Abstract
Pioglitazone (PGT) belongs to thiazolidinedione (TZD) family or insulin sensitizers that are potent ligands for peroxisome proliferator activated receptor gamma and are used in the treatment of type 2 diabetes mellitus. It has been shown that injection of TZD in cattle has some useful effects on blood parameters but there is no published study with respect to the feeding of TZDs in ruminants till now. Therefore, the aim of this study was to determine the bioavailability and several other pharmacokinetic parameters of PGT in sheep. A single intravenous (IV) or oral dose of PGT (10 mg/kg) was administered to five male sheep. Blood samples were collected at various time intervals, and PGT concentration was measured by a validated high-performance liquid chromatography method. The data obtained were best fitted into a two-compartment model for the IV route, and non-compartmental approach for oral route. The bioavailability of PGT was obtained to be approximately 62%. After IV injection of PGT, the elimination half-life (t 1/2β), the volume of distribution at steady-state (V ss) and the elimination rate constant (k el) were obtained to be 4.04±0.88 h, 0.30±0.06 L/kg and 0.47±0.09 h−1, respectively. After oral administration of PGT, highest drug concentration observed in plasma (C max), the time (t max) at which C max occurs, half-life (t 1/2), absorption rate constant (k ab) and elimination rate constant (k el) were obtained to be 10.2±1.3 µg/mL, 6.4±0.3 h, 4.42±0.21 h, 0.16±0.01 h−1 and 0.16±0.01 h−1, respectively.
Introduction
Pioglitazone (PGT) is a member of thiazolidinedione (TZD) drugs used for the treatment of human type 2 diabetes mellitus (Gillies and Dunn Citation2000) that reduces insulin resistance at target tissues, mainly skeletal muscle, adipose tissue and liver (Mudaliar and Henry Citation2001). TZDs are potent, synthetic ligands of the nuclear transcription factor peroxisome proliferator activated receptor gamma (PPARγ) (Lehmann et al. Citation1995). It seems that TZDs exert their biological effects on insulin sensitivity through binding to PPARγ (Tontonoz and Spiegelman Citation2008). PPARs are members of the nuclear receptor superfamily. PPARγ is predominantly found in white and brown adipose tissue, large intestine and spleen (Kliewer et al. Citation2001). PPARγ, as a pivotal regulator of adipocyte differentiation (Houseknecht et al. Citation2002), influences on the storage of fatty acids in the adipose tissue and takes part in adipocyte differentiation (Kersten et al. Citation2000). It has been reported that PPARγ can regulate several adipokines secreted by white adipose tissue, including tumour necrosis factor alpha (TNFα), leptin, resistin and adiponectin (Knouff and Auwerx Citation2004). Several studies indicate that TZDs, as potent PPARγ ligands, increase insulin-stimulated glucose uptake and decrease free fatty acid (FFA) concentrations (Yki-Järvinen Citation2004). In animal studies, TZDs reduce TNFα expression and decrease leptin, FFA and triglyceride levels and improve insulin sensitivity (Mudaliar and Henry Citation2001).
In ruminants, TZDs have been examined in several in vitro (Bionaz et al. Citation2008; Grant, Ortiz-Colón, Doumit and Buskirk Citation2008; Grant, Ortiz-Colón, Doumit, Tempelman, et al. Citation2008; Revelo and Waldron Citation2010) and in vivo studies (Kushibiki et al. Citation2001; Smith et al. Citation2007, Citation2009). It has been reported that TZDs have the potential to improve metabolic health and reproduction in dairy cattle (Smith et al. Citation2007, Citation2009) and may exert useful effects on intramuscular fat (marbling) in some species (Poulos and Hausman Citation2006; Hausman et al. Citation2008; Tontonoz and Spiegelman Citation2008). Several studies have indicated the advantage of TZDs in ruminants, but there is no available data about oral administration and bioavailability of these drugs in ruminant species. With regard to feeding of ruminants with each supplement or drug, we need to know their degradation process by rumen microorganisms due to the high potency of rumen microbes involved in feed degradation. Therefore, it seems that evaluation of pharmacokinetics parameters and bioavailability of drugs can lead us to determine optimal dosage of drugs in ruminants. Pharmacokinetics of PGT has been investigated in humans (Eckland and Danhof Citation2000; Budde et al. Citation2003; Kalliokoski et al. Citation2008), rats (Fujita et al. Citation2003; Umathe et al. Citation2008) and horses (Wearn et al. Citation2010), but there is no published data on the pharmacokinetics of PGT in sheep. Thus, the aim of present study was to determine the bioavailability and some other pharmacokinetic parameters of PGT in sheep, as a ruminant model, to define a dosage regimen for the drug administration in sheep as a potential feed additive (growth promoter) due to its useful effects on blood chemistry (e.g., nonesterified fatty acid [NEFA]), lowering the rate of metabolic disorders, improving reproduction and meat marbling.
Materials and methods
Animals
Five healthy male Naeini (Iranian fat-tailed) sheep were obtained from a flock in dairy facilities of Lavark Research Station (Isfahan University of Technology, Isfahan, Iran) and were kept in a large community pen. Animals were fed with a basal ration consisting of alfalfa hay, wheat straw and concentrate (barley grain and wheat bran) with free access to water and saltlick. Body weights were recorded one day before PGT administration. The mean body weights were 31.5±0.6 and 32.0±0.7 kg (n=5) before intravenous (IV) injection and oral administration of PGT, respectively. Animals were acclimatised to the environment for six weeks prior to the start of the experiment.
Experimental design
All animals received a single dose of PGT (10 mg/kg) through either IV (jugular vein) or oral routes. PGT was dissolved in propylene glycol (Merck, Germany) to prepare a 100 mg/mL solution for the IV injection. After 8 weeks washout period, PGT (mixed in water as a suspension) was given to the animals by drench with an equivalent amount to IV dosing. Blood samples were collected from jugular vein into 6 mL vacuum tubes containing heparin before administration of drug and at 5, 15, 30 min, 1, 1.5, 2, 4, 6, 8, 12, 18, 24 and 48 h after IV injection of PGT. The time intervals for blood sampling after oral dosing were at 0 (pre-treatment), 30, 45 min, 1, 1.5, 2, 4, 6, 8, 12, 18, 24 and 48 h after drenching of PGT. Plasma samples were immediately separated by centrifugation for 10 min at 2000g and were stored at −20°C until analysis.
Pioglitazone assay
Plasma concentration of PGT was determined by a validated high-performance liquid chromatography (HPLC) method with UV detection at a wavelength of 269 nm described by Souri et al. (Citation2008). The mobile phase was acetonitrile and 140 mM KH2PO4 (40:60, vol/vol) with final pH of 4.45. Ethylparaben dissolved in methanol with final concentration of 2 µg/mL was used as an internal standard. An HPLC system consisting of a pumping unit (JASCO PU-980, Japan), detection unit (a UV detector, JASCO UV-2075 plus) and separation unit (Nova-Pak C8, 4 µm column, 250 mm×4.6 mm, Waters, Milford, MA, USA) was used.
The HPLC method for PGT assay showed high selectivity with clear curve for PGT and the internal standard (ethylparaben) comparing the retention times of PGT and internal standard chromatograms with those for blank samples. The calibration curve of PGT was linear with high correlation, presented by r 2=0.9996. The recovery of PGT was approximately 91% and the intra- and inter-assay precisions of the method, expressed as relative standard deviation, with six replicates were less than 10%. The limit of quantification for the assay was 50 ng/mL.
Drug and reagents
Pioglitazone (PGT) hydrochloride was from Hetero Drugs (India; Batch No: PH0040807) that was kindly provided by B.A. Shiraz Co. (Tehran, Iran). All solvents used were from Merck (Germany) and were purchased from local suppliers.
Analysis of data
All data are presented as mean±SEM. Data for the IV route were analysed using WINNONLIN professional software (version 5.2.1; Pharsight Corporation, CA, USA). F-test was used to decide which kinetic model best describes the pharmacokinetics of the drug. The IV data were best fitted into a two-compartment body model. Data for the oral route, however, were analysed using a non-compartmental approach.
All pharmacokinetics parameters including maximum plasma concentration (C max), time at maximum plasma concentration (t max), distribution half-life (t 1/2α), elimination half-life (t 1/2β), elimination rate constant (k el), absorption rate constant (k ab), mean residence time (MRT) and area under the first moment–time curve (AUMC) were calculated using the software afore mentioned. The trapezoidal rule was used to calculate the area under the curve (AUC0 − t). Bioavailability (F) was calculated using the following formula and other pharmacokinetic parameters were corrected for bioavailability.
Results
It should be mentioned that data of one sheep from each group were not taken into consideration due to technical errors as drug was not injected correctly into the vein, therefore, the number of animals for data analysis decreased to four for both groups. The mean plasma concentrations after IV and oral administration of PGT are illustrated in . Various pharmacokinetics parameters for PGT are presented in .
Figure 1. Semilogaritmic plot of mean plasma concentration versus time after a single intravenous injection (•; n=4) and oral administration (o; n=4) of 10 mg/kg PGT in sheep.
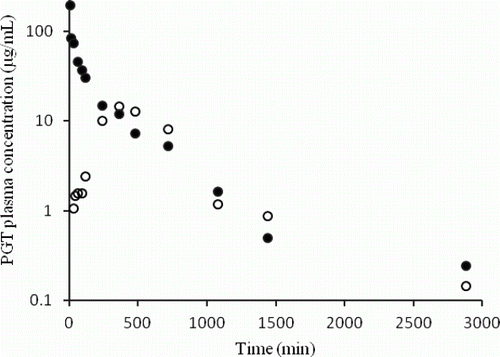
Table 1. Pharmacokinetic parameters [mean±SEM (range); n=4] following a single oral or IV administration of PGT (10 mg/kg) in sheep.
Maximum drug concentration (10.2±1.3 µg/mL) was achieved at 6.4±0.3 h after oral administration of PGT. Both k ab and k el were similar (0.16±0.01 h−1). Half-life, volume of distribution, clearance (CL) and AUC were 4.42±0.21 h, 0.38±0.05 L/kg, 0.06±0.01 Lh−1 kg−1 and 180.3±30.5 h µg/mL, respectively. The mean absolute bioavailability (F) of PGT was calculated to be 62.4±6.9% ().
After IV injection of PGT (10 mg/kg), the zero time intercept of distribution (A) and elimination (B) phases were 71.7±9.4 and 42.0±12.9 µg/mL, respectively. Distribution half-life (t 1/2α) and t 1/2β were 0.49±0.13 and 4.04±0.88 h, respectively. Volume of distribution at steady-state (V ss), volume of the central compartment (V 1) and volume of peripheral compartment (V 2) were found to be 0.30±0.06 L/kg, 0.16±0.04 L/kg and 0.14±0.03 L/kg, respectively. Total clearance was determined to be 0.08±0.02 Lh−1 kg−1. Elimination rate constant (k el), distribution rate constant from central to peripheral compartment (K 12) and distribution rate constant from peripheral to central compartment (K 21) were obtained to be 0.47±0.09, 0.68±0.15 and 0.88±0.41 h−1, respectively. Area under the plasma concentration–time curve from time zero to infinity (AUC0 − ∞), MRT and AUMC were found to be 249±16 h µg/mL, 4.7±1.0 h and 1218±300 h2 µg/mL, respectively ().
Discussion
A few studies have focused on the disposition of PGT in various species. The present study generated new data on the pharmacokinetics of PGT in a ruminant species. C max for PGT after oral administration in sheep was found to be close to the value reported in male rats (10.2 vs. 13.6 µg/mL; ). A relatively smaller value for C max in sheep can be due to the differences in the digestive tract physiology between rats (as a monogastric) and sheep (as a ruminant). Oral doses (15–45 mg) of PGT in human studies will correspondingly give dosage rates of 0.2 to 0.6 mg/kg assuming an average body weight of 70 kg (Wearn et al. Citation2010). It has also been reported that, for single doses between 2 and 60 mg (0.03–0.86 mg/kg), C max increases linearly with increasing dose (Eckland and Danhof Citation2000). Although the administrated dose used in the present study was about 15 folds more than that in humans (10 vs 0.65 mg/kg), the C max achieved in sheep was only approximately seven times more than that in humans (Eckland and Danhof Citation2000; Budde et al. Citation2003) ().
Table 2. Comparison of several pharmacokinetic parameters for PGT in sheep, horse, rat and human following single oral dose administration of the drug.a
The t max value in sheep (approximately 6.5 h; ) is much greater than the reported values in other species (), which may be related to the greater duration of digestion in ruminant compared to the non-ruminant species.
The half-life of PGT in sheep after oral administration was approximately half the period reported in humans (Eckland and Danhof Citation2000) and horses (Wearn et al. Citation2010) (4.42 vs. 9.2 and 9.9 h, respectively; ).
High clearance of PGT in sheep following oral dosing (0.06 Lh−1 kg−1), compared with the value reported in humans (Eckland and Danhof Citation2000) (0.04 Lh−1 kg−1; ), can account for a shorter t 1/2 in sheep. The t 1/2 of PGT after oral administration in male rats (Fujita et al. Citation2003) is less than the value found in sheep (2.5 vs. 4.42 h; ). The clearance of PGT is not reported in the above study. The study by Umathe et al (Citation2008), however, indicates a greater clearance (0.51 Lh−1 kg−1) after oral administration of 10 mg/kg PGT in rats compared to the value found in sheep ().
The k el values in two different studies using rats (Fujita et al. Citation2003; Umathe et al. Citation2008) are actually close to the value in sheep (0.26 and 0.22 vs. 0.16 h−1, respectively; ).
The mean clearance (2.4 L/h) of PGT after a single IV route in our study is similar to the reported value in human study, where 5 mg PGT was injected (Eckland and Danhof Citation2000). Range of clearance in the human study (Eckland and Danhof Citation2000) after injection of 5 mg PGT is reported to be 1.72–4.17 L/h (), which is close to those found for IV administration of PGT in sheep (1.2–4.1 L/h, data not shown). Clearance values of PGT in sheep with both routes (oral and IV) are relatively higher than those in humans administered 45 mg through oral route (0.038 Lh−1 kg−1) or 5 mg through IV injection (0.034 Lh−1 kg−1) (Eckland and Danhof Citation2000) assuming an average weight of 70 kg for human subjects. Elimination of PGT in human is believed to occur with a low hepatic extraction ratio (Budde et al. Citation2003). It has been shown in human that clearance of PGT following oral administration of 2–60 mg (0.03–0.86 mg/kg) is low and independent of the dose administered. Therefore, it seems that the involved hepatic enzymes in the metabolism of PGT does not saturate when the above doses are administered (Eckland and Danhof Citation2000).
It is worth mentioning that elimination rate constant (k) is dependent on both clearance and volume of distribution according to the equation: k=CL/V. Therefore, the difference in clearance can be explained by the differences in protein binding and/or volume of distribution in different animal models.
The volume of distribution of PGT in humans is relatively low. This might be due to extensive binding (>97%) of PGT to plasma proteins, particularly albumin, in humans (Eckland and Danhof Citation2000). The mean volume of distribution (0.30 L/kg) in sheep after a single IV dosing (10 mg/kg) is close to that in humans (0.25 L/kg) after injection of 5 mg PGT (Eckland and Danhof Citation2000). In addition, the volume of distribution of the drug after oral administration in our study is higher than that reported for male rats (Fujita et al. Citation2003) (0.38 vs. 0.27 L/kg; ).
The free fraction of PGT in plasma in rat and human is less than 3% (Eckland and Danhof Citation2000). However, there is no information about the extent of binding of PGT to plasma proteins in sheep. The binding of PGT to plasma proteins is reported to be independent of its concentration at blood levels of 0.034–2.00 µg/mL (Eckland and Danhof Citation2000). In the present study, the plasma concentrations of PGT, after both oral and IV administration, were higher than the above concentration range. This may explain the greater value for volume of distribution in sheep. It should also be mentioned that the overall rate of elimination is related to the free fraction of the drug in plasma and intrinsic capacity of liver to metabolise PGT and so, the extraction ratio of PGT is low in healthy humans (Budde et al. Citation2003).
The mean absorption rate constant (k ab) after oral administration of PGT in sheep is low and was found to be less than that in male rats (0.16 vs. 0.38 h−1; ) (Fujita et al. Citation2003). The values reported for k ab after oral administration of 7.5 mg PGT in humans (Eckland and Danhof Citation2000) comprise a broader range (0.4–1.2 h−1) compared to those in sheep (0.14–0.18 h−1) ().
The bioavailability (F) of PGT after oral administration of 10 mg/kg of the drug was found to be approximately 62% which is in between the values reported for humans (83%) (Eckland and Danhof Citation2000) and female rats (50%) (Umathe et al. Citation2008). The variation may be due to the differences in the physiology and/or anatomy of digestive system in these species.
In conclusion, the results of this study indicate that, following oral administration of PGT, C max will be attainable after a longer t max in sheep compared to that reported for humans. However, PGT has a marked post-ruminal absorption which results in a noticeable bioavailability in sheep and apparently rumen microorganisms exert little degradation effect on PGT. Other studies with more replication and multiple dose of PGT may be appropriate in future researches. TZDs property in the reduction of plasma NEFA in dairy cow can be a novel strategy in the management of some metabolic disorders in transitional cows especially in high producing dairy cows. In addition, proven effects of TZDs on marbling may open a new area in the nutrition of sheep.
Acknowledgements
The authors acknowledge SamiSaz Pharmaceutical Co. and Osvah Pharmaceutical Co. as they supplied PGT for the pilot study. Kind helps of the following colleagues with respect to laboratory and field works of this study is greatly appreciated: H.R. Khoshooee, A. Nowrouzi, E. Ghasemi, S.M. Nasrollahi, A. Shahmoradi, H. Beyranvand, A.A. Hajialiakbari, A. Rahmanian and J. Razavi. This research was financially supported by Isfahan University of Technology.
References
- Bionaz , M , Baumrucker , CR , Shirk , E , VandenHeuvel , JP , Block , E and Varga , GA . 2008 . Characterization of Madin-Darby bovine kidney cell line for peroxisome proliferator-activated receptors: temporal response and sensitivity to fatty acids . Journal of Dairy Science , 91 : 2808 – 2813 .
- Budde , K , Neumayer , HH , Fritsche , L , Sulowicz , W , Stompor , T and Eckland , D . 2003 . The pharmacokinetics of pioglitazone in patients with impaired renal function . British Journal of Clinical Pharmacology , 55 : 368 – 374 .
- Eckland , DA and Danhof , M . 2000 . Clinical pharmacokinetics of pioglitazone . Experimental and Clinical Endocrinology and Diabetes , 108 : S234 – S242 .
- Fujita , Y , Yamada , Y , Kusama , M , Yamauchi , T , Kamon , J , Kadowaki , T and Iga , T . 2003 . Sex differences in the pharmacokinetics of pioglitazone in rats . Comparative Biochemistry Physiology Part C Toxicology Pharmacology , 136 : 85 – 94 .
- Gillies , PS and Dunn , CJ . 2000 . Pioglitazone . Drugs , 60 : 333 – 343 .
- Grant , AC , Ortiz-Colón , G , Doumit , ME and Buskirk , DD . 2008 . Optimization of in vitro conditions for bovine subcutaneous and intramuscular preadipocyte differentiation . Journal of Animal Science , 86 : 73 – 82 .
- Grant , AC , Ortiz-Colón , G , Doumit , ME , Tempelman , RJ and Buskirk , DD . 2008 . Differentiation of bovine intramuscular and subcutaneous stromal-vascular cells exposed to dexamethasone and troglitazone . Journal of Animal Science , 86 : 2531 – 2538 .
- Hausman , GJ , Poulos , SP , Pringle , TD and Azain , MJ . 2008 . The influence of thiazolidinediones on adipogenesis in vitro and in vivo: potential modifiers of intramuscular adipose tissue deposition in meat animals . Journal of Animal Science , 86 : E236 – E243 .
- Houseknecht , KL , Cole , BM and Steele , PJ . 2002 . Peroxisome proliferator-activated receptor gamma (PPARgamma) and its ligands: a review . Domestic Animal Endocrinology , 22 : 1 – 23 .
- Kalliokoski , A , Neuvonen , M , Neuvonen , PJ and Niemi , M . 2008 . No significant effect of SLCO1B1 polymorphism on the pharmacokinetics of rosiglitazone and pioglitazone . British Journal of Clinical Pharmacology , 65 : 78 – 86 .
- Kersten , S , Desvergne , B and Wahli , W . 2000 . Roles of PPARs in health and disease . Nature , 405 : 421 – 424 .
- Kliewer , SA , Xu , HE , Lambert , MH and Willson , TM . 2001 . Peroxisome proliferator-activated eceptors: from genes to physiology . Recent Progress in Hormone Research , 56 : 239 – 265 .
- Knouff , C and Auwerx , J . 2004 . Peroxisome proliferator-activated receptorg calls for activation in moderation: lessons from genetics and pharmacology . Endocrine Review , 25 : 899 – 918 .
- Kushibiki , S , Hodate , K , Shingu , H , Ueda , Y , Shinoda , M , Mori , Y , Itoh , T and Yokomizo , Y . 2001 . Insulin resistance induced in dairy steers by tumor necrosis factor alpha is partially reversed by 2,4-thiazolidinedione . Domestic Animal Endocrinology , 21 : 25 – 37 .
- Lehmann , JM , Moore , LB , Smith-Oliver , TA , Wilkison , WO , Willson , TM and Kliewer , SA . 1995 . An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptorγ(PPARγ) . Journal of Biological Chemistry , 270 : 12953 – 12956 .
- Mudaliar , S and Henry , RR . 2001 . New oral therapies for type 2 diabetes mellitus: the glitazones or insulin sensitizers . Annual Review of Medicine , 52 : 239 – 257 .
- Poulos , SP and Hausman , GJ . 2006 . A comparison of thiazolidinedione- induced adipogenesis and myogenesis in stromal vascular cells from subcutaneous adipose tissue or semitendinosus muscle of postnatal pigs . Journal of Animal Science , 84 : 1076 – 1082 .
- Revelo , XS and Waldron , MR . 2010 . Effects of in vitro insulin and 2,4-thiazolidinedione on the function of neutrophils harvested from blood of cows in different physiological states . Journal Dairy Science , 93 : 3990 – 4005 .
- Smith , KL , Butler , WR and Overton , TR . 2009 . Effects of prepartum 2,4-thiazolidinedione on metabolism and performance in transition dairy cows . Journal Dairy Science , 92 : 3623 – 3633 .
- Smith , KL , Stebulis , SE , Waldron , MR and Overton , TR . 2007 . Prepartum 2,4-thiazolidinedione alters metabolic dynamics and dry matter intake of dairy cows . Journal Dairy Science , 90 : 3660 – 3670 .
- Souri , E , Jalalizadeh , H and Saremi , S . 2008 . Development and validation of a simple and rapid HPLC method for determination of pioglitazone in human plasma and its application to a pharmacokinetic study . Journal of Chromatographic Science , 46 : 809 – 812 .
- Tontonoz , P and Spiegelman , BM . 2008 . Fat and beyond: the diverse biology of PPARγ . Annual Review of Biochemistry , 77 : 289 – 312 .
- Umathe , SN , Dixit , PV , Kumar , V , Bansod , KU and Wanjari , MM . 2008 . Quercetin pretreatment increases the bioavailability of pioglitazone in rats: involvement of CYP3A inhibition . Biochemical Pharmacology , 75 : 1670 – 1676 .
- Wearn , JMG , Crisman , MV , Davis , JL , Geor , RJ , Hodgson , DR , Suagee , JK , Ashraf-khorassani , M and McCutcheon , LJ . 2010 . Pharmacokinetics of pioglitazone after multiple oral dose administration in horses . Journal of veterinary Pharmacology and Therapeutics , 34 : 252 – 258 .
- Yki-Järvinen , H . 2004 . Thiazolidinediones . New England Journal of Medicine , 351 : 1106 – 1118 .