Abstract
The objective of the present study was to evaluate the antinociceptive activity of ficus fraction in thermal as well as chemogenic stimuli models in mice. Alloxan induced diabetic mice showed a significant decrease (p<0.001) in tail-flick and hot-plate latencies and increase in serum glucose level, hind paw cumulative withdrawal latencies in allodynia, formalin pain responses, formalin-induced paw oedema and number of writhers formed indicating the accomplishment of hyperalgesia. The simultaneous treatment with ficus fraction reversed the two thermal thresholds in both tail-flick and hot-plate latencies and consequent decrease in allodynia responses suggesting reduction of hyperalgesic condition. The neutralising effect of chemogenic nociception (pain score due to formalin, harmful effects of acetic acid and paw oedema) is due to stimulation of the autonomic nervous system, which helps in absorbing the acids into nutrient metabolic pathways or due to antioxidant and anti-inflammatory properties of ficus fraction. Overall, the activation of opioidergic systems appears to play a crucial role in ficus-induced antinociception.
Introduction
Diabetes is a chronic endocrine disorder characterised by hyperglycemia and associated with absolute or relative deficiencies in insulin secretion or function (Srinivasan Citation2005) due to progressive deterioration in beta-cell function and cell mass (Wajchenberg Citation2007) resulting in accelerating apoptosis. A common complication associated with diabetes is painful or painless diabetic peripheral neuropathy (DPN; Tracy and Dyck Citation2008). Progressive loss of thermal and tactile pain sensation are the symptoms that contribute to neuropathy (Sugimoto et al. Citation2000). Peripheral nerve damage due to pathological processes (diabetes) can initiate cellular changes in the primary afferent fibres that might account for the symptoms of neuropathic pain such as allodynia, hyperalgesia and ongoing pain (Shim et al. Citation2005).
Clinical treatment with pharmacological therapy has led to harmful side effects that, in turn, lead to the growth of phytotherapeutics (Wills et al. Citation2000) contributing to the prevention and reduction of diabetic complication.
Ficus bengalensis Linn (banyan tree)
Urticaceae is used in the ayurvedic system of medicine for treatment of various ailments like tonic, astringent, cooling and diuretic effects. The plant is known to possess anti-inflammatory (Lansky et al. Citation2008), antihyperglycemic (Musabayane et al. Citation2006) and antioxidant (Palasuwan et al. Citation2005) activities. The literature survey revealed that there are limited scientific studies carried out regarding antinociceptive activity on the bark of Ficus bengalensis to substantiate their therapeutic claim. Therefore, the present investigation made an endeavour to test the ability of the potent fraction of ficus for antihyperglycemic and antinociceptive property against ALX-D mice for the period of 28 days.
Materials and methods
Swiss albino mice (Mus musculus) belonging to either sex, weighing about 30±2 g were procured from the breeding unit (NCLAS) of NIN, Hyderabad. The departmental ethics committee approved the safety of the animals and protocols accordingly. The animals were maintained following standard laboratory practices.
Fleshy ficus barks were powdered soaked in petroleum ether in a closed flask (12 h) and solvent fraction decanted. This process was repeated three or four times. The residue left behind was similarly extracted with rectified spirit (95% ethanol) and then with ethyl acetate so that one active fraction ‘Leucocyanidin’ (the ethyl acetate soluble fraction) may be removed. The chemical extraction procedure was as described by (Augusti Citation1996). A pilot study was conducted to determine the effective dose (ED50) of ficus fraction and 150 mg/kg body wt were considered to be minimum effective doses for long-term treatment of 28 days.
Thirty Albino mice were equally divided into three groups: The first group was used as control, second group alloxan (45 mg/kg) induced diabetic animals ALX-D, and third group of four animals injected with alloxan further received ficus extract (150 mg/kg orally in saline for 28 d) ALX-D + F. Serum glucose levels were determined by Kurt Dubinsky, the 1962 method.
The three groups were exposed to various thermal as well as chemogenic noxious and non-noxious stimuli for assessment of pain. Tail-flick test (TFT; Sewell and Spencer Citation1974), hot-plate latency (HPLs; Hiura et al. Citation1992), allodynia (Bennett and Xie Citation1988), formalin test (Dubuisson and Dennis Citation1977), paw oedema test (weight average and relative thickness method, 0.05 ml of 2% of formalin was inoculated intraplantar region and after 48 h the mice were sacrificed by chloroform inhalation. The paw was amputated from ankle, weighed and thickness was measured by vernier calipers) and Acetic acid writhing tests (Koster et al. Citation1959) are the different nociceptive tests used.
For statistical analysis two-way ANOVA following multiple comparison was used. The differences were considered significant if p was <0.001.
Results and discussion
The ALX-D mice had shown a marked increase in the serum glucose levels after 1st, 14th and 28th day indicating the hyperglycemia when compared to control. The increase in SGLs was observed due to diabetogenic action of alloxan (Czerwiñska et al. Citation2006). After simultaneous treatment with ficus bark extract, the SGL decreased significantly (). This observation confirms an earlier report (Edwin et al. Citation2008). The hypoglycaemic action of leucocyanidin, fraction from ficus bark stimulates and releases the insulin from the pancreatic remnants of β-cells (Daniel et al. Citation2003) in ALX-D diabetic mice.
Table 1. Serum glucose level, tail-flick test (sec), hot-plate latency (fore paw cumulative latency in sec), allodynia (hind paw cumulative withdrawals in sec) and acetic acid writhing response (number of writhers produced in 20 min) in control, ALX-D mice and ALX-D + ficus extract treated groups on 1st, 14th and 28th day.
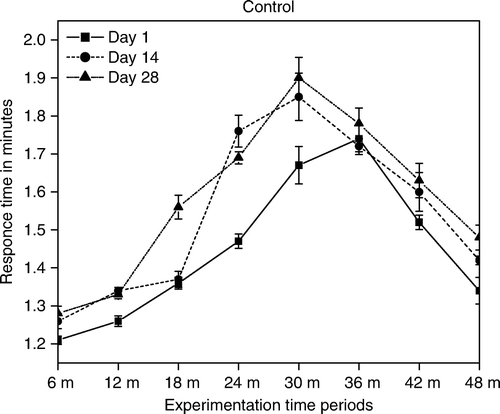
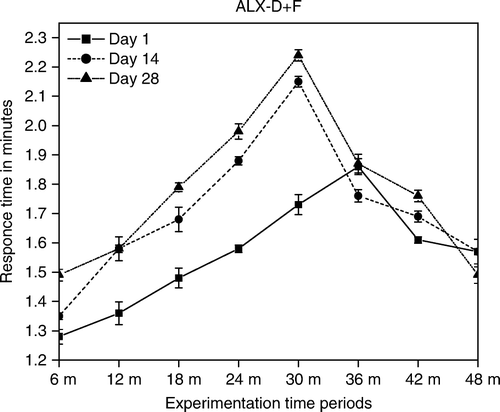
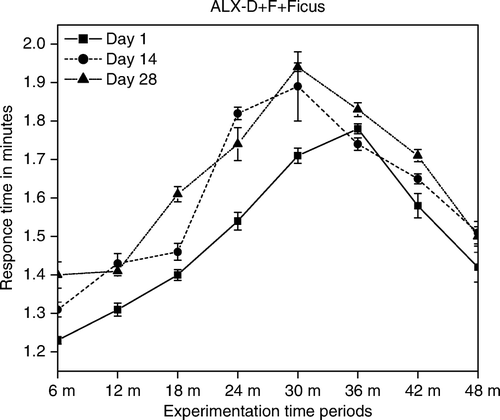
Diabetic mice had shown a significant decrease in tail-flick test, hot-plate latencies (Kuhad et al. Citation2008) and increase in hind paw cumulative withdrawal in allodynia responses () showing hyperalgesia (Kuhad and Chopra Citation2008). Further ficus administration showed an antinociceptive effect on the acute noxious stimulation. The tail-flick (TF) response has been believed to be a spinally mediated reflex and the hot-plate response is more complex supraspinally organised behaviour. Our results demonstrate that ficus has the ability to prolong response latencies indicating its analgesic condition (Malairajan et al. Citation2006). The increase in the threshold of pain to thermal stimuli by ficus fraction may be due to its hyperinsulinemic raising effect, which in turn increases the β-endorphin concentration in the hypothalamus to a level higher than normal.
The formalin test produced a distinct biphasic nociceptive response generally regarded as the early phase that persists for five min and late phases that appears between 15 and 60 min after formalin administration ( and ) indicating accomplishment of hyperalgesia (Malmberg and Yaksh Citation1992). The ability of ficus to inhibit both phases of formalin test () suggests the involvement of central mechanism (i.e. opioids) and also may be due to its anti-inflammatory activity (Lansky et al. Citation2008). The decrease in weight and relative thickness of the hind paw () may be due to potent superoxide anion scavenging activity (Lee et al. Citation2006).
Table 2. Paw oedema test (weight, mg and relative thickness, mm) in control, ALX-D mice and ALX-D + ficus extract treated groups on 1st, 14th and 28th day.
The sensitivity of mice to writhing response significantly decreased in alloxan-treated hyperglycemia when compared to control (), simultaneous treatment with the ficus extract inhibited the abdominal constrictions induced by acetic acid and also increased the pain threshold of mice towards the thermal source. The decrease in writher count may be due to the action of drug on autonomic nervous system neutralising the acid into nutrient metabolic pathways and might also be due to antioxidant, antiperoxide (Rao et al. Citation2008) and the anti-inflammatory property of ficus.
In conclusion, Ficus bengalensis fraction (leucocyanidin) has proved to possess a significant role in regaining thermal thresholds to normal confirming its hypoalgesic property. Further studies on ficus fraction have to be undertaken to know its ameliorative effects.
Acknowledgements
This part of the research is supported by funds from research grants UGC-DRS (SAP), Department of Zoology, Osmania University.
References
- Augusti , KT. 1996 . Therapeutic values of onion (Allium cepa L.) and (Allium sativum L.) . Indian Journal of Experimental Biology , 34 : 634 – 640 .
- Bennett , J and Xie , YK. 1988 . A peripheral neuropathy in rat produces disorder of pain sensation like those seen in man . Pain , 33 : 87 – 107 .
- Czerwiñska , M , Sikora , A , Szajerski , P , Adamus , J , Marcinek , A and Gebicki , J. 2006 . Mechanistic aspects of alloxan diabetogenic activity: a key role of keto-enol inversion of dialuric acid on ionization . Journal of Physical Chemistry A , 110 : 7272 – 7278 .
- Daniel , RS , Devi , KS , Augusti , KT and Sudhakaran Nair , CR. 2003 . Mechanism of action of antiatherogenic and related effects of Ficus bengalensis Linn. flavonoids in experimental animals . Indian Journal of Experimental Biology , 41 : 296 – 303 .
- Dubinsky , K. 1962 . An O-toluidine method for body fluid glucose determination . Clinical Chemistry , 8 : 215 – 235 .
- Dubuisson , D and Dennis , SG. 1977 . The formalin test: a quantitative study of the analgesic effects of morphine, mepedrine and brainstem stimulation in rats and cats . Pain , 4 : 161 – 174 .
- Edwin , E , Sheela , E , Chaturvedi , M , Sharma , S and Gupta , VB. 2008 . A comparative study on antihyperglycemic activity of Ficus bengalensis, Linn aerial roots and barks . Pharmacognosy Magazine , 4 : 95 – 97 .
- Hiura , A , Villalobos , EL and Ishizuka , H. 1992 . Age dependent attenuation of the decrease of C-fibers by capsaicin and its effects on response to nociceptive stimuli . Somatosensory and Motor Research , 9 : 37 – 43 .
- Tracy , JA and Dyck , PJ. 2008 . The spectrum of diabetic neuropathies . Physical Medicine and Rehabilitation Clinics of North America , 19 : 1 – 26 .
- Koster , R , Anderson , M and De Beer , EJ. 1959 . Acetic acid for analgesic screening . Federation Proceedings , 18 : 412 – 418 .
- Kuhad , A and Chopra , K. 2008 . Lycopene ameliorates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rat . Indian Journal of Experimental Biology , 46 : 108 – 111 .
- Kuhad , A , Sharma , S and Chopra , K. 2008 . Lycopene attenuates thermal hyperalgesia in a diabetic mouse of neuropathic pain . European Journal of Pain , 12 : 624 – 632 .
- Lansky , EP , Paavilainen , HM , Pawlus , AD and Newman , RA. 2008 . Ficus spp. (graph): ethnobotany and potential as anticancer and anti-inflammatory agents . Journal of Ethnopharmacology , 119 : 195 – 213 .
- Lee , MH , Kim , JY , Yoon , JH , Lim , HJ , Kim , TH and Jin , C. 2006 . Inhibition of nitric oxide synthase expression in activated microglia and peroxynitrite scavenging activity by opuntia ficus indica var. saboten . Phytotherapy Research , 20 : 742 – 747 .
- Malairajan , P , Gopalakrishnan , G , Narasimhan , S and Jessi Kala Veni , K. 2006 . Analgesic activity of some Indian medicinal plants . Journal of Ethnopharmacology , 106 : 425 – 428 .
- Malmberg , AB and Yaksh , TL. 1992 . Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rats . Journal of Pharmacology and Experimental Therapeutics , 263 : 136 – 146 .
- Musabayane , CT , Bwititi , PT and Ojewole , JA. 2006 . Effects of oral administration of some herbal extracts on food consumption and blood glucose levels in normal and streptozoticin-treated diabetic rats . Methods and Findings in Experimental and Clinical Pharmacology , 28 : 223 – 228 .
- Palasuwan , A , Soogarun , S , Lertlum , T , Pradniwat , P and Wiwanitkit , V. 2005 . Inhibition of Heinz body induction in an in vitro model and total antioxidant activity of medicinal Thai plants . Asian Pacific Journal of Cancer Prevention , 6 : 458 – 463 .
- Rao , CV , Verma , AR , Vijayakumar , M and Rastogi , S. 2008 . Gastroprotective effect of standardized extract of ficus glomerata fruit on experimental gastric ulcers in rats . Journal of Ethnopharmacology , 115 : 323 – 326 .
- Sewell , RA and Spencer , PS. 1974 . Proceedings: modification of the antinociceptive activity of narcotic agonists and antagonists by intraventricular injection of biogenic amines in mice . British Journal of Pharmacology , 51 : 140 – 141 .
- Shim , B , Kim , DW , Kim , BH , Nam , TS , Leem , JW and Chung , JM. 2005 . Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy . Neuroscience , 132 : 193 – 201 .
- Srinivasan , K. 2005 . Plant foods in the management of diabetes mellitus: spices as beneficial anti diabetic food adjuncts . International Journal of Food Sciences and Nutrition , 56 : 399 – 414 .
- Sugimoto , K , Murakawa , Y and Sima , AA. 2000 . Diabetic neuropathy – a continuing enigma . Diabetes Metabolism Research and Reviews , 16 : 408 – 433 .
- Wajchenberg , BL. 2007 . Beta-cell failure in diabetes and preservation by clinical treatment . Endocrine Reviews , 28 : 187 – 218 .
- Wills , RBH , Bone , K and Morgan , M. 2000 . Herbal products: active constituents, modes of action and quality control . Nutrition Research Reviews , 13 : 47 – 77 .