Abstract
Paired box (Pax) proteins 3 and 7, which are two members of the PAX gene family, are important to animal skeletal muscle development acting their roles by mediating the expression of MyoD. These three genes expressed in several of tissues and organs in vertebrates. In order to investigate whether or not the relationships between Pax3, Pax7 and MyoD in other tissues are still similar with that in skeletal muscle, we detected the expression of Pax3, Pax7 and MyoD in duck different tissues and organs using real-time PCR technical. Results showed that these three genes not only expressed in skeletal muscle tissues, but also expressed in other tissues like heart, brain, spleen, fat and so on. The Pax3 and Pax7 also have coordinate roles in heart, spleen and brain, which are similar with that in skeletal muscle. Results indicate that the MyoD, Pax3 and Pax7 may involve in other tissues development besides skeletal muscle in duck and may also act on a similar mechanism between Pax3/7 and MyoD in skeletal muscle.
Introduction
Paired box (Pax) proteins 3 and 7, which are two members of the PAX gene family, play essential roles in commitment of cells to a myogenic lineage during skeletal muscle development and regeneration (Charytonowicz et al. Citation2011). It has been proved that the Pax genes also play key roles in the formation of tissues and organs during embryogenesis in mammals (Buckingham and Relaix Citation2007). In chick, Pax3 and Pax7 negatively regulate the expression of each other gene in the dermomyotome, and have essential roles in developing chick somites and limbs (Galli et al. Citation2008). When mouse are absent both of Pax3 and Pax7, myotube differentiating to myofibre is arrested and only the early embryonic muscle of the myotome can form (Relaix et al. Citation2005). During myogenesis, the myogenic regulation factors family (MRFs), which includes MyoD, Myf5, MyoG and MRF4, is responsible for all the process of muscle development, including myoblast migration and proliferation, cell fusion to form myotubes and myotube terminal differentiation to form mature myofibre (Rudnicki and Jaenisch Citation1995). Recent evidences have proposed that Pax3 and its paralogue Pax7 were functionally by initiating the expression of MRFs during the early myogenesis. Normally, Pax3/7-positive skeletal muscle progenitor cells, which are derived from the central dermomyotome region of the somites (Ben-Yair and Kalcheim Citation2005; Gros et al. Citation2005), activate the myogenic regulatory genes and differentiate into skeletal muscle fibres or remain as a proliferating reserve cell population within the muscle mass (Kassar-Duchossoy et al. Citation2005; Relaix et al. Citation2005). Kander further found that Pax3 and Pax7 participate in adult skeletal muscle plasticity during the initial responses to chronic overload and appear to coordinate with MyoD expression (Hyatt et al. Citation2008). Dominant-negative forms of both Pax3 and Pax7 repressed MyoD, but do not interfered with the expression of the other myogenic determination factor (Kander et al. Citation2007; Hyatt et al. Citation2008), indicating that the MyoD was important to Pax3/7 acting their functions during muscle development.
It is clearly that it has coordinate roles of Pax3/7 and MyoD in controlling muscle development. However, all of the three genes Pax3/7 and MyoD expressed in many different tissues (Gerhart et al. Citation2001; Gotensparre et al. Citation2006; Gang et al. Citation2008), it is hypothesised that it has a similar coordinated role in other tissues with in skeletal muscle tissues. Therefore, in order to find out the possible relationships between the Pax3/7 and MyoD in adult duck tissues, the expression of Pax3/7 and MyoD were determined using qRT-PCR method. These works may give a further knowledge about the relationships between Pax3/7 and MyoD in bird different tissues.
Materials and methods
Ten ducks (Anas platyrhynchos) including five male and five female at 4 wk of age were selected for sampling. All of the birds were provided by the Sichuan Agricultural University Waterfowl Breeding Experimental Farm. The birds were maintained under the same treatment, which was approved by the Beijing Animal Welfare Committee. For each bird, the tissues including breast muscle, leg muscle, heart, liver, spleen, lung, kidney, small intestine, stomach, abdomen fat, skin fat, brain and pancreas were divided into pieces with RNase free scalpels and scissors, and immediately wrapped by aluminium foil and frozen in liquid nitrogen, stored at −80°C for RNA extraction.
Total RNA was extracted using Trizol (Invitrogen, New York, USA) according to the manufacturer's instructions. The total RNA was treated with DNase I (Takara, Dalian, China) for 10 min and quantified by spectrophoto-metric absorbance at 260 nm and 280 nm. First-strand cDNA was obtained from 10 µg of total RNA using a cDNA synthesis kit (Takara, Dalian, China) according to the manufacturer's instruction.
The mRNA expression levels of the Pax3/7 and MyoD in duck different tissues were measured by the real-time PCR using 96-well iCycler IQ5 (Bio-Rad, Hercules, USA) with Takara ExTaq RT-PCR kit and SYBR green as the dye (Takara, Dalian, China). The volume was 25 µl: SYBR Premix EX Taq 12.5 µl, forward primer (10 pmol) 1 µl, reversed primer (10 pmol) 1 µl, cDNA 2 µl and dH2O 8.5 µl. One sample was collected from a bird at each tissue for RNA extraction, and repeated two times for qRT-PCR assays. Duck β-Actin (EF667345) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, AY436595) sequences were obtained from GenBank and were used as internal controls. The real-time PCR primers were designed using the Primer 5 software (Primer Biosoft International, Palo Alto, USA) and were shown in . The primers were synthesised by Dalian TAKARA Biology Company. Amplifying corresponding to each target gene was examined by agarose gel electrophoresis to confirm the presence of a unique band of the expected size. The procedure for real-time PCR was as follows: 10 s of pre-denaturation reaction at 95°C, followed by 45 cycles of 95°C for 5 s and 60–65°C for 30 s.
Table 1. Primers for real time PCR.
The2−Δ ΔCt method of quantification (Livak and Schmittgen Citation2001) was used to calculate the relative expression levels of Pax3/7 and MyoD.
Results and discussion
MyoD plays essential roles in muscle development, and it was proved to have an expression in many tissues (Gerhart et al. Citation2001). In this research, we also found that the MyoD was expressed in several tissues in duck, expressed at the highest level in post-hatching duck skeletal muscle, and have a higher expression in spleen, lung and fat tissues (), indicating that the MyoD may have extensive roles in these tissues besides of in skeletal muscle. Pax3 and Pax7 are another two transcription factors involving in muscle development. From the real-time PCR results, we can see that these two genes have an expression in duck heart, spleen, lung, kidney, stomach, fat, brain and pancreas ( and ). Some of these findings about the tissue specific expression of MyoD, Pax3 and Pax7 consisted with the results of many researches, which also show that there are extensive expression in different tissues and organs for these three genes (Kocaefe et al. Citation2005).
Figure 1. The relative mRNA expression level of MyoD in duck different organs and tissues.
Note: Blue bar, expression level in male duck; red bar, expression level in female duck; green bar, the means of male and female. The different letter on each bar means significant different (p<0.05).
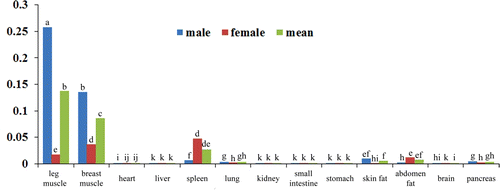
Figure 2. The relative mRNA expression level of Pax3 in duck different organs and tissues.
Note: Blue bar, expression level in male duck; red bar, expression level in female duck; green bar, the means of male and female. The different letter on each bar means significant different (p<0.05).
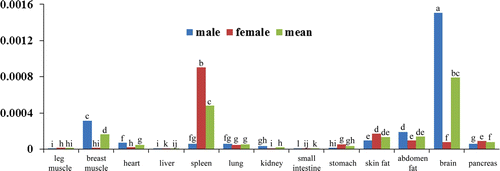
Figure 3. The relative mRNA expression level of Pax7 in duck different organs and tissues.
Note: Blue bar, expression level in male duck; red bar, expression level in female duck; green bar, the means of male and female. The different letter on each bar means significant different (p<0.05).
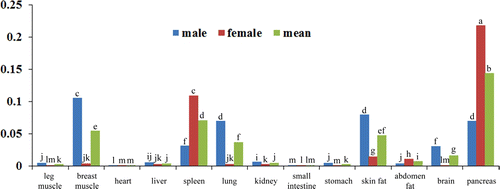
Paired box 3 and paired box 7 are key determinants for skeletal muscle development by initiating MRFs genes expression. However, recent evidence showed that Pax3 and Pax7 only interacted with MyoD, whereas the Myf5, MRF4 and MyoG are not included during embryogenesis (Relaix et al. Citation2006). Hyatt al (2008) examined the time course of expression of Pax3, Pax7 and MyoD during the early phase (≤7 days) of mechanical overload of the rat plantaris muscle, and found that Pax3 and Pax7 coordinate the expression of MRFs (Hyatt et al. Citation2008). Our results showed that in the breast muscle, higher expression of Pax3 and Pax7 companied with the higher expression of MyoD (), consisting with the previous findings in rat plantaris muscle from developmental studies, indicating that Pax3 and Pax 7 coordinate the expression of MRFs. Besides of the skeletal muscle tissues, we also found similar relationships between the MyoD and Pax3, Pax7 in duck heart, spleen and brain (). It can be concluded that these three transcription factors play important roles in these tissues, and the mechanism of them for regulating these tissues development maybe in a similar way with that in skeletal muscle tissues. In other tissues such as small intestine, abdomen fat, pancreas, there is no similar expression patterns between MyoD and Pax3, Pax7 (), and whether or not these three genes functioned in these tissues need a further investigation.
Table 2. Correlation analysis of Pax3/7 and MyoD in 13 tissues.
However, the precise influences of Pax3 and Pax7 on myogenic progression and on the MRFs remain a subject of debate. Some researchers supported that high-levels of Pax gene activation increase proliferative rate and prevent precocious myogenic differentiation (Collins et al. Citation2009). And meanwhile, expression of Pax3 or Pax7 dominant-negative constructs results in a down regulation of Myf5, MyoD and MyoG and prevents myogenic differentiation from proceeding (Collins et al. Citation2009). Here, we showed that the MyoD and Pax3, Pax7 had a coordinate role in skeletal muscle, and also had a similar expression pattern in heart, spleen and brain in 4 wk duck. Some people support that the ability of Pax3 to activate the myogenic programme in various embryonic tissues, including neural cells and in the pluripotent P19 stem cell line, were well documented (Maroto et al. Citation1997; Ridgeway and Skerjanc Citation2001). However, in adult-derived cells, there are conflicting reports of the effects of Pax3, with both an inhibition of myogenic differentiation and no effects (Epstein et al. Citation1995; Miller and Hollenbach Citation2007), having been recorded. Therefore, these different results may be contributed by the different stages researched, and these three transcription factors MyoD, Pax3 and Pax7 may play different roles in different stages in animal.
Additionally, Pax3 and Pax7 showed some overlap roles in tissues specificity and spatiotemporal expression (Vorobyov and Horst Citation2006). It is reported that Pax7 played important roles in satellite cells’ activation in skeletal muscle. Mice lacking Pax7, satellite cells are progressively lost in both muscles when Pax3 absent or not (Relaix et al. Citation2006). Research by Radix also found that Pax3 is also present in both quiescent and activated satellite cells in many skeletal muscles. In duck, both of the Pax3 and Pax7 expressed in duck breast muscle, heart, spleen, kidney, small intestine, abdomen fat, brain and pancreas, and their expression levels have the significantly correlation relationship (<0.01) (), indicating the overlap roles of Pax3 and Pax7 in duck.
Although both of the transcription factors Pax3 and Pax7 involve in muscle development, the roles of them also have some differences. The expression of Pax3 is up-regulated in the vitro lateral dermomyotome, while Pax7 expression becomes down regulated (Goulding et al. Citation1994). Pax3 are also detected in the precursor cells of the developing limb musculature (Williams and Ordahl Citation1994), while Pax7 is not expressed in the limb. In the present study, we also found that Pax3 and Pax7 are widely expressed in tissues, but the expression levels are extremely different ( and ). Pax3/7 both has high expression level in breast muscle, and low in leg muscle. And interestingly, Pax3 is expressed highly in the brain tissue, whereas the Pax7 is lowly; and Pax7 is highly expressed in the lung, whereas the Pax3 is lowly ( and ), indicating the two genes also play a differential roles in different tissues.
In conclusion, we detected the expression of Pax3/7 and MyoD gene in duck different tissues, and found these three genes are not only expressed in skeletal muscle tissues, but also expressed in other tissues like heart, brain, spleen, fat and so on. To compare their relationships, we detected these three genes’ expression patterns. It is easily to find that at this time point, they have coordinate roles in heart, spleen and brain, which is similar with that in skeletal muscle. Moreover, the tissues expression patterns of Pax3 and Pax7 also have differences in other tissues.
Acknowledgements
The work was supported by the National High Technology Research and Development Program of China (No.2010AA10A109), and Chinese AgricultureResearch Service (CARS-43-6)
References
- Ben-Yair , R and Kalcheim , C . 2005 . Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates . Development , 132 ( 4 ) : 689 – 701 . doi: 10.1242/dev.01617
- Buckingham , M and Relaix , F . 2007 . The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions . Annual Review of Cell and Developmental Biology , 23 : 645 – 673 . doi: 10.1146/annurev.cellbio.23.090506.123438
- Charytonowicz , E , Matushansky , I , Castillo-Martin , M , Hricik , T , Cordon-Cardo , C and Ziman , M . 2011 . Alternate PAX3 and PAX7 C-terminal isoforms in myogenic differentiation and sarcomagenesis . Clinical & Translational Oncology , 13 ( 3 ) : 194 – 203 . doi: 10.1007/s12094-011-0640-y
- Collins , CA , Gnocchi , VF , White , RB , Boldrin , L , Perez-Ruiz , A , Relaix , F , Morgan , JE and Zammit , PS . 2009 . Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation . PLoS One , 4 ( 2 ) : 4475 doi: 10.1371/journal.pone.0004475
- Epstein , JA , Lam , P , Jepeal , L , Maas , RL and Shapiro , DN . 1995 . Pax3 inhibits myogenic differentiation of cultured myoblast cells . The Journal of Biological Chemistry , 270 ( 20 ) : 11719 – 11722 . doi: 10.1074/jbc.270.20.11719
- Galli , LM , Knight , SR , Barnes , TL , Doak , AK , Kadzik , RS and Burrus , LW . 2008 . Identification and characterization of subpopulations of Pax3 and Pax7 expressing cells in developing chick somites and limb buds . Developmental Dynamics , 237 ( 7 ) : 1862 – 1874 . doi: 10.1002/dvdy.21585
- Gang , EJ , Bosnakovski , D , Simsek , T , To , K and Perlingeiro , RC . 2008 . Pax3 activation promotes the differentiation of mesenchymal stem cells toward the myogenic lineage . Experimental Cell Research , 314 ( 8 ) : 1721 – 1733 . doi: 10.1016/j.yexcr.2008.02.016
- Gerhart , J , Bast , B , Neely , C , Iem , S , Amegbe , P , Niewenhuis , R , Miklasz , S , Cheng , PF and George-Weinstein , M . 2001 . MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle . The Journal of Cell Biology , 155 ( 3 ) : 381 – 392 . doi: 10.1083/jcb.200105139
- Gotensparre , SM , Andersson , E , Wargelius , A , Hansen , T and Johnston , IA . 2006 . Insight into the complex genetic network of tetraploid Atlantic salmon (Salmo salar L.): description of multiple novel Pax-7 splice variants . Gene , 373 : 8 – 15 . doi: 10.1016/j.gene.2005.12.026
- Goulding , M , Lumsden , A and Paquette , AJ . 1994 . Regulation of Pax-3 expression in the dermomyotome and its role in muscle development . Development , 120 ( 4 ) : 957 – 971 .
- Gros , J , Manceau , M , Thome , V and Marcelle , C . 2005 . A common somitic origin for embryonic muscle progenitors and satellite cells . Nature , 435 ( 7044 ) : 954 – 958 . doi: 10.1038/nature03572
- Hyatt , JP , McCall , GE , Kander , EM , Zhong , H , Roy , RR and Huey , KA . 2008 . PAX3/7 expression coincides with MyoD during chronic skeletal muscle overload . Muscle Nerve , 38 ( 1 ) : 861 – 866 . doi: 10.1002/mus.21006
- Kander EM , McCall GE , Zhong H , Roy RR , Hyatt1 J-PK. 2007 . Early time course of Pax3, Pax7, and MyoD protein content in the functionally overloaded rat plantaris muscle . The FASEB Journal 21 : 769.25 .
- Kassar-Duchossoy , L , Giacone , E , Gayraud-Morel , B , Jory , A , Gomes , D and Tajbakhsh , S . 2005 . Pax3/Pax7 mark a novel population of primitive myogenic cells during development . Genes & Development , 19 ( 12 ) : 1426 – 1431 . doi: 10.1101/gad.345505
- Kocaefe , YC , Israeli , D , Ozguc , M , Danos , O and Garcia , L . 2005 . Myogenic program induction in mature fat tissue (with MyoD expression) . Experimental Cell Research , 308 ( 2 ) : 300 – 308 . doi: 10.1016/j.yexcr.2005.03.038
- Livak , KJ and Schmittgen , TD . 2001 . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method . Methods , 25 ( 4 ) : 402 – 408 . doi: 10.1006/meth.2001.1262
- Maroto , M , Reshef , R , Munsterberg , AE , Koester , S , Goulding , M and Lassar , AB . 1997 . Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue . Cell , 89 ( 1 ) : 139 – 148 . doi: 10.1016/S0092-8674(00)80190-7
- Miller , PJ and Hollenbach , AD . 2007 . The oncogenic fusion protein Pax3-FKHR has a greater post-translational stability relative to Pax3 during early myogenesis . Biochimica et Biophysica Acta , 1770 ( 10 ) : 1450 – 1458 . doi: 10.1016/j.bbagen.2007.06.016
- Relaix , F , Montarras , D , Zaffran , S , Gayraud-Morel , B , Rocancourt , D , Tajbakhsh , S , Mansouri , A , Cumano , A and Buckingham , M . 2006 . Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells . The Journal of Cell Biology , 172 ( 1 ) : 91 – 102 . doi: 10.1083/jcb.200508044
- Relaix , F , Rocancourt , D , Mansouri , A and Buckingham , M . 2005 . A Pax3/Pax7-dependent population of skeletal muscle progenitor cells . Nature , 435 ( 7044 ) : 948 – 953 . doi: 10.1038/nature03594
- Ridgeway , AG and Skerjanc , IS . 2001 . Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2 . The Journal of Biological Chemistry , 276 ( 22 ) : 19033 – 19039 . doi: 10.1074/jbc.M011491200
- Rudnicki , MA and Jaenisch , R . 1995 . The MyoD family of transcription factors and skeletal myogenesis . Bioessays , 17 ( 3 ) : 203 – 209 . doi: 10.1002/bies.950170306
- Vorobyov , E and Horst , J . 2006 . Getting the proto-Pax by the tail . Journal of Molecular Evolution , 63 ( 2 ) : 153 – 164 . doi: 10.1007/s00239-005-0163-7
- Williams , BA and Ordahl , CP . 1994 . Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification . Development , 120 ( 4 ) : 785 – 796 .