Abstract
An outbreak of acute trypanosomosis caused by Trypanosoma evansi in Punjab, a northern state of India was investigated in a cattle farm comprising 78 adults (lactating and dry) and 27 calves. The outbreak in the affected animals exhibiting high fever (105°F), circling, tremors, convulsions and death within 24–48 hours was confirmed based on examination of Wright-Giemsa stained blood smears, morpho-metric measurements and by polymerase chain reaction by using T. evansi specific primers yielding species specific 227 bp PCR product. Pretreatment parasitaemia estimated in three clinical cases revealed 12.5×104, 11.2×104 and 16.7×104 tryps/µl of blood and cow that was treated with isometamedium chloride revealed parasitemia of 1.4×104 tryps/µl of blood 24 hours after treatment while no parasitemia was found after 24 hrs of treatment in other two cows, treated with quinapyramine sulphate and chloride combination (Triquin). Post-mortem revealed moderate enlargement of spleen. Biochemical parameters revealed increase in cholesterol, bilirubin and total proteins.
Introduction
Trypanosomosis (Surra) caused by the haemo-flagellate protozoan Trypanosoma evansi, is a major constraint on the health and productivity of domestic animals throughout the tropics and subtropics (Gill Citation1991; Singla et al. Citation2004; Da Silva et al. Citation2010). In India, T. evansi is the commonest and the most prevalent trypanosome of livestock, although isolated cases of Trypanosoma theileri have also been encountered (Sood et al. Citation2011). The organism is transmitted mechanically by tabanid flies (Singh et al. Citation1993; Desquesnes et al. Citation2009) and disease is more common in areas where environment for the breeding of the fly vectors is most suitable (Bhatia et. al. Citation2006). The disease in cattle and buffaloes usually occurs in chronic form as an asymptomatic infection (Aulakh et al. Citation2005, Bharadwaj and Randhawa Citation2010). Present investigation deals with an outbreak of acute trypanosomosis caused by T. evansi in crossbred cattle.
Materials and methods
Case history and symptoms
An outbreak was attended in a crossbred cattle farm having 105 cattle comprising 78 adults (lactating and dry) and 27 calves during rainy season in the month of August 2009 in village Heran, District Jalandhar of Punjab State, India. The farm was surrounded by paddy fields having tabanid flies in the visinity. Farmer started silage feeding to animals one week before the onset of disease. Death of cattle after 24–48 hrs of fever and nervous signs compelled the farmer to approach this Department for investigation. A team of experts from the Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, visited the farm for investigation. At the time of visit three adult cattle were sick, out of which two were seriously affected and recumbent and three had already succumbed to disease. Another two cases were observed in due course of investigation.
Parasitological and hemato-blood cellular studies
Wright-Giemsa stained thin blood smears were examined for presence of any blood protozoa. Morphological and micrometric measurements of parasites were carried out in microscope fitted with micrometry unit by using Software DP2-BSW(OLYMPUS). Parasitic load was calculated by counting number of trypanosomes/µl of blood to assess the status of infection in the infected animals. Blood samples collected in EDTA at 1.5 mg/ml were also subjected to complete blood count viz. haemoglobin (Hb), total leucocytes count (TLC) and differential leucocytes count as per Feldman et al. (Citation2000). Plasma was analyzed for biochemical changes viz. total proteins, albumin, alkaline phosphatase, total bilirubin, creatinine and cholesterol by using auto analyzer.
Molecular studies
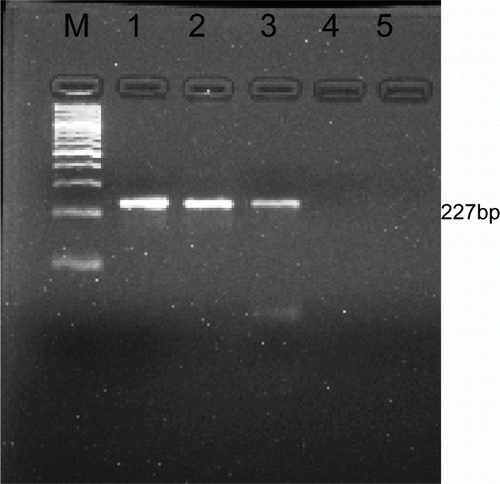
DNA extraction was carried out from whole blood by standard phenol-chloroform-isoamylalcohol (PCI) method (Sambrook et al. Citation1989). DNA pellet was dissolved in TE buffer and stored at −20 °C until further use. DNA amplification was done using 5 µl of DNA template extracted by PCI. The primers for PCR (Bangalore Genei, India) from a repetitive sequence probe pMuTec 6.248 (Wuyts et al. Citation1994) having forward and reverse sequence of 5′- TGCAGACGACCTGACGCTACT-3′and 5′- CTCC TAGAAGCTTCGGTGTCCT-3′, respectively, were used. The reaction was performed using Taq DNA polymerase (2.5 U), PCR buffer (1×) with MgCl2 (1.5 mM), dNTPs (200µM) and 0.5 µM of each primer to make final volume of 50 µl. The reaction was performed as per method of Basagoudanvar et al. (Citation1998). The amplified product was analysed on 1.5% agarose gel (50 min at 70 V) by electrophoresis and visualized by using gel documentation system (Bio rad, USA)
Treatment administered
Local veterinarian treated four affected animal with isometamedium chloride at 0.5 mg/kg body weight (Surral; Alembic Pharma) on the basis of clinical signs. After confirmation of presence of trypanosomes in the blood of all affected animals, quinapyramine sulphate and chloride combination at 2.5 g/adult cow (Triquin; Wockhardt Limited) was administered by subcutaneous route in four cases (one already treated with isometamedium chloride and three untreated) and results were compared.
Pathological examination
Post-mortem was performed on one dead animal and detailed gross post-mortem lesions were recorded and tissues of vital organs were collected in 10% formalin for histopathology. H&E stained tissue sections were examined for pathological changes.
Results and discussion
Clinical examination of affected animals revealed high fever (105° F), sudden onset of circling movement and muscle tremors. Sometimes pressing head against the manger followed by convulsions leading to recumbency and death within 24–48 hours were observed. Clinical symptoms indicated acute form of trypanosomosis (Bhatia et al. Citation2006).
Epidemiological findings
Outbreak was reported during rainy season (conducive for the breeding of tabanids) in the month of August 2009 with average relative humidity, rain fall and temperature of 77%, 2.3 mm and 30.5°C, respectively. Moreover, the cattle farm was surrounded by paddy fields which remained studded with water which helped in building up of large number of Tabanus flies in and around the farm. Isolated cases of T. evansi were observed previously from the area surrounding the farm. Chronic cases with mild or moderate clinical signs of trypanosomosis are commonly reported from all over the Punjab State (Aulakh et al. Citation2005). As farmer had purchased some new animals, the possibility of entry of carrier animal entering the herd responsible for the onset of outbreak can not be ruled out. In this first outbreak of T. evansi at this farm, eight adult animals were affected out of the 78 adult cattle with morbidity, mortality and case fatality rate (CFR) of 10.3%, 5.1% and 50%, respectively. CFR of 50% seems to be very high which may be due to failure of isometamedium chloride to combat the infection quickly or due to very acute nature of disease. Young animals and calves which were kept in confinement remained healthy probably not exposed to the tabanid fly.
Parasitological and Hemato blood cellular findings
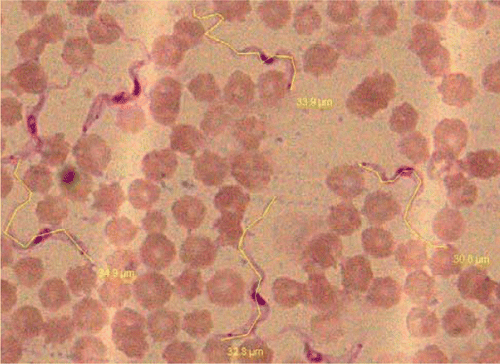
Morphologic examination of peripheral thin Giemsa stained blood smears indicated that the nucleus in the trypanosomes was in central position with a small kinetoplast visible at the posterior position (. Micrometric measurements revealed average size of parasite 29.08µm (Range 22.0–34.2µm, ). The measurements were similar to reported by John et al. Citation1992. Affected animals revealed heavy parasitemia (7–43 tryps/high power field (HPF) in clinically infected cows (). Parasites were showing active multiplication, elongation with two nuclei and kinetoplast indicating acute nature of disease. Pretreatment parasitemia estimated in three clinical cases (Case No.27, 49, 50) revealed 12.5×104 , 11.2×104 and 16.7×104 tryps/µl of blood, respectively. Case no.27 was treated with isometamedium chloride and revealed significant parasitemia of 1.4×104 tryps/µl of blood with an average of 5 tryps/HPF (range1–7 tryps/HPF) 24 hours after treatment. A total of eight crossbred cattle were infected with acute trypanosomosis and four were treated with isometamedium chloride and all died within 24–48 hrs post treatment despite decrease in parasitemia. In comparison all the four cattle treated with Triquin were parasitologically negative after 24 hrs of treatment and 100% survival rate indicating better efficacy and fast parasiticidal activity. Eisler et al. (Citation1996) has evaluated chemoprophylactic regime of isometamidium chloride and has been reported to have very good prophylactic activity but there is no evaluation of the chemotherapeutic efficacy of this drug in clinical or experimentally induced infection of T. evansi.
Figure 1. Blood film showing actively multiplying stages and micrometric measurements of Trypanosma evansi (Giemsa×100X).
Hematological findings revealed slight anaemia as indicated by mild decrease in haemoglobin (9.1±0.17 g/dl) as compared to reference value (12.0 g/dl). Blood cellular changes revealed lymphocytosis in infected cow treated with isometamedium while there was severe neutrophilia in clinically infected and non-treated cow (). Total proteins were on the higher side along with increase in bilirubin level. There was significant increase in cholesterol (mean 229.66 mg/dl) in all the infected cases (). Increased levels of total proteins may be due to increase in immunoglobulin in response to antibody production to different antigenic variants of T. evansi (Singla et al. Citation2000). Increase in bilirubin indicated increased destruction of erythrocytes which is commonly reported due to hemolytic crisis during the period of infection and was also supported by decrease in haemoglobin (Youssif et al. Citation2008).
Table 1. Sero-biochemical and haematological findings of acute clinical cases T. evansi.
Molecular studies
Amplification of DNA from whole blood yielded PCR product of 227 bp () which confirmed the species of parasite to be T. evansi. Molecular methods are very sensitive and specific in diagnosis when parasitemia is very low which may not be detected by routine diagnostic techniques.
Pathological studies
Microbiological studies revealed no growth of infectious organism on blood agar from heart blood and jugular blood collected from dead animal and clinically infected animals. No organism was isolated on cold enrichment method that ruled out the suspicion of listeriosis.
Gross pathological lesion observed on post-mortem of dead animal was moderate enlargement of spleen. Histopathologically marked congestion and hemorrhages were visible in spleen and most of the organs were showing autolytic changes. In brain no lesion indicating listeriosis i.e. micro abscess or perivascular cuffing was observed.
Acknowledgement
Thanks are due to Dean, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University for necessary facilities to carry out the work.
References
- Aulakh , GS , Singla , LD and Singh , J . 2005 . “ Bovine trypanosomosis due to Trypanosoma evansi: clinical, haematobiochemical and therapeutic studies ” . In New horizons in animal sciences , Edited by: Sobti , RC and Sharma , VL . 137 – 144 . Jalandhar : Vishal Publishing and Co .
- Basagoudanavar , SH , Rao , JR , Omanwar , S , Singh , RK and Butchaiah , G . 1998 . Sensitive Polymerase Chain Reaction for detection of Trypanosoma evansi in Camels (Camelus dromdarius) . Journal of Parasitic Diseases , 22 : 40 – 43 .
- Bharadwaj , RK and Randhawa , CS. 2010 . Chronic trypanosomiasis in crossbred cattle . Indian Veterinary Journal , 87 : 408
- Bhatia , BB , Pathak , KML and Banerjee , DP. 2006 . Text book of veterinary parasitology , 2nd ed , 304 – 315 . Ludhiana : Kalyani Publishers .
- Da Silva , AS , Wolkmer , P , Costa , MM. , Tonin , AlA , Eilers , TL , Gressle , LT , Otto , MA , Zanette , RA , Santurio , JM Lopes , STA . 2010 . Biochemical changes in cats infected with Trypanosoma evansi . Veterinary Parasitology , 17 : 48 – 52 .
- Desquesnes , M , Biteau-Coroller , F , Bouyer , J , Dia , ML and Foil , LD . 2009 . Development of a mathematical model for mechanical transmission of trypanosomes and other pathogens of cattle transmitted by tabanids . International Journal of Parasitology , 39 : 333 – 346 .
- Eisler , MC , Maruta , J , Nqindi , J , Connor , RJ , Ushewokunze-Obatolu , U , Holmes , PH and Peregrine , AS . 1996 . Isometamidium concentrations in the sera of cattle maintained under a chemoprophylactic regime in a tsetse-infested area of Zimbabwe . Tropical medicine & international health , 1 ( 4 ) : 535 – 541 .
- Feldman , BF , Zinker , JG and Jain , NC. 2000 . Schalm's Veterinary haematology , 5th ed , 351 Baltimore , MD : Lippin Cott Williams and Walkins .
- Gill , BS . 1991 . Trypanosomes and Trypanosomosis of Indian Livestock , 192 New Delhi : ICAR Publication .
- John , MC , Nedunchelliyan , S and Venkataraman , KS . 1992 . Biometrical observations on different strains of Trypanosoma evansi . Veterinary Parasitology , 43 : 143 – 145 .
- Sambrook , J , Fritch , EF and Maniatis , T . 1989 . Molecular cloning: a laboratory manual , 2nd ed , New York , NY : Cold spring harbor Laboratory press .
- Singh , B , Kalra , IS , Gupta , MP and Nauriyal , DC . 1993 . Trypanosoma evansi infection in dogs: seasonal prevalence and chemotherapy . Veterinary Parasitology , 50 : 137 – 141 .
- Singla LD , Aulakh GS , Juyal PD , Singh J. 2004 . Bovine trypanosomosis in Punjab, India . Proceeding 11th International Conference of the Association of Institutions for Tropical Veterinary Medicine and 16th Veterinary Association Malaysia Congress 2004 Aug 23–27 , Malaysia: Sunway Pyramid Convention Centre , Petaling Jaya , 283 – 285 .
- Singla , LD , Juyal , PD and Ahuja , SP. 2000 . Serum protein changes of cross-bred calves experimentally infected with Trypanosoma evansi and immuno-modulated with levamisole . Indian Veterinary Journal , 77 : 172 – 174 .
- Sood , NK , Singla , LD , Singh , RS and Uppal , SK . 2011 . Association of Trypanosoma theileri with peritonitis in a pregnant cross-bred cow: a case report . Veterinarni Medicina , 56 : 82 – 84 .
- Wuyts , N , Chokesajjawatee , N and Panyim , S . 1994 . A simplified and highly sensitive detection of Trypanosoma evansi by DNA amplification . Asian Journal of Tropical Public Health , 25 : 262 – 271 .
- Youssif , FM , Mohammed , OSA and Hassan , T . 2008 . Efficacy and toxicity of cymelarsan® in Nubian goats infected with Trypanosoma evansi . Journal of Cell and Animal Biology , 2 : 140 – 149 .