Abstract
Understanding the nature of the interaction of the plant alkaloid ryanodine with its receptor channel (RyR) is important to aid interpretation of physiological studies and provide structure-function information about RyR. We present here the first quantitative description of the relative single-channel kinetic effects of a single-point mutation in RyR2. We exploit the well-characterized ryanoid 8β-amino-9α-hydroxyryanodine that displays reversible kinetics with RyR2. We explicitly show that the effect of the Q4863A mutation is to increase the apparent dissociation constant by increasing the apparent dissociation rate of the ryanoid. The voltage-dependence of the interaction displays no change. We infer that Q4863 is not involved with the voltage-drop but is able to influence ryanoid-bound structural changes. We discuss structural mechanisms by which this mutation could affect ryanoid interaction.
Introduction
The plant alkaloid ryanodine is an extract from the powdered stems of the tropical American shrub Ryania speciosa. It was initially investigated for its insecticidal properties in the 1940s and has seen limited, though effective, use as a ‘natural’ pesticide since Citation[1].
Ryanodine causes dysfunction in muscle contraction through disruption of a critical step in excitation-contraction coupling. Studies with tritiated ryanodine were pivotal in enabling the identification and purification of the specific molecular target of ryanodine – the ryanodine receptor channel (RyR). The protein has been cloned and expressed Citation[2]. Functional studies elucidated its mechanism of action Citation[3]. Located in sarco(endo)plasmic reticulum membranes, RyR channels mediate the regulated release of Ca2 + from intracellular stores into the cytoplasm. RyRs have a fundamental role in coupling excitation to contraction in muscle as well as contributing to a variety of other processes Citation[4]. Ryanodine continues to be an invaluable pharmacological tool to probe the role of RyRs in physiology.
The interaction of RyR with ryanodine is highly specific and dependent on concentration. At low, nM, ryanodine concentrations, channel function is modified characterized by both a reduced single-channel conductance and an increased probability of being in the ion-conducting state Citation[5], Citation[6]. This ‘high affinity’ interaction lasts for tens of minutes to hours. At higher concentrations, µM to mM, the channel is rendered closed to ion flow Citation[7].
Understanding the nature of the RyR/ryanodine interaction is important to aid interpretation of physiological studies and provide structure-function information about RyR. Some information has been gained from work with ryanoids – natural congeners and chemical derivatives of ryanodine synthesized from the extracted compound Citation[8–10]. Single channel studies with ryanoids have shed light on the mechanisms involved in the ‘high affinity ryanoid/RyR’ interaction. The altered ligand structure causes changes both to timescales and conductance of the modified state. In the absence of ryanoids, modified events are not observed suggesting that the conformation of the channel underlying modified function either does not exist (i.e., requires the ligand bound) or is at least very unlikely without ryanoid bound. It was proposed that the modified state represents a change in channel structure rapidly formed by ligand-binding. By interpreting the modified state as the ligand-bound channel, dwell times were measured. As ligand-binding and the functional change in channel properties appear concomitant under current recording conditions, the kinetic parameters associated are preceded with ‘apparent’. The simplest model to account for the measurements suggests a bimolecular and state-dependent reaction: the ryanoid can only access the modified state through the open conformation of the channel. Furthermore, the channel is able to close within the modified state i.e. with ligand bound. Together, these observations led to a working model of ryanoid interaction (see and Methods). The interaction is dependent upon electrical holding potential. As the trans-membrane potential increases positively (relative to the luminal side of the channel), the apparent rate of association increases while the apparent rate of dissociation decreases. A 3-D QSAR analysis of RyR function with several ryanoids Citation[11] has identified significant features of the ryanodine molecule involved in the interaction. The pyrrole group is the primary locus of molecular recognition Citation[12] whilst distal changes around positions 9 and 10 have a strong effect on the ryanoid-modified conductance Citation[11].
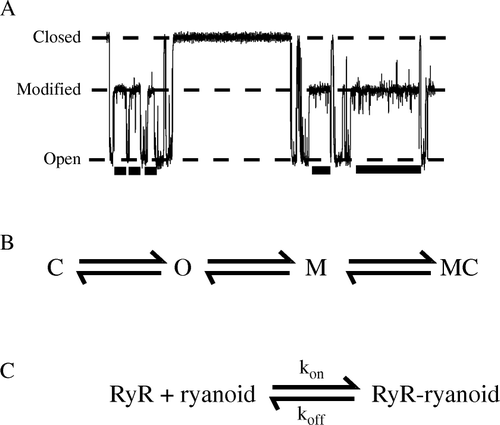
Figure 1. The working model of the high-affinity interaction of a ryanoid with a RyR2 channel. A section of trace with ryanoid-bound ‘modified’ sections marked as a bar beneath is shown in (A). Open, closed, and modified conductance levels are marked. (B) RyR2, in the absence of ryanoid, flickers between open (O) and closed (C) states. The ryanoid only modifies and unmodifies from the open (O) state. When the ryanoid is bound to the channel, the channel is described as modified (M) and may close from and to the modified level (MC). The ryanoid-bound and ryanoid-unbound closed states are indistinguishable. (C) The simple, bimolecular scheme that describes the reversible interaction. kon and koff are the apparent rates of association of the ryanoid and RyR2 and dissociation respectively.
Photoaffinity and tryptic digest studies localized the ryanodine binding site to the pore-forming C-terminal of RyR Citation[13], Citation[14]. More recently, molecular biology with cloned RyR has yielded further information. Mutagenesis studies identify several residues in the putative channel-forming region that affect ryanodine-binding. We focus, in this study, on glutamine 4863 located in the middle of predicted trans-membrane helix 10 thought to line the ion-conducting pathway Citation[15]. We have established that mutation to alanine abolishes 3H-ryanodine binding to populations of RyR2 Citation[16]. At the single-channel level, it dramatically shortens dwell times in ryanodine Citation[16] and ryanoid modified states with additional effects on some ryanoid modified conductances Citation[17]. In the present study we exploit the well-characterized ryanoid 8β-amino-9α-hydroxyryanodine that displays measurably reversible kinetics with both native sheep wtCitation[18] and cloned mouse Q4863A RyR2 Citation[17] to present the first quantitative description of the relative single-channel kinetic effects of a single-point mutation in RyR2.
Materials and methods
Materials
Phosphatidylethanolamine was purchased from Avanti Polar Lipids (Alabaster, AL, USA). Ryanodine was supplied by Agrisystems International (Wind Gap, PA, USA) and 3H-ryanodine by Amersham Biosciences, UK (Little Chalfont, Buckinghamshire, UK). 8β-amino-9α-hydroxyryanodine was synthesized as previously described Citation[11] and stored as a stock solution in 50% ethanol at −20°C. All other reagents were of the highest grade available and obtained from either VWR (Lutterworth, Leicestershire, UK) or Sigma-Aldrich (Poole, Dorset, UK).
Site-directed mutagenesis, DNA transfection and protein purification
The methodology of purified, recombinant RyR2 channel preparation has been described in detail previously Citation[16]. Briefly, the Q4863A mutation was introduced into mouse RyR2 DNA via polymerase chain reaction. The method of calcium phosphate precipitation was employed to transfect human embryonic kidney 293 cells with mouse DNA. Purification of recombinant channels was achieved by sucrose density centrifugation monitored by 3H-ryanodine-binding or Western blotting.
Planar phospholipid bilayer formation and single-channel measurements
Phospholipid bilayers were formed from suspension of phosphatidylethanolamine in n-decane (35 mg.ml−1) dispersed over a hole of diameter 200 µm in a partition separating a 0.5 ml cis chamber from a 1 ml trans chamber. The trans chamber was held at virtual ground and the cis chamber voltage-clamped to the desired electrical potential with an operational amplifier in the current to voltage configuration Citation[19], Citation[20] in a custom-built set-up. The recording solution was made with 600 mM KCl, 20 mM HEPES titrated to pH 7.4 with KOH adding a further ∼10mM K+; contaminant Ca2 + levels were ∼10 µM. The purified channel preparation was added solely to the cis chamber. Under these conditions, RyR channels preferentially insert into the bilayer such that the cytosolic face of the channel is exposed to the cis chamber. Up to two 100 µl aliquots of 3M KCl were added to the cis (cytosolic) chamber to create an osmotic gradient across the bilayer to assist insertion. Following channel incorporation, the cis chamber was perfused with fresh recording solution to remove the gradient and excess channels. Channels were then activated by addition of up to 200 µM EMD 41000 to the cis chamber Citation[21]. 8β-amino-9α-hydroxyryanodine was added to the cis (cytosolic) chamber at the required concentration. All experiments were conducted at room temperature (22±2°C).
Single-channel data acquisition
Single-channel currents were low-pass pre-filtered at 1 kHz with an eight-pole Bessel filter and sampled at 20 kHz with a PCI-6036i AD board (National Instruments, Austin, TX) for viewing and acquisition with Acquire 4.0 (Bruxton Corporation, Seattle, WA).
Measurement of single-channel kinetics parameters
In the presence of 8β-amino-9α-hydroxyryanodine, RyR2 reversibly converts between a reduced-conductance state as well as the full conductance and closed states Citation[22] (Figure 1A). The observations that ryanoid binding (modification) and unbinding occur from the open state of the channel and that the channel can close from the modified state have defined a working model of ryanoid interaction Citation[23] (Figure 1B). The timescales of bilayer recording limit the number of events that may be observed. In this study, we have chosen to focus on the effects of trans-membrane voltage measuring 10s-100s events under each condition. Previous studies are consistent with a simple bimolecular combination of ligand and receptor Citation[23] (Figure 1C) and data in this work have been analysed under this assumption. Apparent rate constants were obtained from the following relationships:where kon and koff are the apparent association and dissociation rate constants and τunmod and τmod are characteristic dwell times- estimated from fitting of single exponential distributions to dwell time distributions- in the unmodified and modified states respectively.
Dwell times in unmodified and modified states were calculated from steady-state recordings of approximately one minute duration for Q4863A and minutes with wt RyR2 in order to obtain estimates of kinetic parameters. The apparent association rate constant of the molecules is dependent on channel open probability, Po. To minimize the effects of the natural variability of Po between channels, experiments were performed in the presence of the activating agent EMD 41000. In addition, apparent association rate constants were normalized to a Po of 1 based on estimates of Po in unmodified sections of recording under identical conditions. Note that the uncertainty in Po estimation is greater the shorter the length of recording: in correspondence with this, normalization of the Q4863A association rates is less reliable.
Analysis of kinetic parameters
In previous studies with ryanoids, dwell time measurements were made manually with cursors Citation[23]. The kinetics of the ryanoid/Q4863A interaction are greatly increased when compared to those of the wt channel Citation[17] rendering this method impractical. In order to measure multiple states objectively at differing timescales within the confines of our ryanoid interaction model (see below), we carried out analysis as described.
Sections of recorded data were converted to open binary format with an in-house Visual Basic program (R.A.P. Montgomery) for importing into Mathematica 5.2 (Wolfram Research, Champaign, IL). Subsequent analysis with user-defined functions followed standard methods Citation[24]. Sampled data points were assigned as open, modified, and closed based on their position relative to 50% thresholds between these states. Thresholds were estimated from all points amplitude histograms. Data points consecutive in time were joined and a finite, minimum time resolution (tmin) of 3 ms was imposed to produce an events list. The value of tmin was selected as the lowest time at which no events of reduced current amplitude due to the recording system were identified as modified on analysis of a stretch of RyR2 in the absence of ryanoid. Regions were identified as unmodified or modified based on the working model of ryanoid interaction (Figure 1B) and dwell times calculated. Random sections of data were visually inspected at each stage of analysis. Characteristic time constants were calculated by maximum likelihood fitting with missed events correction (tmin 3 ms, tmax 10000 s). These were calculated for each channel under each condition and errors (standard deviations of the means- SEM) estimated from the variance between channels. Kinetic models were fitted by weighted, least-squares non-linear regression. Further details on the method used for the analysis of kinetic parameters can be found in the supplementary data provided online.
The influence of trans-membrane holding potential on the interaction of 8β-amino-9α-hydroxyryanodine with RyR2
The probability of the channel being in the ryanoid-modified state (Pmod) increases with increasing trans-membrane electrical potential (V). This dependence is modelled as a Boltzmann distribution Citation[23]:where F is the Faraday constant, R the gas constant, T the temperature (295 K), z the valence of the reaction and Gi the Gibbs free energy of the equilibrium in the absence of a trans-membrane electrical potential.
Within this framework, the apparent association (kon) and dissociation (koff) rate constants are described thus:where kon(0) and koff(0) are the apparent rate constants in the absence of a trans-membrane potential and zon and zoff the valence of the appropriate reaction.
The apparent dissociation constant, Kd, is calculated as the ratio koff/kon.
Results
Our data with cloned wt mouse RyR2 displays kinetics of the 8β-amino-9α-hydroxyryanodine interaction that are comparable to earlier work with native sheep RyR2 Citation[18]. Modified events induced by 8β-amino-9α-hydroxyryanodine are noticeably shorter with the Q4863A RyR2 channel compared to wt (A and A).
Figure 2. The interaction of mouse wt RyR2 with 8β-amino-9α-hydroxyryanodine is dependent on trans-membrane holding potential. (A) Representative traces of mouse wt RyR2 in the presence of 4 µM cytosolic 8β-amino-9α-hydroxyryanodine at −40, −20 and 20 mV. (B) The probability of being in the ryanoid-bound modified state (Pmod) increases with increasing trans-membrane potential (V) and is well fit by a Boltzmann distribution (solid line). Each point is the mean±SEM of n=6 channels. (C) Variation of the rate of association (kon) and dissociation (koff) with holding potential. (D) Variation of the apparent dissociation constant (Kd) with holding potential (V). Each point represents the mean±SEM of n=3–6 channels. The lines represent weighted linear regressions to the points (kon – dashed; koff, Kd – solid). Parameters are quoted in the .
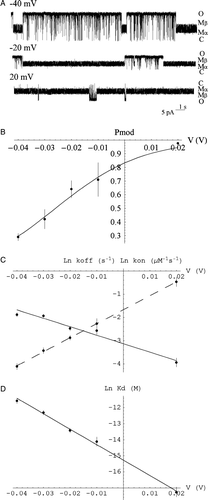
As the trans-membrane potential is raised relative to the luminal face of RyR, the length of time spent in the ligand-bound modified state, Pmod, increases for mutant and wt. The voltage-dependence of this equilibrium is well-described by a Boltzmann distribution (Figure 2B and 3B) yielding comparable values of 2.4±0.3 (n=6) and 1.9±0.1 (n=4) for z in wt and mutant respectively: the voltage-dependence of the ryanoid interaction is retained and unchanged by this RyR2 mutation.
Apparent association and dissociation rates display similar voltage-dependence in mutant and wt
Analysis of dwell times in ligand-bound modified and unbound unmodified states allows us to investigate the underlying kinetic parameters of the interaction. The apparent rates of association and dissociation of 8β-amino-9α-hydroxyryanodine with both wt and Q4863A RyR2 show a clear dependence on trans-membrane holding potential (Figures 2C and 3C). As the potential becomes more positive, the apparent rate of association of the ryanoid increases while the apparent rate of dissociation decreases in both cases. The valence of dissociation, zoff, is comparable whilst zon is slightly lower for the mutant than for wt ().
Table I. Kinetic parameters describing the interaction of 8β-amino-9α-hydroxyryanodine with single cloned mouse Q4863A and wt RyR2 channels.
Q4863A confers an increase in the apparent rate of dissociation of 8β-amino-9α-hydroxyryanodine
The apparent dissociation constant of both reactions decreases with increasing holding potential (Figures 2D and 3D). Extrapolated to 0 mV, the apparent Kd for the interaction with Q4863A RyR2 is of the order of 200 times greater than that with wt. The extrapolated values for the underlying reaction parameters display a small increase in apparent association rate constant (<10-fold) and a large (several 100-fold) increase in apparent dissociation rate constant ().
The 8β-amino-9α-hydroxyryanodine induced β modified state is rarely observed in Q4863A RyR2
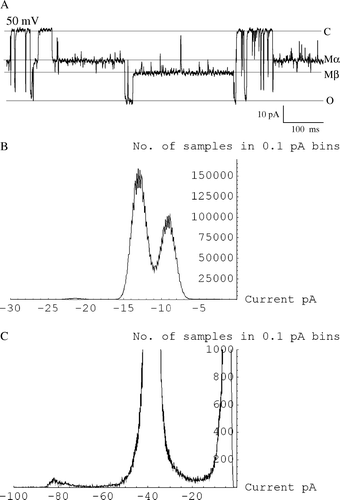
The modified state induced by 8β-amino-9α-hydroxyryanodine in wt RyR2 is noisy (Figure 2A) due to a rapid voltage-dependent flickering between two states labelled α and β (as previously observed with native sheep RyR2 Citation[18]). In contrast, modified events in Q4863A are not obviously noisy (A) although transitions to long-lived β states were observed very rarely (3 individual observations in 4 channels, e.g., A). All points amplitude histograms clearly resolve the underlying α and β states of the noisy, wt modified state (Figure 4B). Very brief transitions to a β-like state appear with increased positive potential with the Q4863A mutant channel, however this putative state cannot be discerned above bilayer noise and filter-truncated events in corresponding all points amplitude histograms (Figure 4C) and so is not amenable to analysis.
Figure 3. The interaction of mouse Q4863A RyR2 with 8β-amino-9α-hydroxyryanodine is dependent on trans-membrane holding potential. (A) Representative traces of mouse Q4863A RyR2 in the presence of 5 µM cytosolic 8β-amino-9α-hydroxyryanodine at −40, −20 and 20, 40, 60, 80 mV. (B) The probability of being in the ryanoid-bound modified state (Pmod) increases with increasing trans-membrane potential (V) and is well fit by a Boltzmann distribution (solid line). Each point is the mean±SEM of n=4 channels. (C) Variation of the apparent rate of association (kon) and dissociation (koff) with holding potential (V). (D) Variation of the apparent dissociation constant (Kd) with holding potential. Each point represents the mean±SEM of n=2–4 channels. The lines represent weighted linear regressions to the points (kon – dashed; koff, Kd – solid). Parameters are quoted in the .
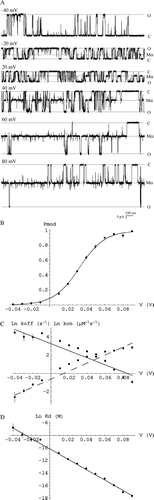
Figure 4. The β modified state of 8β-amino-9α-hydroxyryanodine is only occasionally observed with Q4863A RyR2. (A) Trace showing one occurrence of occasional β modified state. (B) All points amplitude histogram of mouse wt RyR2 in the presence of 8β-amino-9α-hydroxyryanodine at 20 mV showing two clear levels in the modified state. (C) All points amplitude histogram of mouse Q4863A RyR2 in the presence of 8β-amino-9α-hydroxyryanodine at 90 mV showing only a single level in the modified state.
Discussion
The Q4863A mutation profoundly alters the dissociation rate of 8β-amino-9α-hydroxyryanodine from its binding site on RyR2
The Q4863A mutant illustrates important features of RyR channel function. There is no alteration of single-channel conductance Citation[16] or, qualitatively, in gating between the unmodified, ligand-free channel in the presence or absence of ryanoids/ryanodine. The effect of the mutation is only prominent when the channel is modified and the ligand is bound. The dominant effect of this mutation is to clearly shorten the duration of ryanoid-bound modified events with little effect on apparent association rate.
We have measured explicitly the single-channel kinetics of the ryanoid 8β-amino-9α-hydroxyryanodine with both wt and mutant to establish that the 200-fold increase in apparent Kd is predominantly due to a 600-fold increase in the apparent dissociation rate constant. Qualitative observations suggest that the change in apparent dissociation constant is uniform irrespective of ryanoid structure Citation[17]. Therefore these measurements describe a generally valid characteristic of this mutation.
It is of interest that the major effect of the mutation is on apparent dissociation rate. Studies with several ryanoids suggest that structural changes in the ryanodine molecule affect both apparent dissociation and association rates Citation[9]. In contrast, this structural change in the receptor affects the energetic stability of the ligand-bound state with little effect on access to the binding site in the ligand-free state.
Bioinformatics studies suggest that the RyR pore may bear the same fold as potassium channels Citation[2], Citation[25]. A putative molecular model has been built in which Q4863 is located on the inner, pore-lining helix with the side chain orientated along the circumference of these helices Citation[26]. Located just below the filter region, these residues make frequent bonds to other Q4863 residues as well as filter, inner helix and outer helix residues in molecular dynamics simulations of the model (Welch, unpublished results). Whilst preliminary, such studies suggest possible interactions: thus these polar Gln residues may play an important role in inter-helical and inter-subunit interactions that are disrupted by mutation to the smaller Ala which also lacks hydrogen-bonding potential. We propose that the tetrameric RyR forms a single-binding site for a ryanoid that is disrupted by the putative pore mutation Q4863A which destabilizes intersubunit interactions.
There are several polar groups on the ryanoid molecule that could potentially interact with the receptor. Whilst it remains possible that they could interact with Q4863 residues directly, the effect may be indirect caused by a knock-on change in receptor structure due to the mutation.
The voltage-dependence of the interaction is not altered by the mutation
The interaction's dependence on trans-membrane potential persists regardless of the formal charge on the ryanoid leading to the hypothesis that it is, in large part, due to a potential driven change in receptor affinity Citation[9]. The additional small changes seen with ligands of differing formal charge may indicate some interaction with the voltage-drop Citation[9]. We unequivocally demonstrate that the voltage-dependence of the 8β-amino-9α-hydroxyryanodine interaction with RyR2 is not affected by mutation of Q4863 to alanine. This suggests that this residue cannot sense the voltage-drop.
One unavoidable complicating factor is the high activation state of the channel and the presence of the activating ligands EMD41000 and Ca2 + . As the ryanoid interaction is state-dependent, the channel's Po needs to be raised in order to measure sufficient numbers of modified events. It is likely that the ion-conducting state is an aggregate of several open states each of which may have differing affinity for ligands and/or voltage dependence Citation[27], Citation[28]. It may well be the case that this combination of ligands or the high activation state of the channel in these studies contributes to the voltage-driven change in affinity for ryanoids. An explicit test of this is lengthy and outside the scope of this study.
It is expected that the voltage-drop is concentrated over the relatively narrow confines of the filter with less influence in the wider, aqueous cavity Citation[29]. Positioning of Q4863 in the cavity is consistent with the interpretation of our data and we hypothesize that the voltage-drop is unaffected. The bound ryanoid may fall within the voltage-drop or interact with other parts of the channel that are in contact with the voltage-drop.
The β state is energetically de-stabilized by the mutation
The drastic reduction in observation of the β state in the 8β-amino-9α-hydroxyryanodine-induced modified state with the mutant indicates a considerably lower stability of this state. In a previous study with native sheep RyR2, it was hypothesized that the β state could only be accessed through the α state based on observations and modelling studies Citation[18]. If this model holds for the mutant, destabilization of the α state naturally leads to the observed destabilization of the β state. The data presented do not contradict this however, as we are unable to measure β transitions with the mutant, we cannot test and investigate the hypothesis further.
Approaching the molecular mechanism of high-affinity ryanoid/RyR2 interaction
The molecular mechanism of the ryanoid-RyR2 interaction remains elusive largely due to experimental limitations on measurement timescale. The relatively slow kinetics of modified events together with the fast kinetics of RyR2 and modified state substructure gating require fast sampling and recording times beyond typical bilayer experiments. In addition, as the model of gating of RyR2 in the presence of activating ligands is not resolved, the background setting of RyR2 gating is not yet known. Modern single-channel analysis methods Citation[30], Citation[31] are able to explicitly test different reaction mechanisms given a sequence of channel data. Current ryanoid/RyR2 recordings, in order that they contain sufficient numbers of events, are too lengthy for such analysis to be feasible at present. Perhaps this might be possible in the near future and with the advent of more solid models of RyR gating. In which case several remaining questions might be answered: Does the ryanoid stabilize an existing conformation of the receptor or induce a new state? Can we separate ligand binding from the channel conformation associated with the modified state? How do channel mutations precisely affect the reaction? Until then, this work to date on ryanoid/RyR2 interaction represents a step towards this goal.
In conclusion, we present the first single-channel, quantitative comparison of the properties of a mutant on the ryanoid/RyR2 interaction. We demonstrate that the 8β-amino-9α-hydroxyryanodine is a useful, quantitative probe of this relationship and describe analysis methods to exploit this. Such methods may be employed with other ryanoid/RyR mutation combinations and analysed with 3-D QSAR methods to provide a detailed understanding of the ligand-receptor relationship. We explicitly show that the effect of the Q4863A mutation is to increase the dissociation rate of the ryanoid from its binding site and destabilizes the ryanoid-bound β-modified state. We infer that Q4863 is not involved with the voltage-drop but is able to influence ryanoid-bound structural changes.
Supplementary Data
Further details on analysis of kinetic parameters
Open (O), closed (C) and modified (M) levels were estimated as the centre of the peaks calculated by non-linear fitting of a sum of Gaussian functions to all points histograms. These fits were checked by eye and where the distribution was skewed, due to truncation by filtering Citation[24], the position of the peaks was approximated visually. The modified level was set halfway in between the α and β states for wt channels and at the putative, single α state for the Q4863A mutant.
Two thresholds were set 50% between O and M levels and 50% between M and C levels. Sampled data points were assigned as O if they lay between minimum and the O/M threshold, modified if between O/M and M/C thresholds, and closed if between M/C threshold and maximum. Minimum and maximum were either +200 or –200 pA depending on the polarity of the command potential and the subsequent direction of current flow. Data points of the same assigned level that were consecutive in time were joined to form events. A minimum time resolution of 3 ms was imposed.
Within the working model of ryanoid/RyR2 interaction (Figure 1A), the channel may close with ryanoid bound and the ryanoid binds to/from the open state (O) of the channel. To identify which closings occurred with ryanoid bound, the preceding and following events were inspected. As sojourns in the open state (O) are, in general, much shorter than those in the modified state (M), it is assumed that openings are more likely to be missed than modifications. OCM-ryanoid unbound closing/missed opening after closing. OCO-ryanoid unbound closing. MCO-ryanoid unbound closing/missed opening before closing. MCM-ryanoid bound closing.
The events list was re-cast as ryanoid unbound (unmodified) and ryanoid bound (modified) events by assimilating the closings appropriately and dwell times were measured. The dwell time distributions were fit by maximum likelihood fitting with missed events correction with one or two exponential components and the fits viewed with log dwell times distribution. The data were best fit with a single exponent consistent with previous studies.
The Mathematica analysis package and worksheets may be requested from the corresponding author.
This paper was first published online on iFirst on 03 May 2007.
Acknowledgements
We are grateful to Mr Richard A. P. Montgomery and Ms Tracy M. Moreno-King for expert technical assistance. We thank Drs Ruiwu Wang and Bhavna Tanna for molecular biology and Q4863A RyR2 channel preparation. This work was supported by grants from the British Heart Foundation (AJW), the Canadian Institutes for Health Research (SRWC), the Stroke Foundation of Alberta, Northwestern territories and Nunavut (SRWC), and the National Science Foundation (WW).
References
- Banerjee A. Plants and the heart. Dialog Cardiovas Med 2000; 5: 239–241
- Williams AJ, West DJ, Sitsapesan R. Light at the end of the Ca2 + -release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels. Quart Rev Biophys 2001; 34: 61–104
- Marban E, Wier WG. Ryanodine as a tool to determine the contributions of calcium entry and calcium release to the calcium transient and contraction of cardiac purkinje fibers. Circ Res 1985; 56: 133–138
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003; 4: 517–529
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behaviour of single Ca2 + release channel. Am J Physiol 1987; 253: C364–368
- Ashley RH, Williams AJ. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J Gen Physiol 1990; 95: 981–1005
- Tinker A, Sutko JL, Ruest L, Deslongchamps P, Welch W, Airey JA, Gerzon K, Bidasee KR, Besch HR, Jr, Williams AJ. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys J 1996; 70: 2110–2119
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev 1997; 49: 53–98
- Williams AJ, Tanna B. The interaction of ryanoids with individual ryanodine receptor channels. Biol Res 2004; 37: 527–538
- Welch W. Quantitative relationships between ryanoids, receptor affinity and channel conductance. Front Biosci 2002; 1: 1727–1742
- Welch W, Williams AJ, Tinker A, Mitchell KE, Deslongchamps P, Lamothe J, Gerzon K, Bidasee KR, Besch HR, Jr, Airey JA, Sutko JL, Ruest L. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochem 1997; 36: 2939–2950
- Welch W, Sutko JL, Mitchell KE, Airey JA, Ruest L. The pyrrole locus is the major orienting factor in ryanodine binding. Biochem 1996; 35: 7165–7173
- Witcher DR, McPherson PS, Kahl SD, Lewis T, Bentley P, Mullinnix MJ, Windass JD, Campbell KP. Photoaffinity labeling of the ryanodine receptor/Ca2 + release channel with an azido derivative of ryanodine. J Biol Chem 1994; 269: 13076–13079
- Callaway C, Seryshev A, Wang J-P, Slavik KJ, Needleman DH, Cantu C, III, Wu Y, Jayaraman T, Marks AR, Hamilton SL. Localization of the high and low affinity [3H]Ryanodine binding sites on the skeletal muscle Ca2 + release channel. J Biol Chem 1994; 269: 15876–15884
- Wang R, Bolstad J, Kong H, Zhang L, Brown C, Chen SRW. The predicted TM10 transmembrane sequence of the cardiac Ca2 + release channel (ryanodine receptor) is crucial for channel activation and gating. J Biol Chem 2004; 279: 3635–3642
- Wang R, Zhang L, Bolstad J, Diao N, Brown C, Ruest L, Welch W, Williams AJ, Chen SRW. Residue Gln4863 within a predicted transmembrane sequence of the Ca2 + release channel (ryanodine receptor) is critical for ryanodine interaction. J Biol Chem 2003; 278: 51557–51565
- Ranatunga KM, Moreno-King TM, Tanna B, Wang R, Chen SRW, Ruest L, Welch W, Williams AJ. The Gln4863Ala mutation within a putative, pore-lining trans-membrane helix of the cardiac ryanodine receptor channel alters both the kinetics of ryanoid interaction and the subsequent fractional conductance. Mol Pharmacol 2005; 68: 840–846
- Tanna B, Welch W, Ruest L, Sutko JL, Williams AJ. Voltage-sensitive equilibrium between two states within a ryanoid-modified conductance state of the ryanodine receptor channel. Biophys J 2005; 88: 2585–2596
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Phil Trans Roy Soc Lon B 1982; 299: 401–411
- Williams AJ. An introduction to the methods available for ion channel reconstitution. Microelectrode techniques: the Plymouth Workshop handbook, D Ogden. The Company of Biologists, Cambridge 1994; 79–99
- McGarry SJ, Williams AJ. Activation of the sheep cardiac sarcoplasmic reticulum Ca2 + -release channel by analogues of sulmazole. Br J Pharmacol 1994; 111: 1212–1220
- Tanna B, Welch W, Ruest L, Sutko JL, Williams AJ. Ryanoid modification of the cardiac muscle ryanodine receptor channel results in relocation of the tetraethylammonium binding site. J Gen Physiol 2001; 117: 385–393
- Tanna B, Welch W, Ruest L, Sutko JL, Williams AJ. Interactions of a reversible ryanoid (21-amino-9alpha-hydroxy-ryanodine) with single cardiac ryanodine receptor channels. J Gen Physiol 1998; 112: 55–69
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel recording. Single-channel recording, B Sakmann, E Neher. Plenum, New York & London 1983; 191–263
- Shah PK, Sowdhamini R. Structural understanding of the transmembrane domains of inositol triphosphate receptors and ryanodine receptors towards calcium channeling. Prot Eng 2001; 14: 867–874
- Welch W, Rheault S, West DJ, Williams AJ. A model of the putative pore region of the cardiac ryanodine receptor channel. Biophys J 2004; 87: 2335–2351
- Saftenku E, Williams AJ, Sitsapesan R. Markovian models of low and high activity levels of cardiac ryanodine receptors. Biophys J 2001; 80: 2727–2741
- Zahradnik I, Gyorke S, Zahradnikova A. Calcium activation of ryanodine receptor channels – reconciling RyR gating models with tetrameric channel structure. J Gen Physiol 2005; 126: 515–527
- Hille, B. 1984. Ionic channels of excitable membranes. 1st ed. Sunderland, MA: Sinauer. pp 426.
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J 1996; 70: 264–280
- Venkataramanan L, Sigworth FJ. Applying hidden Markov models to the analysis of single ion channel activity. Biophys J 2002; 82: 1930–1942