Abstract
Arg-βNA hydrolysing aminopeptidase B was purified from a goat brain with a purification fold of ∼280. As the enzyme is reported to hydrolyse bradykinin and angiotensins, the effect of commonly used anti-inflammatory and antihypertensive drugs on activity of this enzyme was studied. Amongst the studied anti-inflammatory drugs, analgin had the highest potency with Ki of 171 µM followed by diclofenac Na and nimesulide with Ki values of 209 and 252 µM, respectively. Amlodipine besylate was the most potent among studied antihypertensive drugs. Ki values for amlodipine besylate, labetalol and atenolol are 200, 500 and 568 µM, respectively. Analgin and diclofenac sodium showed competitive inhibition, whereas, nimesulide showed mixed type of inhibition. All the studied antihypertensive drugs competitively inhibited the enzyme.
Introduction
Aminopeptidase B (EC.3.4.11.6; arginyl aminopeptidase), which acts on Arg-βNA at PH 7.4 was purified from goat brain with a purification fold of ∼280 (Bogra et al. Citation2009a) and immobilised in calcium alginate beads (Bogra et al. Citation2009b). The enzyme is a metalloenzyme with direct or indirect involvement of the thiol group in regulation of catalysis. The activity of enzymes is reported to increase in patients with cancer (Martinez et al. Citation1999). Recently, advances have been made in understanding the catalytic mechanism and structural features of proteases and their inhibitors. This has significant implications in the medicinal field in designing therapeutic modalities. In the present study we demonstrate the effect of different antihypertensive and anti-inflammatory drugs on aminopeptidase B activity.
Materials and methods
Aminopeptidase B was purified from a goat brain by the method of Bogra et al. (Citation2009a). The homogeneity and monomeric nature of the enzyme was checked by native and SDS-PAGE according to the method of Davis (Citation1964) and Laemmli (Citation1970), respectively.
Aminopeptidase B activity was determined spectrophotometrically using L-Arg–βNA as substrate as explained by Bogra et al. (Citation2007). One unit of enzyme activity was defined as the amount of enzyme that released one nmol of βNA/minute from L-Arg–βNA as substrate at 37°C under the assay conditions.
In order to study the effect of anti-inflammatory and antihypertensive drugs, the enzyme assays were carried out in the presence of different concentrations of these compounds (0.1–5.0 M). Stock solutions of various anti-inflammatory compounds viz. nimesulide, diclofenac sodium, analgin, piroxicam, paracetamol and hydroxycortcosterone as well as antihypertensive drugs viz. amlodipine besylate, atenolol and labetalol were prepared in dimethylsulfoxide (DMSO). Control assays were carried out under a similar condition.
For determining the inhibition constant (Ki) and type of inhibition of aminopeptidase B by these drugs, stock solutions of (1.0 M, 20 mM and 10 mM) various drugs were prepared in DMSO so that the required final concentrations (0.2–1.0 M) in 1.0 ml assay mixture could be obtained by the addition of an appropriate volume of drug stock solution. The 200 µl of purified enzyme was incubated with the 750 µl of assay buffer (50 mM sodium phosphate buffer, pH 7.4 containing 50 mM NaCl) at 37°C for 5 min in seven sets. To the first batch, 10 µl of substrate stock solution was added to give 100 µM final substrate concentrations. Similarly, in second and third batches 10 µl of substrate stock solution was added to effect final substrate concentrations of 200 and 300 µM, respectively. The reaction rates were calculated in terms of nmols of βNA liberated per ml of enzyme.
Results and discussion
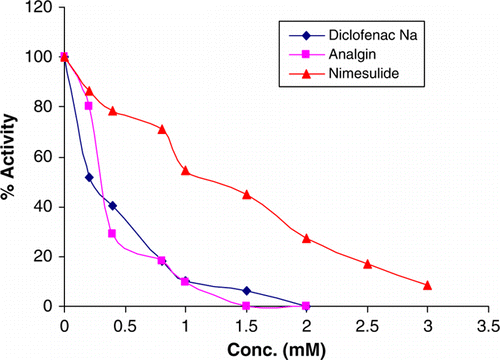
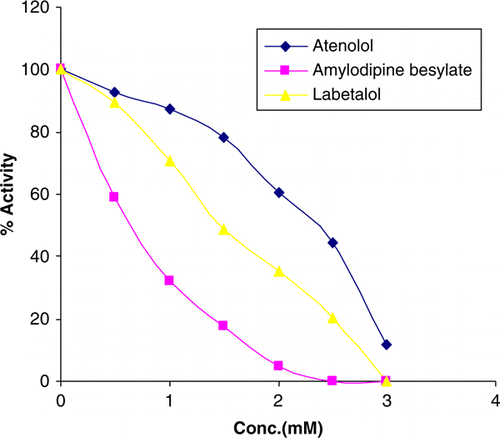
The role of aminopeptidase B has been studied in inflammation and hypertension so any inhibitor of the enzyme may serve as the potential anti-inflammatory and antihypertensive compounds. Analgin had maximum potency followed by diclofenac sodium and nimesulide (; ). Paracetamol, piroxicam and hydroxycorticosterone did not inhibit the enzyme. Among antihypertensive drugs, amlodipine besylate, a calcium channel blocker, caused a maximum inhibition of enzyme activity at 1.0 mM concentration ().
Table 1. Interaction of goat brain aminopeptidase B with anti-inflammatory and antihypertensive drugs.
The mechanism of inhibition of aminopeptidase B activity by these drugs still remains to be elucidated. Sulfonamide group in the nimesulide may interact with enzyme's active site and cause inhibition. Though the mechanism of action of these drugs is not known, but inhibition of aminopeptidase B by these drugs may affect the concentration of bradykinin and substance P, which are known to exhibit edemagenic effects (Rubinstein Citation2007). The enzyme is also known to be involved in metabolism of angiotensin-III, the peptide effector of the brain rennin angiotensin system that participates in the control of blood pressure and vasopressin release (Goazigo et al. Citation2005). So any inhibitor to the enzyme may prove to be an effective anti-inflammatory and/or antihypertensive, respectively.
Among anti-inflammatory compounds, analgin and diclofenac competitively inhibited the enzyme (). Nimesulide showed a mixed type of inhibition. All the antihypertensive drugs amlodipine besylate, labetalol and atenolol competitively inhibited the enzyme (). The enzyme is widely distributed in all the tissues so any drug that is inhibitory may have a wide spread effect with the condition that the receptor of the drug does not perform a special function.
It is concluded that the enzyme aminopeptidase B is effectively inhibited by the studied drugs, but detailed examination of the structure-activity relationship needs to be done for designing the therapeutic modalities.
Acknowledgements
Pushpa Bogra is thankful to Kurukshetra University, Kurukshetra for providing University Research Scholarship.
References
- Bogra , P , Singh , J and Singh , H . 2007 . Aminopeptidase B: regional and subcellular distribution in goat brain tissue . Journal of the Indian Chemical Society , 84 : 598 – 602 .
- Bogra , P , Singh , J and Singh , H . 2009a . Purification and characterization of aminopeptidase B from goat brain . Process Biochemistry , 44 : 776 – 780 .
- Bogra , P , Singh , J and Singh , H . 2009b . Immobilization of goat brain aminopeptidase B in calcium alginate beads . Biocatalysis and Biotransformation , 27 ( 2 ) : 96 – 101 .
- Davis , BJ . 1964 . Disc electrophoresis II. Methods and application to human serum proteins . Annals of the New York Academy of Sciences , 121 : 404 – 427 .
- Goazigo , R , Iturrioz , X , Massot , C , Claperon , C , Roques , BP and Cortes , CL . 2005 . Role of angiotensin III in hypertension . Current Hypertension Reports , 7 : 128 – 134 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of bacteriophage T 4 . Nature , 227 : 680 – 685 .
- Martinez , JM , Prieto , I , Ramirez , MJ , Cueva , C , Alba , F and Ramirez , M . 1999 . Aminopeptidase activities in breast cancer tissue . Clinical Chemistry , 45 : 1797 – 1802 .
- Rubinstein , I . 2007 . Bradykinin and substance P-induced edema formation in the hamster cheek pouch is tyrosine kinase dependent . Journal of Applied Physiology , 103 : 184 – 189 .