Abstract
Perinatal cerebral ischemia is a major cause of brain damage in the human newborn. Animal models of the brain ischemia provided important roles for extension of the pathophysiological knowledge and evaluating of the efficacy of therapeutic interventions. The purpose of the present study was to introduce a cerebral ischemia model of rabbit neonates which provides useful clues for assessing the ischemic insult in human newborns. Different time occlusion of maternal uterine arteries in five groups of 10 timed pregnant rabbits (n=2) was done to induce cerebral ischemia in their neonates. Twenty-eight neonates with ischemic time schedules including 0 (control group), 6, 8, 10 and 15 minutes were delivered, left to recover, and clinically evaluated. After decapitation, cerebral lesions were assessed pathologically. Microscopic analyses of brain sections from experimental groups showed degrees of swelling and deformity in neurons of hippocampus which increased by increasing of time of uterine artery occlusion (p<0.01). Ischemic changes were apparent after 8 minutes of hypoxia. Sever necrosis and massive neuronal loss were observed at 10 minutes of hypoxia and over. Occlusion of maternal uterine arteries in timed pregnant rabbit for 8 minutes can be used as an animal model for evaluation of brain ischemia in human investigations.
1. Introduction
Hypoxic-ischemic brain injury is one of the major nervous system injuries and is a serious complication in neonatal wards, which may result in global hypoxic-ischemic encephalopathy. It occurs in uterus from different intrapartum conditions (Badawi et al. Citation1998). It can cause infant's death as well as mental retardation and cerebral palsy. Systemic asphyxia in newborns has been reported 20 in 1000 live births (Parer Citation1998). Although depending on severity and duration of asphyxia, age, sensitivity and etc., some infants recover completely from an asphyxic episode, others develop permanent neurological deficit and developmental disorders or even die (Parer Citation1998).
Cerebral ischemia has a complex pathophysiology involving the interplay of many different cells and tissues such as neurons, glial cells, endothelium and the immune system. When the brain is exposed to hypoxic-ischemia, the injury evolves in two phases (McLean and Ferriero Citation2004; Perlman Citation2006; Volpe Citation2008). The first injury is the primary local injury that affects the exposed part of the brain and mainly includes necrosis (Northington et al. Citation2001; Volpe Citation2008). The second one is described as delayed energy depletion and occurs as the blood flow and energy supplies are restored during the reperfusion phase. This, however, is not equivalent to restored mitochondrial function and injured cells may suffer from delayed injury. This phase mainly includes a process consisting of inflammatory activation and predominantly apoptosis (Lorek et al. Citation1994; Azzopardi and Edwards Citation1995; Northington, et al. Citation2001). These events could not be mimicked satisfactorily in vitro. Thus, large portions of researches are conducted on animal model procedures inducing cerebral ischemia. As animals contain whole elements, neurons, glial cells, vasculature and cerebrospinal fluid which make them more close to the human system; therefore, significant efforts have been made by the neuroscientists to develop models that mimic closely the physiological and pathophysiological changes associated with brain ischemia (Gupta et al. Citation2004).
Several models in different species are currently known to produce cerebral ischemia (Tamura et al. Citation1979; Beech et al. Citation2001). The most significant mechanism causing perinatal hypoxic-ischemic injury is defects of cerebral blood supply due to maternal placental uterine unit disorder (Parer Citation1998). Most previous studies have employed models of cerebral hypoxia-ischemia in postnatal animals (Parer Citation1998). Other methods of establishing hypoxia have worked on adult animals which reduced the accuracy of the study due to differences between foetuses and new-borns and adults (Parer Citation1998). Many methods have been used to establish hypoxia of brain like permanent obstruction of middle cerebral artery (Tamura, et al. Citation1979), injection of embolic substances into middle cerebral artery or common carotid artery (Tamura et al. Citation1981; Zivin et al. Citation1987; de Leciñana et al. Citation2001). However, high mortality rate, complicated surgery methods and variations in injured region had limited the usage of these methods (Hossmann Citation1998). Global ischemia models, both complete and incomplete, tend to be easier to perform. However, they are more relevant to neonatal asphyxia than the focal stroke models. Different species also vary in their susceptibility to the various types of ischemic insults.
There has been no suitable research done on producing perinatal ischemia in foetus of animals with physiologic similarities to human. The aim of the present study was to develop an animal model that incorporates etiological, structural and functional features providing an ideal guide to investigate mechanisms of prenatal hypoxia-ischemia and to test both prenatal and postnatal therapeutic interventions.
2. Materials and methods
2.1. Animals
This study was approved by the Ethics Committee for Animal Experiments in Yasuj University of Medical Sciences. In vivo global hypoxia-ischemia of foetuses was induced by uterine ischemia in timed pregnant New Zealand white rabbits. Ten young and healthy New Zealand pregnant rabbits purchased from Razi Institute were used. They were housed in condition of 25±2°C temperature and humidity 60%, 12-hour light/dark cycle. They had free access to food and water. Carrot and parsley were added to their food for better supplementation. Accurate timing of gestation was ensured by observing the mating of the rabbits and limiting the exposure to male rabbits.
2.2. Induction of cerebral ischemia
The pregnant rabbits were initially divided into five groups (n=2). About one day before expected delivery, hysterotomy was performed on dams. Briefly, the dams were anaesthetised and sedated with a single intramuscular dose of a combination of ketamine 10% (35–40 mg/kg, Alfasan™, The Netherlands), acepromazine (0.75–1.0 mg/kg, Ayeirst™, USA) and Xylazine (3–5 mg/kg, Merk™, Germany) and continued with diazepam (1 mg/kg, Chemidaru™, Iran). Non-ischemic group, tagged as control group, only underwent the stress of caesarean section. Both uterine arteries of dams in other four groups were occluded by putting pressure on artery's wall with non-traumatic clips to establish ischemic condition for their foetuses. Obstruction durations were randomly selected from 6, 8, 10 and 15 minutes. Then clips were removed for 10 minutes to allow cerebral reperfusion and foetuses were expulsed. Then uterus was repaired with 2-0 vicryl sutures, and the skin was closed with 2-0 silk sutures. At the end of caesarean operation, rabbits received intramuscular diclofenac (1.1 mg/kg, Caspian–tamin™, Iran) immediately and then every 12 hours in 4 doses. Penstrep-400 (Nasr™, Iran) was injected as an antimicrobial, just after the operation and continued for 3 days.
2.3. Cerebral ischemia evaluation in neonates
Following the caesarean operation, neonates of control (n=6), 6 (n=6), 8 (n=5), 10 (n=6) and 15 (n=5) minutes groups were exposed to room air (21% oxygen). They were left for recovery under a heating lamp and stimulated to breathe by cleaning up fluids, removal of placenta and performing tactile stimulation on their oral region. Neonates of five groups evaluated clinically.
After ensuring their viability after birth, live neonates were sacrificed 10 minutes with a high dose of pentobarbitone (1g, Specia™, France). The head of the neonates were immersion fixed for two weeks in 10% paraformaldehyde in 0.1 M phosphate buffer. Then the brains were systematically cut into 5 mm thick coronal blocks with a matrix tissue slicer to uniformly sampled similar regions in each brain. Thus, each region of rabbit's brain sampled consists of multiple sections including the cortex, the cerebellum and medulla oblongata. Alternate 2 mm blocks of tissue were embedded in paraffin and sectioned at 5 micron range with a vibrating microtome. Two sections of a slide rule and brain cortex were prepared. To assess brain destruction and to determine the presence of cellular hypoxia and degenerating neurons, brain sections were stained with haematoxylin and eosin. Neuronal density (neurons per millimeter) in the brain was determined under a light microscope (×400) by a pathologist who was blind to the experimental groups.
2.4. Statistical analysis
Histopathologic scores were presented as median, range and mean on graphs which were designed by GraphPad Prism software (version 5.01, USA) and were statistically analysed using non-parametric Mann–Whitney U test by SPSS software (version 11.5, Chicago, IL, USA). p<0.05 was statistically considered significant.
3. Results
Uterine contractions were raised about 1–1.5 minutes after arterial blockage, which were minimised after 2 minutes. Uterine colour changed from light pink to dark pink through the first 3 minutes after the operation, and after that, change was not obvious either in uterine colour or in temperature. Ten minutes after release of the arterial blockage, contractures, colour and temperature became normal ().
Figure 1. Gravid uterus of rabbit; both branches of uterine artery have been closed for 3 and 9 minutes; colour changed but no remarkable change was observed from the third minute.
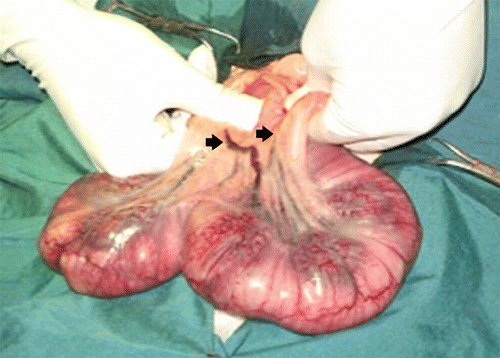
Twenty-eight neonates were born. All of the non-ischemic neonates in the control group born live (). In 6 minutes group, one of the neonates was born dead and five others were alive. One out of five had irregular breathing at the beginning, but became normal after 10 minutes. Four of them had normal movements and breathing. In 8 minutes group, two neonates did not start breathing even after resuscitation and were expired. Three others started irregular breathing after about 30 seconds of resuscitation, but only two of them remained alive with regular breathing. In the other one, the intensity and rate of respiration decreased through 10 minutes and then stopped. In 10 minutes group, four rabbits did not respond to resuscitation but one of the neonates started breathing after about 30 seconds that was disrupted after 5 minutes and then stopped. The last animal, despite having apparently normal breathing initially, the respiratory rate diminished in 6 minutes and finally stopped. In 15 minutes group, none of the rabbits had response to resuscitation and did not start breathing.
Table 1. Number of rabbit's neonates with different intensity of hypoxia in study groups based on clinical findings.
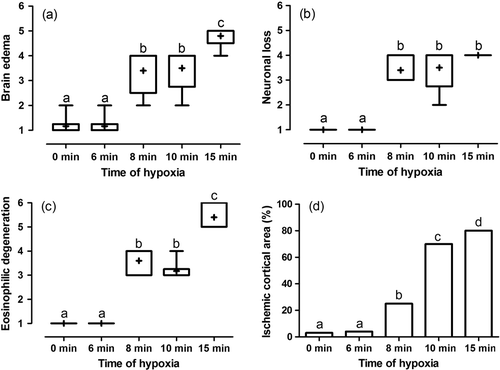
Microscopic analyses of brain sections from experimental groups showed degrees of swelling and deformity in neurons of hippocampus significantly increased by increasing of time of uterine artery occlusion (p<0.01, a–c). Ratio of damaged cortical area due to ischemia to normal cortical area was less than 3% in 6 minutes group which was not significant in comparison with the control group (p=1.0, d). Twenty-five percent to fifty percent of cortical area was injured by ischemia in 8 minutes group which significantly more than control group (p=0.004). In 10 minutes group, cortical ischemia spread over about 70% of the area and also about 80% of the cortex was involved in 15 minutes ischemic damage which both were more than control (p=0.002 and p=0.004, respectively). As pathologic findings claim, this study revealed that more than 8 minutes total uterine arteries occlusion caused measurable cerebral damage in the late gestational ages in neonates of rabbits. More than 10 minutes total uterine artery occlusion caused the most significant cerebral damage in the late gestational ages, predominantly to the hippocampus. The damage may be induced by the hypoxic-ischemic episode itself and/or by the subsequent reperfusion. Because the mortality rose to more than 98% after 15 minutes of ischemia no animal was obtained to experience ischemia for this duration.
Figure 2. Box and whisker plot of median, mean and range of changes in brain oedema (a), neuronal loss (b), eosinophilic degeneration (c) and mean of percent of ischemic cortical area (d) of brain sampled of rabbit's neonates with different intensity of hypoxia. Different superscript letters indicates significant statistical difference (p<0.05) between groups using Mann–Whitney U test. Score 1 means 0–4%; score 2, 5–24%; score 3, 25–49%; score 4, 50–74%; score 5, 75–99%; and score 6, 100% histopathologic changes.
4. Discussion
Since the mortality rose to more than 98% after 15 minutes of ischemia due to arterial occlusion, taking animals that had experienced this duration of ischemia into an experiment would not be useful except for pathologic demonstration. Cerebral ischemia in neonates is a major cause of stillbirths and early neonatal deaths, and is an important cause of hypoxic-ischemic encephalopathy (Gunn and Cable Citation1984).
Animal models will be essential to pursue this vital course of study with the proficiency and accuracy that the field requires. Thus, it is important to have an economical and readily available animal model to study the cerebral ischemia in the perinatal period and to evaluate potential neuroprotective agents. However, the most common animal models are postnatal in origin, and stroke pathophysiology had been used as their method; examples are found in rats, mice, pigs, sheep and baboons (Gupta et al. Citation2004; Derrick et al. Citation2007). Results of the present study showed that based on pathologic findings, the control group had no hypoxia. In the control group, which was undergone surgical stress without inducing of ischemia, the clinical and pathological findings revealed no traces of ischemia that means that anaesthesia and caesarean section did not produce any ischemic changes and did not affect results of the study. Moreover, the neonate rabbits experienced 6 minutes of hypoxia were labelled as low intensity hypoxia; 8 minutes group was tagged as moderate hypoxia; and 10 and 15 minutes groups were marked as high intensity hypoxia. The complexity and diversity of cerebral ischemia pathology will ensure a continuing role for animal models to define the cascade of morphologic and biochemical events occurring after an ischemic insult more accurately (Gupta et al. Citation2004).
Disorders of any of the components of the maternal-placental-foetal unit can damage the foetal brain (Derrick et al. Citation2007). Thus, various animal models can be categorised according to the subunits of the maternal-placental-foetal unit studied. For example, maternal and foetal models have been used in guinea pigs, mice, rats and sheep (Derrick et al. Citation2007). One of the most commonly used animal models of hypoxia-ischemia was originally described by Levine (Citation1960) and later refined by Rice et al. (Citation1981). Those approaches were useful to study hypoxia-ischemia in the developing brain, since newborn rat pups are utilised in those models. Briefly, 7-day old rat pups underwent a permanent unilateral carotid artery ligation with a subsequent 3 hours exposure to a hypoxic environment (8% oxygen); those models created a unilateral infarct in the hemisphere ipsilateral to the ligation and the area of injury was typically concentrated in periventricular regions of the brain, especially cortical and hippocampal areas. Those models offered the distinct advantages that are similar to the human newborn. Those studies provided an animal model in which antecedent insult during development of the foetal brain caused by maternal uterine ischemia in the dams resulted in distinct pathologic changes in the newborn pups (Levine Citation1960; Rice et al. Citation1981)
Lind et al. (Citation1975) reported an acceptable dog model for short-term studies by clamping the ascending aorta for 15 minutes. Sixteen minutes of high-pressure neck occlusion with a tourniquet and special intensive care resulted in major neurological deficit with survival for at least 7 days, and proved suitable for simultaneous study of neurological, physiological, and histological changes (Lind et al. Citation1975). In a model presented by Koizumi et al. (Citation1986) reversible obstruction of middle cerebral artery was performed using silicon covered nylon suture. Longa et al. (Citation1989) in order to make obstruction of middle cerebral artery, used nylon suture that had its end rounded by heat. The first method of Koizumi et al. (Citation1986) was more successful although it had limitations (Laing et al. Citation1993; de Leciñana et al. Citation2001). Belayev et al. (Citation1996) presented intravascular method using thicker nylon suture covered by poly-l-lysine; they had reported larger lesions in their model. In a research by Parer (Citation1998) effects of asphyxia of embryo on structure and function of cerebral cells of some animals was studied. Parer (Citation1998) used umbilical artery obstruction to establish asphyxia. Spandou et al. (Citation2005) studied the preventive effect of erythropoietin in prolonged reduction of motor-sensory and cerebral injury in ischemic-hypoxic model of rat embryos. They had used 7-day old Wistar rats and established hypoxia by permanent obstruction of left common carotid artery and putting animals in 37°C with 8% oxygen and 92% nitrogen air condition for an hour (Spandou et al. Citation2005).
In the present study, we generated a model of hypoxia-ischemia in the near-term foetal rabbit subjected to uterine ischemia that resulted in brain damage. The perinatal rabbit offers several distinct advantages relative to other animal models of hypoxic-ischemic brain injury. Rabbits and humans are perinatal brain developers, unlike rodents (postnatal) and sheep (prenatal) (van Marthens et al. Citation1975). Thus, this presented model provided further advantages. It was a true foetal model, involving global hypoxia-ischemia that the dam was not significantly affected by the uterine ischemia. Rabbits were relatively inexpensive with an average of 6–8 foetuses per dam and dams fully recovered from surgeries and could give birth normally afterwards.
5. Conclusion
The presented method can be used to produce brain hypoxia in newborn rabbits with acceptable limitations, in order to investigate the effect of various resonator or attenuator factors in both the acute and delayed phases of hypoxia. In addition, the results can be used in reducing the level of severity of hypoxic complications and mortality in human infants noting that the superiority of this method was ability to create a relation between hypoxia and severity of its complications.
References
- Azzopardi , D and Edwards , D . 1995 . Magnetic resonance spectroscopy in neonates . Current Opinion in Neurology , 8 : 145 – 149 .
- Badawi , N , Kurinczuk , JJ , Keogh , JM , Alessandri , LM , O'Sullivan , F , Burton , PR , Pemberton , PJ and Stanley , FJ . 1998 . Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study . BMJ , 317 : 1549 – 1553 .
- Beech , JS , Williams , SCR , Campbell , CA , Bath , PMW , Parsons , AA , Hunter , AJ and Menon , DK . 2001 . Further characterisation of a thromboembolic model of stroke in the rat . Brain Research , 895 : 18 – 24 .
- Belayev , L , Alonso , OF , Busto , R , Zhao , W , Ginsberg , MD and Hsu , CY . 1996 . Middle cerebral artery occlusion in the rat by intraluminal suture: neurological and pathological evaluation of an improved model . Stroke , 27 : 1616 – 1623 .
- de Leciñana , M , Díez-Tejedor , ED , Carceller , F and Roda , JM . 2001 . Cerebral ischemia: from animal studies to clinical practice. Should the methods be reviewed? . Cerebrovascular Diseases , 11 : 20 – 30 .
- Derrick , M , Drobyshevsky , A , Ji , X and Tan , S . 2007 . A model of cerebral palsy from fetal hypoxia-ischemia . Stroke , 38 : 731 – 735 .
- Gunn , T and Cable , G . 1984 . Perinatal mortality at a level 2 obstetric hospital: problems after 32 weeks gestation . New Zealand Medical Journal , 97 : 862 – 865 .
- Gupta , S , Kaul , C and Sharma , S . 2004 . Neuroprotective effect of combination of poly (ADP-ribose) polymerase inhibitor and antioxidant in middle cerebral artery occlusion induced focal ischemia in rats . Neurological Research , 26 : 103 – 107 .
- Hossmann , K-A . 1998 . Experimental models for the investigation of brain ischemia . Cardiovascular Research , 39 : 106 – 120 .
- Koizumi , J , Yoshida , Y , Nakazawa , T and Ooneda , G . 1986 . Experimental studies of ischemic brain edema: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area . Japan Journal of Stroke , 8 : 1 – 8 .
- Laing , RJ , Jakubowski , J and Laing , RW . 1993 . Middle cerebral artery occlusion without craniectomy in rats. Which method works best? . Stroke , 24 : 294 – 297 .
- Levine , S . 1960 . Anoxic-ischemic encephalopathy in rats . American Journal of Pathology , 36 : 1 – 17 .
- Lind , B , Snyder , J , Kampschulte , S and Safar , P . 1975 . A review of total brain ischaemia models in dogs and original experiments on clamping the aorta . Resuscitation , 4 : 19 – 31 .
- Longa , EZ , Weinstein , PR , Carlson , S and Cummins , R . 1989 . Reversible middle cerebral artery occlusion without craniectomy in rats . Stroke , 20 : 84 – 91 .
- Lorek , A , Takei , Y , Cady , EB , Wyatt , JS , Penrice , J , Edwards , AD , Peebles , D , Wylezinska , M , Owen-Reece , H Kirkbride , V . 1994 . Delayed (‘secondary’) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy . Pediatric Research , 36 : 699 – 706 .
- McLean , C and Ferriero , D . 2004 . Mechanisms of hypoxic-ischemic injury in the term infant . Seminars in Perinatology , 28 : 425 – 432 .
- Northington , FJ , Ferriero , DM , Graham , EM , Traystman , RJ and Martin , LJ . 2001 . Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis . Neurobiology of Disease , 8 : 207 – 219 .
- Parer , JT . 1998 . Effects of fetal asphyxia on brain cell structure and function: limits of tolerance . Comparative Biochemistry and Physiology – Part A: Molecular & Integrative Physiology , 119 : 711 – 716 .
- Perlman , JM . 2006 . Intervention strategies for neonatal hypoxic-ischemic cerebral injury . Clinical Therapeutics , 28 : 1353 – 1365 .
- Rice , JE , Vannucci , RC and Brierley , JB . 1981 . The influence of immaturity on hypoxic-ischemic brain damage in the rat . Annals of Neurology , 9 : 131 – 141 .
- Spandou , E , Papadopoulou , Z , Soubasi , V , Karkavelas , G , Simeonidou , C , Pazaiti , A and Guiba-Tziampiri , O . 2005 . Erythropoietin prevents long-term sensorimotor deficits and brain injury following neonatal hypoxia-ischemia in rats . Brain Research , 1045 : 22 – 30 .
- Tamura , A , Asano , T , Sano , K , Tsumagari , T and Nakajima , A . 1979 . Protection from cerebral ischemia by a new imidazole derivative (Y- 9179) and pentobarbital. A comparative study in chronic middle cerebral artery occlusion in cats . Stroke , 10 : 126 – 134 .
- Tamura , A , Graham , DI , McCulloch , J and Teasdale , GM . 1981 . Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion . Journal of Cerebral Blood Flow and Metabolism , 1 : 53 – 60 .
- van Marthens , E , Harel , S and Zamenhof , S . 1975 . Experimental intrauterine growth retardation . Neonatology , 26 : 221 – 231 .
- Volpe JJ. 2008 . Hypoxic-ischemic encephalopathy: biochemical and physiological aspects . In: Neurology of the newborn , 5th ed . Philadelphia , PA : Saunders . p. 247 – 324 .
- Zivin , JA , DeGirolami , U , Kochhar , A , Lyden , PD , Mazzarella , V , Hemenway , CC and Henry , ME . 1987 . A model for quantitative evaluation of embolic stroke therapy . Brain Research , 435 : 305 – 309 .