, PS externalization was apparent in all membranes (arrows), including the basolateral membrane. In addition, the nucleus was labeled with PI. Two animals were used (3 cells imaged). (G) No significant difference in PS externalization between cells treated with an apoptotic inhibitor (z-VAD-fmk) or with a control solution for a 30-min incubation period (p>0.05; n=3 animals for each condition).
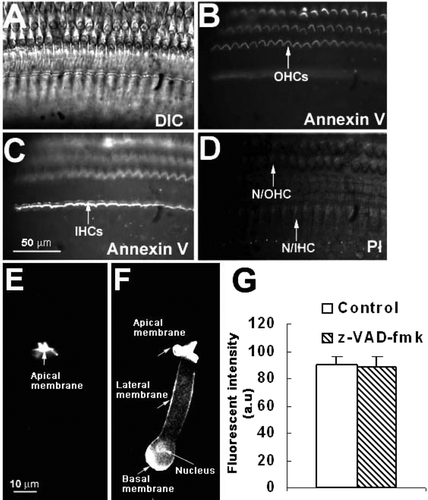
To further test if fluorescent annexin V labeling is an early apoptotic event, isolated tissues were exposed to artificial perilymph contained z-VAD-fmk (50 µM), a broad-spectrum caspase inhibitor Citation[19], Citation[20]. In many cell types, PS externalization is prevented upon inhibition of caspases Citation[21], Citation[22]. In contrast, we found no significant difference in fluorescence between the cells treated with z-VAD-fmk and untreated cells (G).
PS externalization in the normal hair cells
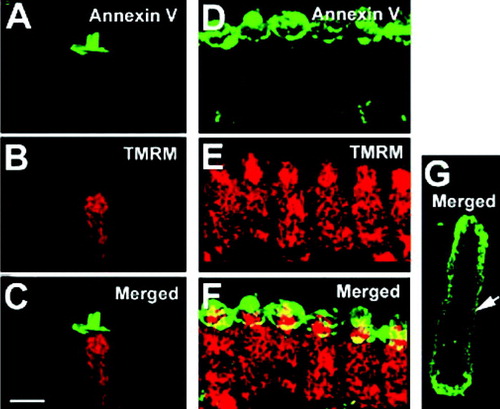
In many types of cells, external exposure of PS correlates with cell damage. In this study, we found that the regional PS externalization occurred in apparently normal hair cells, which lacked cytoplasmic granulations, displaced nuclei, or swelling, and exhibited no organellar Brownian motion under DIC microscopy Citation[23]. A–5F shows that PS was externalized only in the apical domain of an OHC (A) and IHCs (D). PS externalization was correlated with high TMRM+ fluorescence signals where mitochondria were located (B, 5E). The strong TMRM+ signals, combined with normal cellular morphology, indicated that hair cells are not damaged, despite PS externalization in the apical domain. By contrast, in swollen cells with organelles showing Brownian motion and their nuclei displaced, PS exposure was not regional but instead occurred in the entire cell membrane as shown in G. The obviously damaged OHC of G, without a hair bundle, had a significantly reduced mitochondrial membrane potential (no TMRM+ red signal).
Figure 5. Mitochondrial membrane potential is apparently normal in cells showing apical PS externalization. A,D, PS apical externalization in an isolated OHC (A) and a group of IHCs (D) (annexin V-Alexa fluor 488, green). (B, E) normal mitochondria labeling pattern (TMRM+, red). (C, F) Merged images. (G) PS externalization over the entire cellular plasma membrane (annexin V-FITC, arrow) is accompanied with a loss of mitochondrial membrane potential (TMRM+, no red signal) in this damaged cell. Data are representative of ∼10 cells from two animals. Bar: 10 µm.
PS regional externalization is stimulated by intracellular Na+
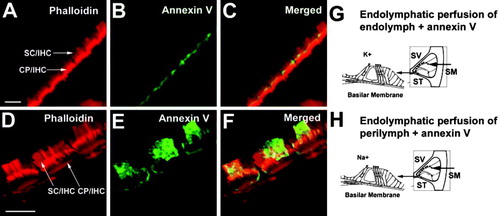
When we, in vivo, perfused the cochlea's scala media compartment, which normally contains endolymph, with artificial endolymph containing fluorescent annexin V, there was very little labeling on apical membrane domains of hair cells (B). By contrast, after endolymphatic perfusion of annexin V-containing artificial perilymph (which includes Na+), surface exposure of PS was considerably higher (E). These results suggest that apical exposure of the organ of Corti to high Na+ induced PS external exposure.
Figure 6. PS externalization after scala media perfusion in vivo. (A–C) scala media perfusion with artificial endolymph. (A) Phalloidin labeling of IHCs to detect F-actin, indicating normal stereocilia morphology. (B) Very weak annexin V-labeling. (C) Merge. In four perfusion experiments with artificial endolymph, we saw weak labeling of hair cells in two experiments and no labeling in two others. (D–F) Scala media perfusion with artificial perilymph. (D) Phalloidin labeling, showing normal stereocilia and cuticular plates. Arrows indicate IHC stereocilia (SC/IHC) and the cuticular plate (CP/IHC). (E) Annexin V labeling is increased in animals perfused with artificial perilymph. (F) Merge. Strong PS labeling in the cochlea's apical turn was observed in two of three perfusion experiments. (G) Experimental design of scala media (SM) perfusion; perfusion with artificial endolymph maintains the normal high K+ concentration at the apical surface of hair cells. (H) Perfusion with artificial perilymph produces a high Na+ concentration at the apical surface of hair cells. The natural perilymph in the scala vestibuli (SV) and scala tympani (ST) is not affected by the SM perfusion. Bar: 5 µm.
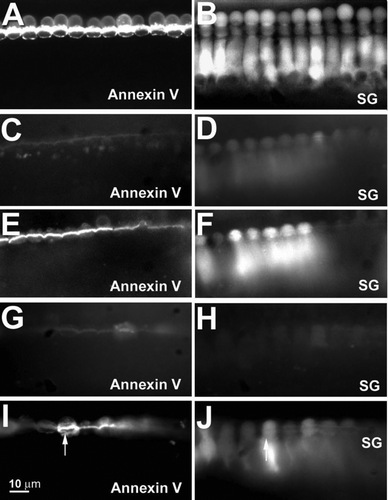
To determine the role of Na+ in PS externalization, we combined fluorescent annexin V labeling with detection of Na+ with Sodium Green dye. When we incubated tissue with an artificial perilymph solution at 60 min, PS labeling was high on the apical hair cell surface (A). Apical blebs were also present, as previously described Citation[24]. At the same time, the intracellular sodium fluorescent signal was high (B). When tissues were incubated with NMDG-Cl solution, where Na+ was replaced by NMDG+, PS externalization was reduced substantially over the 60 min incubation period (C). The intracellular Na+ signal remained low (D). After the NMDG-Cl solution was replaced with standard perilymph solution and maintained for 10 min, annexin V labeling (E) and Sodium Green fluorescence (F) both rapidly increased. In another experiment, we bathed tissues in standard perilymph solution that contained amiloride (to block the mechanoelectrical transduction channel) and PPADS (to block P2X2 channels); this treatment prevents Na+ loading of IHCs (H). Annexin V labeling was greatly decreased after incubation for 60 min with amiloride and PPADS (G). After 10 min washout, Na+ fluorescent signals rapidly increased (J) and annexin V label appeared on the surface of the majority of IHCs (I). Thus, PS externalization was highly correlated with the degree of intracellular Na+ loading.
Figure 7. Na+ entry is correlated with PS externalization in vitro. (A) Annexin V labeling is high in artificial perilymph solution (Na+ 125 mM). (B) Corresponding intracellular Na+ fluorescent signal of Sodium Green detection is high. C, annexin V labeling is reduced in NMDG-Cl (Na+ free) solution. (D) Corresponding Na+ levels are low with Sodium Green detection. (E) Annexin V labeling increases after replacing NMDG-Cl with regular artificial perilymph. F, corresponding increase in Sodium Green signal. (G) Blocking mechanotransduction and P2X2 channels with amiloride and PPADS reduces annexin V labeling. (H) Corresponding reduced Sodium Green signal. (I) Annexin V labeling increases after channel blockers were washed out. (J) Increase in Sodium Green signal. Note correlation of high annexin V labeling with high Sodium Green signal (arrows). Three animals were used for each condition (41 inner hair cells for NMDG treatment; 46 cells for P2X2–amiloride treatment). Bars: 10 µm.
Calcium levels are negatively correlated with PS expression
PS scrambling can be triggered by a rapid rise in cytosolic Ca2 + concentration and ATP depletion Citation[10], Citation[11]. By contrast, using fluorescent annexin V and fluo-3 AM, a calcium indicator, PS externalization was negatively correlated with high intracellular Ca2 + levels: cells with high fluo-3 fluorescence had little or no annexin V label ().
Figure 8. Cells with high levels of Ca2 + have low PS externalization in vitro. (A) Annexin V labeling. PS externalization and blebs occur variably from cell to cell and result from an imbalance of endo- and exocytosis Citation[24]. (B) Fluo-3 AM labeling. Note that high fluorescence signals indicating elevated Ca2 + were associated with cells with no apparent external PS (e.g., cell 3) and vice versa (cell 4).
![Figure 8. Cells with high levels of Ca2 + have low PS externalization in vitro. (A) Annexin V labeling. PS externalization and blebs occur variably from cell to cell and result from an imbalance of endo- and exocytosis Citation[24]. (B) Fluo-3 AM labeling. Note that high fluorescence signals indicating elevated Ca2 + were associated with cells with no apparent external PS (e.g., cell 3) and vice versa (cell 4).](/cms/asset/044e2215-cad4-4f79-afc7-da71eda4aad1/imbc_a_192605_f0008_b.gif)
PS externalization depends on cAMP
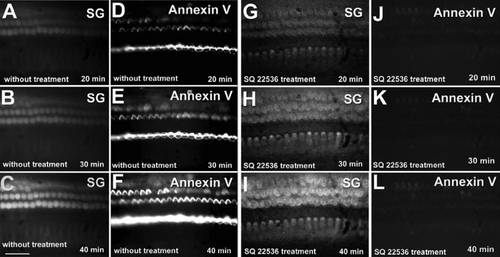
We have shown that bleb formation on apical membranes of IHCs depends on levels of intracellular cAMP Citation[24]. To test whether PS externalization follows adenylyl cyclase activation, we compared externalization with cyclase inhibitors and activators. When tissues were bathed for 40 min in perilymph solution, we observed that the Na+ fluorescent signal gradually increased (A–9C) and correspondingly, PS was exposed on the surface of hair cells (D–9F). However, when tissues were bathed in perilymph solution containing 500 µM SQ 22536, an adenylyl cyclase inhibitor, although intracellular Na+ levels gradually increased (G–9I); annexin V labeling was barely detectable (J–9L).
Figure 9. PS externalization is inhibited by block of adenylyl cyclase with SQ 22536 in vitro. (A, B, C) Sodium Green fluorescence increased when tissue was bathed in artificial perilymph solution. (D, E, F) Annexin V labeling gradually increased. (G, H, I) Sodium Green fluorescence increased during SQ 22536 incubation. (J, K, L) Annexin V labeling did not change during SQ 22536 incubation. Similar results were seen in experiments from three animals. Bar: 50 µm.
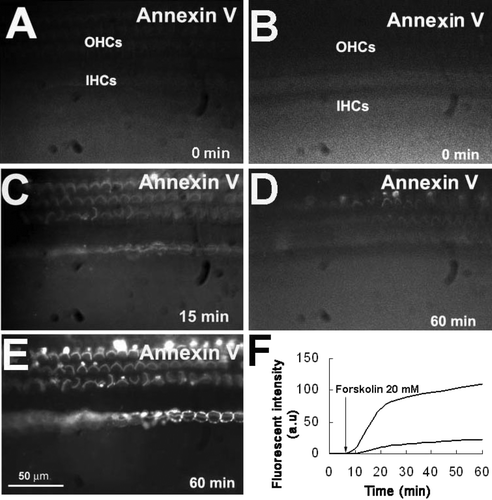
No apical annexin V labeling was seen in organ of Corti cells bathed in NMDG-Cl solution for up to 60 min (D). By contrast, bath application of 20 µM forskolin, an adenylyl cyclase activator, produced a gradual increase in annexin V labeling in every OHC and IHC, but not in supporting cells (C, 10E). Surprisingly, the orientation of the hair bundles of some OHCs in the first and second rows appeared to rotate during the treatment (C, 10E). Cell rotation was never seen when tissue were incubated in artificial perilymph (not visible in A due to labeling intensities). The mechanism of the cell rotation is the subject of a separate report.
Figure 10. PS exposure was stimulated directly by forskolin in vitro. (A–E) Organ of Corti was bathed in an NMDG-Cl solution to prevent Na+ entry. (A, B) Control conditions. No annexin V labeling was seen. (C, E) After introduction of 20 µM forskolin at time 0, annexin V labeling gradually increased (15 and 60 min). (D) In the absence of forskolin, annexin V labeling remained low at 60 min. (F) Annexin V labeling for control (solid line) and forskolin (dotted line). Results are representative of three experiments for each group. Bar: 50 µm.
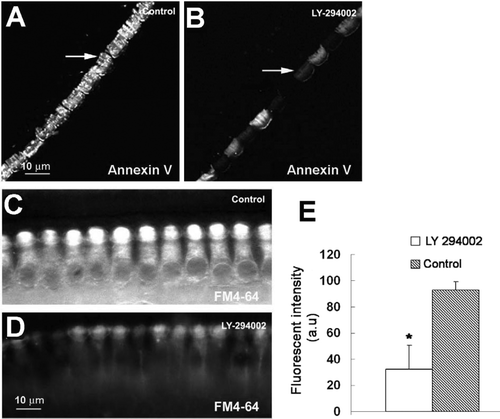
To further test if PS exposure depends on vesicular trafficking, we treated tissues with LY-294002, a PtdIns 3-kinase inhibitor. LY-294002 not only prevents bleb formation initiated by Na+ loading Citation[24], but also significantly decreased fluorescent annexin V labeling in hair bundles. Although it is also possible that LY 294002 may inhibit other pathways related to PS externalization, including tyrosine kinases downstream of PI3-K Citation[25], Citation[26], because LY-294002 inhibits uptake of FM 4-64 (C–11D), our results strongly suggests that PS externalization depends on PtdIns 3-kinase controlled membrane trafficking (A–11B). Under the conditions of our experiments, the principal pathway for FM 4-64 uptake is likely to be through apical endocytosis, not transduction channels Citation[27].
Figure 11. Inhibition of PtdIns 3-kinase with LY-294002 prevents PS externalization and slows endocytosis in vitro. (A) Annexin V labeling under control conditions. (B) Annexin V labeling after LY-294002 treatment. Annexin V labeling was inhibited. (C–D) Endocytosis measured with FM 4-64 uptake. (C) Control. (D) LY-294002. (E) Quantitation. LY-294002 significantly inhibited FM 4-64 uptake (p<0.05). Four experiments were carried out for each treatment.
Discussion
Apical membranes of outer and inner hair cells, including stereocilia, were strongly labeled with fluorescent annexin V, suggesting that these cells display PS on their outer leaflets in this region. Although PS is generally confined to the inner leaflet in mammalian cells, it is externalized to the outer leaflet during membrane fusion, aging, and apoptosis Citation[4], Citation[28]. In hair cells, PS surface exposure was not inhibited by caspase inhibitors, nor did the mitochondrial membrane potential or nuclear accessibility and morphology change during externalization. Thus, PS externalization in these cells is not a harbinger of apoptosis, but instead occurs when cells become Na+-loaded and cAMP levels increase. Our results are consistent with suggestions that membrane turnover in the apical domain of hair cells is extremely rapid Citation[29].
Mechanism of PS externalization
Annexin V binding to PS requires Ca2+ concentrations of 20 µM or more Citation[30]; indeed, we found no significant difference in annexin V fluorescence signals when tissues were bathed with 25 µM–1.5 mM extracellular CaCl2. Rapid PS exposure and scrambling are generally observed after a rapid rise in cytosolic Ca2 + concentration and ATP depletion Citation[10], Citation[11]; by contrast, we found that the highest annexin V fluorescence occurred in cells with the lowest intracellular Ca2 + (), opposite of what is expected for scramblases.
We suggest the following model. PS is thought to be more symmetrically distributed in intracellular vesicles, than in the plasma membrane Citation[5]; following exocytosis, PS will be incorporated into both leaflets of the hair cell apical membrane. Under normal conditions, a flippase activity should rapidly move PS to the intracellular leaflet, so that at steady state, little PS will be exposed to the external environment. In the presence of elevated cAMP, however, either the exocytosis rate is increased or flippase is inhibited, or both, with the consequence that the flippase can no longer match the rate of exocytosis. A new steady state is achieved with a substantially greater PS concentration on the extracellular leaflet. In our experiments, Na+ entry triggers this process, elevating cAMP levels by an unknown mechanism. In turn, cAMP increases apical membrane exocytosis via a mechanism dependent on PtdIns 3-kinase.
Regulation of vesicle transport and exocytosis by cAMP has been reported in many cell types, including neurons Citation[31]. cAMP-dependent PKA phosphorylation can enhance exocytosis by acting at different stages of vesicular transport, e.g., on the rate of vesicle cycling Citation[32]. For example, PKA can affect protein-protein interactions important for vesicle fusion, including those involving trans-soluble N-ethylmaleimide-sensitive fusion protein attachment protein (t-SNARE), vesicle-soluble N-ethylmaleimide-sensitive fusion protein attachment protein (v-SNARE), and vesicle associated membrane protein 2 (VAMP2). Likewise, phosphoinositides modulate membrane trafficking Citation[33]; we found that inhibition of PtdIns 3-kinase decreased PS externalization, consistent with a requirement for vesicular trafficking for PS exposure.
In an alternative model, high levels of Na+ might lead to decreased ATP levels due to overactive Na+/K+-ATPase; aminophospholipid translocase depends on ATP, and the hair bundle may be particularly sensitive to perturbation of cellular energy homeostasis due to its spatial isolation from mitochondria. Under these conditions, the aminophospholipid translocase might be incapable of matching the rate of PS externalization produced by exocytosis. In either case, PS exposure would be a consequence of rapid apical membrane turnover, which is also indicated by the slow formation of hair cell apical membrane blebs under these conditions, resulting from a slight imbalance between exo- and endocytosis Citation[24].
Physiological role for PS externalization
PS is likely present only at low levels under normal conditions in the extracellular leaflet of hair-cell apical membranes. Is outer-leaflet PS physiologically relevant or simply a pathological consequence of Na+ loading? An important issue is under what conditions is Na+ or cAMP elevated in hair cells.
cAMP may be important in regulating hair-cell function. The resting force felt by mechanotransduction channels is reduced by cAMP Citation[34]; similarly, spontaneous oscillations in bullfrog hair cells are inhibited by cAMP analogs Citation[35]. A reduced cytoplasmic leaflet concentration of PS might limit clustering of myosin-1c Citation[36], which would reduce resting force produced by the motor. Elevated cAMP levels could be a mechanism used by the hair cell to reduce the sensitivity of mechanotransduction.
Our experiments highlight the rapid turnover of apical membrane in hair cells, which has been noted repeatedly Citation[27], Citation[29], Citation[37]. Why do these sensory cells turn their membrane over so rapidly? Hair cells release neurotransmitter continuously, and previous experiments have suggested an intimate connection between apical and basolateral membrane endo- and exocytosis in hair cells Citation[27], Citation[29]. Thus although the membrane composition in the apical and basolateral domains is likely to be substantially different, hair cells seem to cycle their membrane compartments in a coupled manner at rates substantially higher than those of most cells. Consistent with a higher total membrane turnover in synaptically active cells, inner hair cells were labeled with annexin V substantially more strongly than outer hair cells.
In conclusion, our data show a high concentration of PS in the apical domain of hair cells and demonstrate that (i) apical PS of hair cells becomes externalized in vitro; (ii) apical PS externalization is independent of cellular apoptosis and necrosis; (iii) apical PS externalization is activated by Na+ loading and a consequent increase in intracellular cAMP, and (iv) apical PS externalization requires membrane trafficking. We suggest that PS exposure reflects the remarkably fast turnover of apical membranes of hair cells Citation[27], Citation[29], which is stimulated to yet greater levels when Na+ enters hair cells.
This paper was first published online on prEview on 27 September 2006.
The authors appreciate the contributions of Bechara Kachar, M.D (NIDCD, National Institutes of Health) on plasma membrane dynamics in sensory hair cells and valuable discussions on the possible mechanisms of phosphatidylserine externalization in the apical area of hair cells. The authors also thank Prof. Robert A. Schlegel (Pennsylvania State University) for his advice and the important question of calcium control of phosphatidylserine externalization. The authors also would like to thank the editor-in-chief and two anonymous reviewers for their scientific critical review of the work. This research was supported by NIH-NIDCD grants P30 DC005983 (A.L.N and P.G.G.), R01 DC000105 (A.L.N), R01 DC000141 (A.L.N), R01 DC002368 (P.G.G.), and R01 DC00702 (P.G.G).
References
- Forge A, Richardson G. Freeze fracture analysis of apical membranes in cochlear cultures: differences between basal and apical-coil outer hair cells and effects of neomycin. J Neurocytol 1993; 22: 854–867
- Williamson P, Schlegel RA. Back and forth – the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol 1994; 11: 199–216
- Boon JM, Lambert TN, Sisson AL, Davis AP, Smith BD. Facilitated phosphatidylserine (PS) flip-flop and thrombin activation using a synthetic PS scramblase. J Amer Chem Soc 2003; 125: 8195–8201
- Cornelissen M, Philippe J, De Sitter S, De Ridder L. Annexin V expression in apoptotic peripheral blood lymphocytes: an electron microscopic evaluation. Apoptosis 2002; 7: 41–47
- Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res 2003; 44: 233–242
- Muller P, Pomorski T, Herrmann A. Transverse movement of NBD-phospholipids in the plasma membrane of HepG2 cells. Prog Biophys Mol Biol 1996; 65: PC440–PC440
- Gadella BM, Harrison RAP. Capacitation induces Cyclic Adenosine 3′,5′-Monophosphate-dependent, but apoptosis-unrelated, exposure of aminophospholipids at the apical head plasma membrane of boar sperm cells. Biol Reprod 2002; 67: 340–350
- Elliott JI, Surprenant A, Marelli-Berg FM, Cooper JC, Cassady-Cain RL, Wooding C, Linton K, Alexander DR. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 2005; 7: 808–816
- Martin S, Pombo I, Poncet P, David B, Arock M, Blank U. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. Int Ach Allergy Immunol 2000; 123: 249–258
- Hampton MB, Vanags DM, Porn-Ares MI, Orrenius S. Involvement of extracellular calcium in phosphatidylserine exposure during apoptosis. FEBS Lett 1996; 399(3)277–282
- Courageot MP, Lepine S, Hours M, Giraud F, Sulpice JC. Involvement of sodium in early phosphatidylserine exposure and phospholipid scrambling induced by P2X7 purinoceptor activation in thymocytes. J Biol Chem 2004; 279: 21815–21823
- Hirono M, Denis CS, Richardson GP, Gillespie PG. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 2004; 44: 309–320
- van Heerde WL, Robert-Offerman S, Dumont E, Hofstra L, Doevendans PA, Smits JF, Daemen MJ, Reutelingsperger CPM. Markers of apoptosis in cardiovascular tissues: focus on Annexin V. Cardiovasc Res 2000; 45(3)549–559
- Shi XR, Ren TY, Nuttall AL. Nitric oxide distribution and production in the guinea pig cochlea. Hear Res 2001; 153: 23–31
- Lin, X.. Thesis: Studies on the ion channels in the plasma membrane of isolated guinea pig outer hair cells ( Thesis). Ann Arbor, MI: The University of Michigan, Publisher: University of Michigan Microfilms; 1993.
- Meyer J, Furness DN, Zenner H-P, Hackney CM, Gummer AW. Evidence for opening of hair-cell transducer channels after tip-link loss. J Neurosci 1998; 18: 6748–6756
- Gabler C, Kalden JR, Lorenz HM. The putative role of apoptosis-modified histones for the induction of autoimmunity in systemic lupus erythematosus. Biochem Pharmacol 2003; 66: 1441–1446
- Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of Poly(ADP-Ribose) Polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002; 297: 259–263
- Clark P, Dziarmaga A, Eccles M, Goodyer P. Rescue of defective branching nephrogenesis in renal-coloboma syndrome by the caspase inhibitor, Z-VAD-fink. J Am Soc Nephrol 2004; 15: 299–305
- He L, Wu X, Meylan F, Olson DP, Simone J, Hewgill D, Siegel R, Lipsky PE. Monitoring caspase activity in living cells using fluorescent proteins and flow cytometry. Am J Pathol 2004; 164: 1901–1913
- Andjelic S, Liou HC. Antigen receptor-induced B lymphocyte apoptosis mediated via a protease of the caspase family. Eur J Immunol 1998; 28: 570–581
- Mandal D, Moitra PK, Saha S, Basu J. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett 2002; 513: 184–188
- Zajic G, Schacht J. Comparison of isolated outer hair cells from five mammalian species. Hear Res 1987; 26: 249–256
- Shi XR, Gillespie PG, Nuttall AL. Na+ influx triggers bleb formation on inner hair cells. Am J Physiol-Cell Physiol 2005; 288: C1332–C1341
- de Vries KJ, Wiedmer T, Sims PJ, Gadella BM. Caspase-Independent Exposure of Aminophospholipids and Tyrosine Phosphorylation in Bicarbonate Responsive Human Sperm Cells. Biol Reprod 2003; 68: 2122–2134
- Sun J, Zhao J, Schwartz MA, Wang JY, Wiedmer T, Sims PJ. c-Abl tyrosine kinase binds and phosphorylates phospholipid scramblase. J Biol Chem 2001; 276: 28984–28990
- Griesinger CB, Richards CD, Ashmore JF. Fm1-43 reveals membrane recycling in adult inner hair cells of the mammalian cochlea. J Neurosci 2002; 22: 3939–3952
- Yasin Z, Witting S, Palascak MB, Joiner CH, Rucknagel DL, Franco RS. Phosphatidylsefine externalization in sickle red blood cells: associations with cell age, density, and hemoglobin F. Blood 2003; 102: 365–370
- Griesinger CB, Richards CD, Ashmore JF. Apical endocytosis in outer hair cells of the mammalian cochlea. Eur J Neurosci 2004; 20: 41–50
- Köhler G, Hering H, Zschörnig O, Arnold K. Annexin V interaction with phosphatidylserine-containing vesicles at low and neutral pH. Biochemistry 1997; 36: 8189–8194
- Gromada J, Rorsman P. New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res 2004; 36: 822–829
- Evans GJ, Morgan A. Regulation of the exocytotic machinery by cAMP-dependent protein kinase: implications for presynaptic plasticity. Biochem Soc Trans 2003; 31: 824–827
- Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta 2001; 1533: 190–206
- Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechanoelectrical transduction in turtle auditory hair cells. J Physiol-London 1997; 501: 111–124
- Martin TFJ. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta (BBA)-Mol Cell Res 2003; 1641: 157–165
- Gillespie PG, Cyr JL. Myosin-1c, the hair cell's adaptation motor. Annu Rev Physiol 2004; 66: 521–545
- Seiler C, Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol 1999; 41: 424–434