Abstract
Days 3–5 post-ovulation is when the embryo is entering the uterus, undergoing genomic activation and progesterone (P4) increases; therefore, this may be a physiologically important time in the cow. The objective of these experiments was to determine the effect on pregnancy rates through the administration of a low dose of P4 or altrenogest (ALT; Regumate) on days 3, 4 and 5 of the estrous cycle among repeat breeder (RB) cattle. In Trial 1, RB cows (n=14), that failed to conceive to 3 breedings with a fertile bull, and fertile control cows (n=14) that were an average of 120 days post-partum were used. In Trial 2, RB cows (n=6), RB heifers (n=4) (identified as in Exp 1) and fertile control cows (n=8) that were an average of 135 days post-partum were used. In Exp 1 and 2, all cattle were exposed to a fertile bull for one estrus and the onset of estrus was considered day 0. The respective treatments and blood sample collections were on days 3–5. In Trial 1, RB cows were administered 15 mg of P4 in 3 ml of ethanol sc and in Trial 2 they were administered 15 mg of ALT (orally) on days 3, 4 and 5. Controls in Trial 1 and 2 were only restrained for blood sample collection to determine plasma P4 levels using radioimmunoassay (RIA). For both trials, pregnancy rates were empirically lower and similar (P>0.05) between treatments vs. controls. Pregnancy rates for Trial 1 controls were 72% (10/14) and 43% (6/14) for Treatment and for Trial 2 controls were 50% (4/8) and 50% (3/6) for Treatment cows. The mean±SE plasma P4 levels were higher (P < 0.05) in pregnant controls (1.87±0.2 ng/ml) compared with nonpregnant controls (0.89±0.2 ng/ml) on day 5. In addition, the mean±SE increase in plasma P4 from day 3–4 was higher (P < 0.05) in pregnant controls (0.42±0.06 ng/ml) compared with nonpregnant controls (0.18±0.11 ng/ml). These resulting pregnancy rates support the hypothesis that P4 supplementation during this time improve pregnancy rate in RB cattle.
Introduction
Problem breeder (PB) cattle are typically cows that fail to become pregnant in a 60–90 day breeding season or to three or more artificial inseminations (AI). These cows can represent a substantial economic loss to the dairy and beef industry and with increased production becoming a necessity for current cattle production industries new methods to improve pregnancy and calving rates to fewer breeding attempts will be required.
In the past there have been many attempts to improve fertility in high producing cows beginning with an early attempt by ovariectomy and steroid replacement therapy which failed (Hawk et al. Citation1963). Later researchers began to investigate differences in the physiology of cattle during fertile and non-fertile matings and several were discovered. During infertile matings cows tend to experience prolonged estrus (Erb et al. Citation1976), delayed pre-ovulatory LH release (Gustafsson et al. Citation1986; Albihn Citation1991) and higher cortisol concentrations (Bage et al. Citation2000; Bage et al. Citation2001). Furthermore, during an infertile mating cows experience delayed post-ovulatory increases in progesterone (P4) and corpora lutea (CL) tissue collected from metestrus in PB cows proved less responsive to luteotropin (e.g. LH and PGE2) stimulation in vitro Shelton et al. Citation1990). Additionally, the total CL volume in PB cows was lower compared with CL volume collected from fertile cows (Albihn Citation1991). These findings demonstrate a pattern of delayed luteal function as well as luteal dysfunction in PB cattle.
Additionally, there has been no correlation found between circulating P4 concentrations and normal embryo morphology in cattle (Linares et al. Citation1982), however, P4 concentrations may still be the primary factor involved in the development of a successful pregnancy (Hasler et al. Citation1980). Using reciprocal embryo transfers among virgin (fertile) heifers and PB cows (transfer embryos from virgin heifers to PB cows and vice versa), it was discovered that PB cows had significantly higher pregnancy rates than virgin heifers (Gustafsson and Larrson Citation1983). In a subsequent study, embryo survival to day 16–17 was lower in PB cows compared with virgin heifers while, more day 7 embryos collected from virgin heifers survived following transfer to PB cows than PB cow embryos transferred to virgin heifers (Gustafsson Citation1985). These results demonstrated that the embryos developing from day 1 to day 7 in PB cows had lowered rates of survival than embryos developing from day 1 to day 7 in fertile cattle. A holistic view of these results indicates that the infertility problems associated with PB cows exists within the initial embryo development period (zygote to blastocyst-stage).
These findings indicate deficient uterine secretion or function may be the cause for decreased embryo survival in PB cows. There are known differences in the number of P4 receptors within the uterus of PB cows (Stanchev et al. Citation1991), however, there may exist a more likely cause of reduced embryo survival. The hypothesis of this research project was that the timing of the P4 increase following mating is the limiting factor for improving embryo development in PB cows. Therefore the objective of this study was to determine if an artificially induced rise in P4, via administration of low doses of P4 or altrenogest (ALT) on day 3–5 following the onset of estrus (day = 0) would increase pregnancy rates in PB cows.
Materials and methods
Animals
Mature crossbred beef cows (from 5 to 10 years old and from 5 to 8 body condition score) were obtained from the Centre for Reproductive Biology at Louisiana State University, St. Gabriel, Louisiana. All of these cows were treated in accordance with guidelines set forth by the Animal Use Committee for Louisiana State University.
Repeat breeder classification
The breeding programme at the Centre for Reproductive Biology was designed so that a group of mature cows will remain nonpregnant for two years in order to serve on AI and/or embryo transfer (ET) experiments. Should some cows not become pregnant to AI or ET within a two-year period then they are pasture-mated with two fertile bulls for a 65-day breeding period in the spring. The PB cows used in this experiment were those cows that failed to become pregnant to various AI or ET for two years and did not become pregnant during the 65-day breeding season. Since these cows were cycling prior to being placed with the bulls there was an opportunity for three to four matings during each spring breeding season. The PB cows were determined nonpregnant 90 days following the end of the breeding season by ultrasound (Aloka 500-V, Corometrics, Wallingford, CT) examination and were maintained on pasture for an additional three months. Due to the fact that PB cows must be determined in hindsight, the 65-day breeding season was considered the nontreatment period.
The cows classified as fertile were used as control cows for bull fertility and were cows that became pregnant to the same 65-day breeding season one year prior to the respective nontreatment breeding season for the corresponding PB cows. All control cows calved, weaned a live calf and were about 130 days post-partum at the initiation of the experimental (treatment) breeding season.
Estrus detection
Prior to the treatment breeding season, all cows were examined via ultrasonography and only cows free from cystic ovarian disease or reproductive tract abnormalities were used in this experiment. To aid in estrus detection, Heat Watch estrus detection aids (Heat Watch, DDX, Boulder, CO) were used in Trial 1, and Estrus Alert patches (Estrus Alert, Apple Valley, MN) were used in Trial 2. In addition to estrus detection aids, visual observations of 45 minutes twice daily were employed.
Treatment and sample collection
In Trial 1, the treatment breeding season lasted only 25 days to allow for pregnancy rates from one estrus. All cows underwent pregnancy examinations via ultrasonography about 30–33 days following the onset of estrus (day 0 = onset of estrus) and throughout this experiment, a pregnancy was defined as the presence of a fetus with a viable heartbeat. During the treatment breeding season, PB cows were treated with 15 mg of P4 (Progesterone, Sigma Chemical, St. Louis, MO) dissolved in 3 ml of 100% ethanol (ETOH) (s.c.) on days 3, 4 and 5 after the onset of estrus (estrus = day 0). The control cows received 3 ml of vehicle (ETOH) on the same days.
In Trial 2, the treatment breeding season length was the same as Trial 1. However, in this trial PB cows received 15 mg of ALT orally on days 3, 4 and 5 following the onset of estrus while cows in the control group were brought to the chute and restrained on the same days but received no further treatment.
Radioimmunoassay (RIA) of P4
In both trials, blood samples were collected immediately prior to treatment via jugular venipuncture, using 10 ml blood tubes containing sodium heparin, on days 3–6 of the estrous cycle. These samples were stored on ice immediately following collection and were later centrifuged at 300× g for 10 minutes. The plasma was collected and stored at 4°C until assay was performed. In Trial 1 and 2 plasma P4 concentrations were determined using a commercial P4 assay kit (Diagnostics Systems Laboratory, Webster, TX). The intra- and inter-assay correlation of variation and assay sensitivites were 5%, 10% and 0.05 ng/ml for progesterone. Additionally there was no cross reactivity in this assay with ALT.
Statistical analysis
Pregnancy rates in this experiment were analysed using procedures for categorical data with Chi-square test in SAS (SAS Institute, Inc., Gary, NC). Plasma P4 concentrations were analysed by repeated measures analysis of variance (ANOVA). Differences in plasma P4 concentrations among pregnant and nonpregnant cows were analysed using a general linear model (Proc GLM) and Tukey's Test. The rise in P4 was determined by subtracting the individual cow plasma P4 value on day 3 from the individual cow plasma P4 value on day 4. Statistical differences in rise of P4 were determined using the LSMeans procedure in SAS. Differences with a probability (P) value of 0.05 or less were considered significant in this study.
Results
In both trials there were no differences (P>0.05) in pregnancy rates among control and treatment groups. In Trial 1 the pregnancy rate for the P4-treated PB cows was 6 of 14 (43%) and for control cows 10 of 14 (72%) and in Trial 2 was 3 of 6 (50%) for ALT-treated PB cows and 4 of 8 (50%) for control cows. However, there was a significant difference (P<0.05) between the pregnancy rate among PB cows during treatment and nontreatment periods with the pooled pregnancy rate for PB cows, regardless of P4 or ALT treatments being 9 of 20 (45%) compared with 0 of 20 (0%) during the nontreatment period ().
Table 1. Pooled pregnancy rates for problem breeder cows and control fertile cows during the non-treatment and treatment breeding periods.
In Trial 1, the mean plasma P4 concentration was significantly lower (P<0.05) on day 3 in pregnant P4-treated PB cows (0.25±0.01 ng/ml) compared with non-pregnant P4-treated PB cows (0.67±0.01 ng/ml) (). However, by day 4, the mean plasma P4 concentration was significantly higher (P<0.05) for pregnant P4-treated PB cows (2.30±0.7 ng/ml) compared with non-pregnant P4-treated PB cows (1.22±0.1 ng/ml). This corresponded to a significantly (P<0.05) greater increase (rise) of mean plasma P4 concentrations from day 3 to day 4 in pregnant P4-treated PB cows (2.07±0.7 ng/ml) compared with non-pregnant P4-treated PB cows (0.55±0.12 ng/ml). This pattern was similar to that observed in the control pregnant and nonpregnant cows, however, the greatest rise was seen from day 4–5 ().
Figure 1. Plasma progesterone (P4) concentrations in pregnant (n=9) and non-pregnant RPB cows (n=11) administered 15 mg of P4 on days 3, 4 and 5 after the onset of estrus.
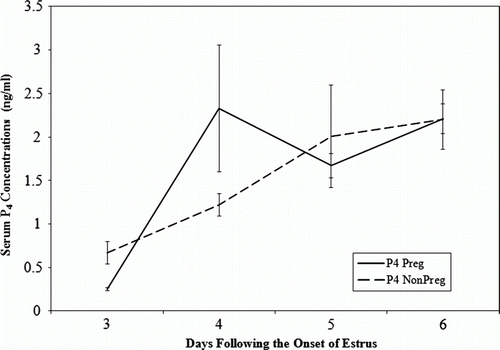
Figure 2. Plasma progesterone (P4) concentrations in pregnant (n=14) and nonpregnant ‘fertile’ control cows (n=8) on days 3, 4 and 5 after the onset of estrus.
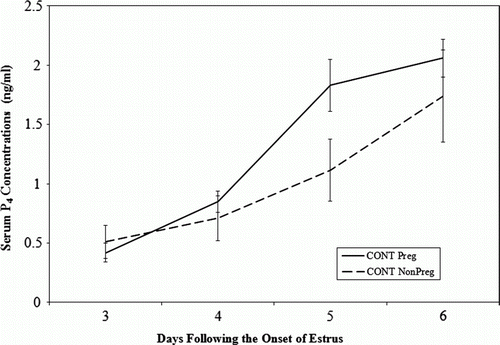
In Trial 2, there were not enough ALT-treated cows to make statistical comparisons so only the mean plasma P4 concentrations are reported; day 3 (0.68±0.28 ng/ml), day 4 (0.87±0.20 ng/ml), day 5 (2.04±0.44 ng/ml) and day 6 (2.35±0.70 ng/ml). In the control group, pregnant cows had a greater (P<0.05) rise in mean plasma P4 concentrations from day 3 to day 4 (0.20±0.09 ng/ml) compared with non-pregnant cows (0.44±0.06 ng/ml) Also, the mean plasma P4 concentrations were greater (P<0.05) in pregnant cows on day 5 (1.87±0.22 ng/ml) compared with nonpregnant cows (0.89±0.16 ng/ml).
Discussion
The results of this experiment demonstrate that administering low doses of P4 or ALT on days 3–5 post-mating improved pregnancy rates in PB cattle. Although administering progestin to improve pregnancy rates in PB cattle is not a new concept, this study evaluated the importance of the timing of P4 administration and dosage of P4. The significant improvement in pregnancy rates among PB cows in this experiment is attributed to the timing and dosage of P4 or ALT treatment.
A series of physiological events occur on day 3, of the estrous cycle, that are critical to normal embryo development. First, at the 8- to 16-cell stage, the bovine embryo leaves the oviduct to reside within the uterus between day 3 and day 4 (El-Banna and Hafez Citation1970; Crisman et al. Citation1980). Also the embryo undergoes genomic activation at this time (Eyestone and First Citation1986). This is also the same stage of the estrous cycle (day 3) the CL was believed to begin producing significant amounts of P4 (Henricks et al. Citation1970; Wettemann and Hafs Citation1973). It is known that P4 concentrations can control the rate of embryo transport into the uterus in the mice (Roblero and Garavagno Citation1979), rat (Forcelledo et al. Citation1982) and the cow (Crisman et al. Citation1980) as well as prepare the uterus to support embryo development (Finn and Martin Citation1967). These findings suggest that day 3 of the estrous cycle may be a time in which a series of critical steps must be completed.
Administration of P4 anytime from day 1 to day 7 is known to increase uterine secretions (Garrett et al. Citation1988; Geisert et al. Citation1992; Barnes Citation2000) increase the rate of embryo transport from the oviduct into the uterus (Crisman et al. Citation1980), increase the rate of embryo development as well as embryo length (Garrett et al. Citation1987), and increase fetal size and crown-rump length later in gestation (Kleeman et al. Citation1994). Also, when embryos were exposed to advance staged uteri there was an increase in pregnancy rates (Wilmut and Sales Citation1981). Collectively these studies demonstrate that early increases in P4 can result in long term beneficial effects for the embryo, however, they involved greater doses of P4.
In this experiment the natural increase in P4 occurred from day 4 to day 5 of the estrous cycle in the control cows. This is one day later than was expected, however, pregnant control cows had a significant increase (rise) in plasma P4 compared with non-pregnant control cows on day 4–5. This greater rise was expected, as this pattern occurred in P4-treated PB cows from day 3 to day 4. It is difficult to explain why non-pregnant PB cows had significantly lower increases in P4 from day 3 to day 4 as they were administered the same amount of P4 as pregnant PB cows. It is possible that in the non-pregnant repeat breeder (RB) cows that the P4 was metabolised more quickly or bound to carrier proteins rendering it biologically inactive. However, the pattern that emerged was that control and PB cows that experienced significant increases in plasma P4 from day 3 to day 5 were cows that were pregnant 30 days later.
The reciprocal embryo transfers between virgin heifers and PB cattle studies appear to support these findings. Embryo development was hindered when early development occurred in PB cattle (Gustafsson and Larrson Citation1983), but occurred normally when early development occurred in virgin-fertile heifers even following transfer on day 7 into PB cattle (Gustafsson Citation1985). These findings seem to support an early pregnancy model where delayed luteal function could be a possible cause to pregnancy failure. More importantly, these findings show the importance of a healthy, fertile reproductive tract for an early stage embryo.
Previous research has demonstrated that P4 supplementation initiated prior to day 6 improved pregnancy rates over controls while supplementation after day 6 did not (Mann and Lamming Citation1999). Unfortunately, the studies involved the administration of a large dose of P4, up to 100 mg per day in some cases which further complicates the dissection of dosage level effects. Also, the treatment protocols initiated prior to day 5 involved a long-term P4 supplementation period lasting through the luteal phase. From these studies it can only be concluded that early P4 is beneficial but not at what time or what level.
In conclusion, supplementation of P4 or ALT to PB cows on days 3–5 following breeding resulted in increased pregnancy rates. These increased pregnancy rates are likely the result of an induced rise in P4 between days 3 and 5. These results indicate that an increase in plasma P4 from day 3 to day 5 of early pregnancy in the cow is beneficial to early embryo development. This effect occurs whether P4 or ALT is administered. Although the described procedures are off label use of ALT this procedure allows for a practical method of treating PB cows because it is orally administered and possibly substituting with other approved progestins for cattle. These findings may offer a practical and effective method for reducing repeated services among beef and dairy cattle.
Acknowledgements
The authors wish to acknowledge Buzz Yancy of DDX, Inc. (Boulder, CO) for use of HeatWatch® transponders. We also wish would like to acknowledge Gary Sides of Intervet, Inc. (Millsboro, DE) for supplying Regumate used in this research. This research was funded, in part, by Federal Regional Project W-171.
References
- Albihn , A . 1991 . Standing oestrus, ovarian function and early pregnancy in virgin and repeat breeder heifers . Journal of Veterinary Medicine , 38 : 212 – 221 .
- Bage , R , Bosu , WT and Rodriguez-Martinez , H . 2001 . Ovarian follicle apoptosis at the onset of standing estrus in virgin and repeat-breeder dairy heifers . Theriogenology , 56 : 699 – 712 .
- Bage , R , Forsberg , M , Gustafsson , H , Larsson , B and Rodriguez-Martinez , H . 2000 . Effect of ACTH-challenge on progesterone and cortisol levels in ovariectomised repeat breeder heifers . Animal Reproduction Science , 63 : 65 – 76 .
- Barnes , FL . 2000 . The effects of the early uterine environment on the subsequent development of embryo and fetus . Theriogenology , 53 : 649 – 658 .
- Crisman , RO , McDonald , LE and Thompson , FN . 1980 . Effects of progesterone or estradiol on uterine tubal transport of ova in the cow . Theriogenology , 13 : 141 – 154 .
- El-Banna , AA and Hafez , ES . 1970 . Egg transport in beef cattle . Journal of Animal Sciences , 30 : 430 – 432 .
- Erb , RE , Garverick , HA , Randel , RD , Brown , BL and Callahan , CJ . 1976 . Profiles of reproductive hormones associated with fertile and nonfertile inseminations of dairy cows . Theriogenology , 5 : 227 – 242 .
- Eyestone , WH and First , NL . 1986 . A study of the 8- to 16-cell developmental block in bovine embyros cultured in vitro . Theriogenology , 25 : 152 abstr
- Finn , CA and Martin , L . 1967 . Patterns of cell division in the mouse uterus during early pregnancy . Journal of Endocrinology , 39 : 593 – 597 .
- Forcelledo , ML , Morales , P , Vera , R , Quijada , S and Croxatto , HB . 1982 . Role of ovarian and adrenal progesterone in the regulation of ovum transport in pregnant rats . Biology of Reproduction , 27 : 1033 – 1041 .
- Garrett JE , Geisert RD , Morgan GL. 1987 . Effect of exogenous progesterone on cycle length, embryonic development and maintenance of pregnancy in the bovine . Journal of Animal Science 65 : 418 abstract .
- Garrett , JE , Geisert , RD , Zavy , MT and Morgan , GL . 1988 . Evidence for maternal regulation of early conceptus growth and development in beef cattle . Journal of Reproduction and Fertility , 84 : 437 – 446 .
- Geisert , RD , Morgan , GL , Short , EC Jr. and Zavy , MT . 1992 . Endocrine events associated with endometrial function and conceptus development in cattle . Reproduction Fertility and Developoment , 4 : 301 – 305 .
- Gustafsson , H . 1985 . Characteristics of embryos from repeat breeder and virgin heifers . Theriogenology , 23 : 487 – 498 .
- Gustafsson , H and Larrson , K . 1983 . Reciprocal embryo transfer between repeat breeder and virgin heifers-An experimental model . Acta Veterinaria Scandinavica , 24 : 59 – 64 .
- Gustafsson , H , Larrson , K , Kindahl , H and Madej , A . 1986 . Sequential endocrine changes and behevior during oestrus and metoestrus in repeat breeder and virgin heifers . Animal Reproduction Science , 10 : 261 – 273 .
- Hasler , JF , Bowen , RA , Nelson , LD and Seidel , GE Jr . 1980 . Serum progesterone concentrations in cows receiving embryo transfers . Journal of Reproduction and Fertility , 58 : 71 – 77 .
- Hawk , HW , Brinsfield , TH , Tuner , GD , Whitmore , GE and Norcross , MA . 1963 . Embryo survival in first-service and repeat-breeder cattle after ovariectomy and hormone therapy . Journal of Dairy Science , 46 : 1397 – 1401 .
- Henricks , DM , Dickey , JF and Niswender , GD . 1970 . Serum luteinizing hormone and plasma progesterone levels during the estrous cycle and early pregnancy in cows . Biology of Reproduction , 2 : 346 – 351 .
- Kleemann , DO , Walker , SK and Seamark , RF . 1994 . Enhanced fetal growth in sheep administered progesterone during the first three days of pregnancy . Journal of Reproduction and Fertility , 102 : 411 – 417 .
- Linares , T , Larsson , K and Edqvist , L . 1982 . Plasma progesterone levels from oestrus though day 7 after AI in heifers carrying embryos with normal or deviating morphology . Theriogenology , 17 : 125 – 132 .
- Mann , GE and Lamming , GE . 1999 . The influence of progesterone during early pregnancy in cattle . Reproduction in Domestic Animals , 34 : 269 – 271 .
- Roblero , LS and Garavagno , AC . 1979 . Effect of oestradiol-17 beta and progesterone on oviductal transport and early development of mouse embryos . Journal of Reproduction and Fertility , 57 : 91 – 95 .
- Shelton , K , Gayerie De Abreu , MF , Hunter , MG , Parkinson , TJ and Lamming , GE . 1990 . Luteal inadequacy during the early luteal phase of subfertile cows . Journal of Reproduction and Fertility , 90 : 1 – 10 .
- Stanchev , PD , Rodriguez-Martinez , H , Albihn , A , Eriksson , H , Gustafsson , H and Larsson , K . 1991 . Concentrations of nuclear progesterone receptors in endometrium of virgin and repeat breeder heifers after embryo transfer . Journal of Veterinary Medicine Series A , 38 : 271 – 280 .
- Wettemann , RP and Hafs , HD . 1973 . LH, prolactin, estradiol and progesterone in bovine blood serum during early pregnancy . Journal of Animal Science , 36 : 51 – 56 .
- Wilmut , I and Sales , DI . 1981 . Effect of an asynchronous environment on embryonic development in sheep . Journal of Reproduction and Fertility , 61 : 179 – 184 .