Abstract
In porcine reproductive and respiratory syndrome virus (PRRSV)-infected farms, there is an increase in the female culling rate, mainly due to reproductive problems and culling of young females. This has significant economic importance, as the low female culling rate is an important management factor. In the present study, in a farrow-to-finish farm with 1100 sows, all gilts and sows were vaccinated with a PRRS killed virus (PRRS KV) vaccine (PROGRESSIS®/Merial SAS, France) for a period of 18 months. For each gilt and sow, reproductive data were collected starting from 1-year prior until 18 months after the start of vaccination. Culling rate and the causes of culling (reproductive failure, death, old age, locomotor problems and other) were recorded. Blood samples from non-vaccinated animals were collected prior and after the start of vaccination. The purpose of this field study was to evaluate the sow longevity in a PRRSV-infected farm after their long-term vaccination with a PRRSV KV vaccine. The results indicated that the vaccination leads to a significant reduction (P < 0.001) of culling rate due to reproductive failure 1.5 years after the start of vaccination and an increase of old age (P < 0.001) totally 1.5 years after the start of vaccination. Eventually, culling rates due to deaths (P = 0.066), locomotor problems (P = 0.264) and other causes (P = 0.894) did not significantly differ per semester and totally prior and after the start of vaccination. In conclusion, the long-term vaccination of breeding stock with a PRRSV KV vaccine can lead to decrease of culling rate due to reproductive failure and improvement of the sow longevity.
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is one of the most significant and economically important infectious diseases in global pig populations (Neumann et al. Citation2005). PRRS virus (PRRSV) is a significant cause of production losses in most swine producing countries, inducing reproductive failure in sows, which is mainly characterised by reduction of their fertility and longevity (Meredith Citation1995).
Sows are culled when they are considered unsuitable for further production. Poor sow longevity in commercial breeding herds can lead to economic inefficiency and animal welfare concerns (D'Allaire and Drolet Citation2006). In PRRSV-infected farms, there is an increase in the female culling rate due to reproductive failure (Benfield et al. Citation1992, Citation1999). This has significant economic importance, as the female culling rate is an important management factor (King et al. Citation1998). A high removal rate requires many replacement animals, which will increase disease risks and the cost of production, decreasing herd productivity by influencing the herd age distribution and the number of non-productive sow days (D'Allaire and Drolet Citation1999).
The measures used currently to control PRRS include management (e.g. whole herd depopulation/repopulation and herd closure), bio-security, test and removal and vaccination (Corzo et al. Citation2010). Vaccination aims to the reduction of clinical losses, but not of prevention of virus infection. There are commercially available two types of PRRS vaccines; modified live virus (MLV) and killed virus (KV) vaccines. Many studies have shown beneficial effects of MLV vaccines on PRRS-infected farms (Scortti et al. Citation2006; Lowe et al. Citation2006; Martelli et al. Citation2009; Zhao et al. Citation2012). However, MLV vaccines are of concern, for its immunogenicity, cross-protective efficacy and safety (Labarque et al. Citation2004; Prieto et al. Citation2008; Kimman et al. Citation2009). PRRS KV vaccines, on the other hand, is well known for its safety (Plana-Duran et al. Citation1997; Papatsiros et al. Citation2006, Citation2011), but their capacity to induce a protective immunity against challenge with wild-type virus or heterologous strains has been questioned (Scortti et al. Citation2007; Zuckermann et al. Citation2007; Kim et al. Citation2011). However, field studies reported that the KV vaccines did not induce reproductive failure in vaccinated sows and improved efficiently the reproductive parameters at a farm level (Joisel et al. Citation2001; Papatsiros et al. Citation2006).
The aim of present study was to evaluate the sow longevity in a PRRSV-infected swine herd after the long-term vaccination with a PRRSV KV vaccine.
Materials and methods
Experimental substance
The commercial PRRSV KV vaccine (PROGRESSIS®, Merial, SAS) based on the European P120 strain was used. The vaccine dose contains ≥102,5 IF units and is suspended in 2 ml of an oily adjuvant (hydrogenated polyisorbutene is the oily part of the emulsion of mineral oil in water) for intramuscular injection behind the ear.
Trial farm
The trial has been performed in a commercial all-in, all-out farrow-to-finish farm with a capacity of 1100 sows located in North Greece. A grandparent nucleus of 70 sows was kept in the farm for producing its own gilts, and these animals were separately housed, but in the same premises as the commercial herd. The farm had its own feed mill and artificial insemination laboratory. Records in the farm were electronically kept.
All gilts/sows were vaccinated against Aujeszky's disease (AD), swine influenza (SI), parvovirus infection, atrophic rhinitis, erysipelas, Escherichia coli and Clostridium perfringens (type A and C). All boars were vaccinated every 6 months against erysipelas, AD and SI, fattening pigs against AD and SI and weaners against Mycoplasma hyopneumoniae. For the antiparasitic control, all breeding females were treated with a single ivermectin injection 14 days prior to each farrowing, while the boars twice a year. The feed provided to the animals were self-prepared based on a corn/barley/wheat–soya meal, depending on the season.
The farm had suffered an acute PRRSV infection 5 years prior to the initiation of the trial. Since then, the herd had been infected with PRRSV for some years and had never been vaccinated before against PRRSV. For at least 1-year prior to the initiation of the trial, the farm was diagnosed PRRS-positive, based on clinical signs (low reproductive performance as was evidenced by increased returns to oestrus, small litters, weak piglets and increased piglet mortality), serological examinations of blood samples and detection of viral ribonucleic acid by polymerised chain reaction from fetuses and newborn piglets. In addition, blood samples of sows were examined for antibodies against a European PRRSV by using indirect immunofluorescence assay in US- or EU-type PRRSV-infected MA104 cells. It was shown that the circulating strain in the farm was a European strain. The trial was performed from November 2001 and lasted till August 2003.
Experimental protocol
Primarily, all gilts/sows of the herd received two injections of ‘PROGRESSIS®’ in 3–4 weeks apart at any stage of production, except those being 1 week prior to 2 weeks post-service. The skipped females have been subjected to primary vaccination, but starting 3 weeks later. All previously vaccinated animals received a booster vaccination between 55 and 60 days of next gestation, and thereafter at each gestation for a period of 18 months. The replacements gilts after the start of vaccination were vaccinated twice at a 3- to 4-week interval at least 3 weeks before the first service and boostered in each pregnancy as described previously.
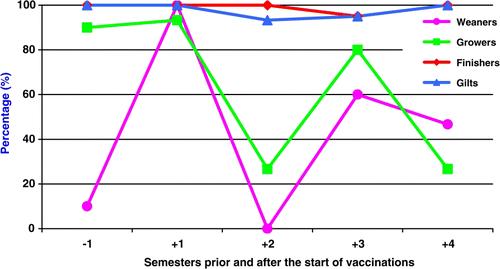
Blood samples from non-vaccinated sows of parities 1, 2, 3 and 4–6 (five samples per parity group), gilts (nine samples) weaners, growers and finishers (1–21 samples per group) were collected one semester (−1) prior to the start of vaccination (). Subsequently, 10–20 blood samples from each age group (gilts, weaners, growers and finishers) were collected at 6 (+1 semester), 12 (+2 semesters), 18 (+3 semesters) and 24 (+4 semesters) months after the start of vaccination. All serum samples were examined for PRRSV-specific antibody titres using the immunoperoxidase monolayer assay (IPMA) technique described by Wensvoort et al. (Citation1991). The lower positive dilution of the test was 1:40.
Table 1. Results of routine mycotoxicological analysis in feed raw materials.
During the monitoring period, there were no significant changes in the management, nutrient specification and feeding schedule, genetics of sows, housing of pigs or vaccination schemes other than PRRS. In order to exclude other factors that may cause reproduction problems, routine serological Leptospira spp. testing in blood samples of pigs from all ages was performed. Also routine mycotoxicological analysis [zearalenone, aflatoxines B1, B2, G1, G2, deoxynivalenol (DON), 3-acetyl-DON, 15-acetyl-DON, nivalenol] in feed raw materials was performed, using High Pressure Liquid Chromatography (HPLC).
Parameters recorded and calculated
For each gilt and sow, reproductive data were collected starting from 12 months prior (−1 and −2 semesters) until 18 months (+1, +2 and +3 semesters) after the start of vaccination. Culling rate was calculated. Also, the causes of culling of each gilt and sow (reproductive failure, death, old age, locomotor problems and other) were recorded during the trial. Reproductive failure was used to define a variety of cases: onset of puberty, anestrus, returns to estrus, negative pregnancy diagnosis, failure to farrow, premature farrowing, abortion, small litter size at farrowing, high preweaning mortality, hypogalactia/agalactia. The culling rate due to old age was referred to sows, which with completed eight reproductive cycles. Other causes of culling included sporadic cases of injuries, poor body condition, uterine prolapses, urinary tract infections (e.g. cystitis-pyelonephritis) and heat stroke.
Data analysis – statistical evaluation
To see the effect of vaccination on sow longevity over time, data were analysed temporally by semester, for example two and one semesters prior to, and one, two and three semesters after the beginning of vaccination. One-way analysis of variance (SYSTAT® version 5.0, Richmond, CA, USA) was used. After checking the normality of variables, the mean of variable was compared between each time or between each vaccination period (prior or after the start of vaccination) using Tukey's test. Kruskall–Wallis analysis was used in cases that the transformations of values did not bring about homogeneity of variations. Fisher's test was also used for the parameters expressed as frequencies. The serological results were analysed by the Statistical Analysis System SAS® (release 8.01 SAS, Institute, Inc., Cary, NC, USA). The level of significance was set at P=0.05.
Table 2. Percentage of infected non-vaccinated animals (PRRSV-specific antibody titres >1:40) per semester prior and after the start of vaccination.
Results
Routine Leptospira spp. and mycotoxin examinations
Laboratory examinations during the trial period did not reveal Leptospira spp. at detectable levels. HPLC results indicated mycotoxins levels that do not induce reproductive disorders in pigs ().
Serological results
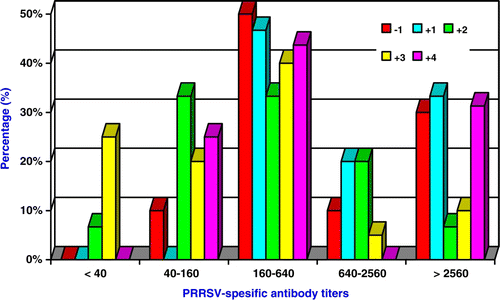
The serological profile of the herd has shown that, one semester prior to the start of vaccination 73.7% of the sows and 100% of replacement gilts have been infected by PRRSV (). The prevalence of PRRSV-infected non-vaccinated animals after the start of vaccinations is shown in . The prevalence of PRRSV-infected weaners and growers was highly variable, while PRRSV-infected non-vaccinated replacements gilts and finishers remained always higher than 93.3% (P > 0.05). Moreover, the distribution of PRRSV-specific antibody titres per semester in gilts prior and after the start of vaccinations, as it is shown in , indicated that the prevalence of PRRSV-infected gilts with high PRRSV-specific antibody titres (≥1:2560), increased during the semesters +1, +3 and +4 after the start of vaccinations.
Results regarding longevity of female breeding stock
Culling rate of female breeding stock (sows/gilts) per semester and totally prior and after the start of vaccination are shown in and the cause of culling rate in the same periods are shown in .
Figure 2. Distribution of serological results based on PRRSV-specific antibody titres per semester in gilts prior and after the start of vaccinations (–1, +1, +2, +3, +4).
As presented in , the vaccination of the female breeding stock leads to significant reduction (P < 0.001) of culling rate due to reproductive failure 1.5 years after the start of vaccination and a tendency of reduction was performed from semester to semester after the start of vaccination without statistical significance. Furthermore, culling rate to old age (sows with completion of eight reproductive cycles) increased (P < 0.001) totally 1.5 years after the start of vaccination, even if a tendency of increase was noticed from semester to semester after the start of vaccination without statistical significance. Finally, culling rates due to deaths (P = 0.066), locomotor problems (P = 0.264) and other causes (P = 0.894) did not significant differ per semester and totally prior and after the start of vaccination.
Discussion
It is known that culling policies influence herd economic performance since removal rates influence the herd age distribution and the number of non-productive sow days. For this reason, culling strategy is a very important part of herd health management (D'Allaire and Drolet Citation2006).
Table 3. Culling rate of female breeding stock (sows/gilts) per semester and totally prior and after the start of vaccination.
Reproductive failure is the main reason for culling, followed by old age, locomotor problems, death and other problems (Paterson et al. Citation1996, Citation1997; Tummaruk et al. Citation2001; D'Allaire and Drolet Citation2006; Serenius and Stalder Citation2006). In PRRSV-infected farms, there is an increase in the female culling rate due to reproductive problems (Benfield et al. Citation1992, Citation1999). This has significant economic importance, as the low female culling rate is an important management factor (King et al. Citation1998). In the present study, the vaccination of female breeding stock leads to significant reduction (P < 0.001) of culling rate due to reproductive failure 1.5 years after the start of vaccination and a tendency of reduction was performed from semester to semester after the start of vaccination without statistical significance (). In addition, regarding to reproductive failure in this farm, in previous studies (Plana-Duran et al. Citation1997; Joisel et al. Citation2001; Papatsiros et al. Citation2006), it was indicated the use of the same KV vaccine in the gilts/sows proved to reduce the negative effects of the virus on the breeding herd, improving significantly the sows’ reproductive performance (e.g. reduction of premature farrowings, abortions and increase of farrowing rate) and their litter characteristics (e.g. increase of the number of live born and weaned pigs and decrease of stillborn, mummified, weak and splay-legged piglets). According to Anil et al. (Citation2009), the farrowing performance of sows is a main factor that influencing the sow longevity in a herd. Therefore, in this study, the vaccination of breeding herd with a KV vaccine has positive effects on sow longevity.
Table 4. Causes of culling in female breeding stock (sows/gilts) per semester and totally prior and after the start of vaccination.
Distinct patterns of immune response to a field PRRSV strain are recognised in PRRS-vaccinated and naive pigs, which probably underlie fundamental differences in the development and differentiation of PRRSV-specific immune effect (Dotti et al. Citation2011). Many studies have shown beneficial effects of MLV vaccines on PRRS clinical disease occurrence and severity, the duration of viremia and the virus shedding (Scortti et al. Citation2006; Lowe et al. Citation2006; Martelli et al. Citation2007, Citation2009), as well as improvement of health status, performance of gilts/sows and their litters (Alexopoulos et al. Citation2005; Zhao et al. 2011). Previous studies with KV vaccine reported that it only confers limited protection (Nilubol et al. Citation2004; Scortti et al. Citation2007; Zuckermann et al. Citation2007). In contrast, the present study shows that the use of PRRSV KV vaccine had beneficial effects in a PRRSV-infected farm, in which during the vaccination period the prevalence of seropositive non-vaccinated replacements gilts remained always higher than 93.3% (P > 0.05) (). However, the prevalence of PRRSV-infected gilts with high PRRSV-specific antibody titres (≥1:2560), increased during the semesters +1, +3 and +4 after the start of vaccinations (). The present study was performed in a closed single-site farrow-to-finish PRRSV-infected farm, a common situation for many swine herds in Europe. In the farm where our experiment took place gilts were produced internally and were introduced into the breeding herd directly from the grower or finisher unit. PRRSV tends to circulate within a herd, due to the persistent PRRSV infection of carrier animals and the continual availability of susceptible animals by their commingling with infected animals in later stages of production (Zimmerman et al. Citation2006). The sow population constitutes a reservoir for a continuous circulation of respiratory pathogens and needs to be properly considered in control strategies (Fablet et al. Citation2011). Therefore, some PRRSV-infected gilts that entered the breeding population in this farm may actually re-introduce the virus in that population. In general, closed-herd systems do not eliminate PRRSV, although they do achieve a level of immunity because replacement animals usually have previous exposure to pathogens circulating in the herd (Dee and Joo Citation1994a, Citation1994b).
During the trial period of this study, there were no evidence (laboratory results and clinical signs) that new PRRSV strains were introduced in the herd. Moreover, no acute outbreaks of PRRS were noticed after the application of vaccinations in breeding herd. A typical epidemic of PRRSV-induced reproductive failure, but during the acute stage of infection with PRRSV, gilts and sows may have few, if any, clinical signs, or they may be severely affected and even die. This difference largely reflects the relative virulence of the strain of PRRSV causing the epidemic (Mengeling et al. Citation2000). The benefit of PRRS-killed vaccine is seen more obviously in virus-infected animals. In these cases, PROGRESSIS® proved to reduce the negative effects of the virus on the breeding herd, improving reproductive performance, for example increased farrowing rate, number of weaned pigs and health status of piglets born to vaccinated sows (Papatsiros et al. Citation2006). However, in the same study, Papatsiros et al. (Citation2006) is found that the humoral response of sows following two vaccinations was short, lasting 40 days after the booster vaccination. Due to the great variability of PRRSV strains, it is not known whether the present results are applicable to all farms. However, it is reasonable to assume that its efficacy will be determined by the homology between the vaccine strain and the strain of the prevalent virus in the farm. In contrast to PRRS MLV vaccine, vaccination with PRRS KV vaccine does not elicit detectable antibodies (Kim et al. Citation2011), but barely elicits cell-mediated immune response to the infecting virus as determined by lymphocyte proliferation and IFNγ production in recall response (Bassaganya-Riera et al. Citation2004; Piras et al. Citation2005; Kim et al. Citation2011). These findings lead to the potential application of PRRS KV vaccine as a therapeutic vaccine in PRRSV-positive farms.
A high removal rate is generally associated with less productivity, an increase in the number of non-productive sow days and an increase in the number of replacement gilts (Dijkhuizen et al. Citation1986). Many studies have reposted that high removal rates are associated with a decrease in litters per sow per year and pigs weaned per sow per year, increasing disease risks and the cost of production (Kroes and van Male Citation1979; Benfield et al. Citation1999). So, based on the findings of our study, the vaccination of breeding population with a PRRSV KV vaccine can improve the sow longevity in a herd, improving the herd age distribution and reducing the number of non-productive sow days. This observation has an important economical impact for swine industry (Dijkhuizen et al. Citation1990), as sow longevity plays an important role in economically efficient piglet production because sow longevity is related to the number of piglets produced during its productive lifetime. Sow mortality can have a large economic impact on commercial pork operations, as many of the sow mortalities occur after substantial costs have already been incurred by the pork operation. Schultz et al. (Citation2001) reported that 38–40% of sow deaths occurred 100–125 days post-breeding, a time at which a substantial gestational economic investment had already been made. Considering the relatively high feed costs experience by producers today, the cost of sow mortalities occurring this late in gestation is magnified.
In conclusion, the long-term vaccination of the female breeding stock in a farm where PRRS is endemic can lead to a significant decrease of culling rate due to reproductive failure and improve sow longevity. These specific effects of vaccination are of high economic interest to pork producers, especially in closed single-site farrow-to-finish farms.
Acknowledgements
This work was supported by Merial SAS (Lyon, France) through the Research Committee of the Aristotle University of Thessaloniki. The present study was performed under the license for experimenting on animals from the local Veterinary Administration Office (License No. 07/1855). All procedures during this clinical study were carried out according to the Code of Practice for the Conduct of Clinical trials for Veterinary Medical Products and the animals were maintained in accordance with national and European animal welfare requirements.
References
- Alexopoulos , C , Kritas , SK , Kyriakis , CS , Tzika , E and Kyriakis , SC . 2005 . Sow performance in an endemically porcine reproductive and respiratory syndrome (PRRS)-infected farm after sow vaccination with an attenuated PRRS vaccine . Veterinary Microbiology , 111 ( 3–4 ) : 151 – 157 . doi: 10.1016/j.vetmic.2005.10.007
- Anil , SS , Anil , L and Deen , J . 2009 . Effect of lameness on sow longevity . Journal of the American Veterinary Medical Association , 235 : 734 – 738 . doi: 10.2460/javma.235.6.734
- Bassaganya-Riera , J , Thacker , BJ , Yu , S , Strait , E , Wannemuehler , MJ and Thacker , EL . 2004 . Impact of immunizations with porcine reproductive and respiratory syndrome virus on lymphoproliferative recall responses of CD8+ T cells . Viral Immunology , 17 : 25 – 37 . doi: 10.1089/088282404322875430
- Benfield , DA , Collins , JE , Dee , SA , Halbur , PG , Joo , HS , Lager , KM , Mengeling , WL , Murtaugh , MP , Rossow , D Stevenson , GW . 1999 . “ Porcine reproductive and respiratory syndrome ” . In Diseases of swine , 8th ed , Edited by: Straw , BE , D'Allaire , S , Mengeling , WL and Taylor , DJ . 201 – 232 . Ames , IA : Iowa State University Press .
- Benfield , DA , Nelson , E , Collins , JE , Harris , L , Goyal , SM , Bobinson , D , Christianson , WT , Morrison , RB , Gorcyca , D and Chladek , D . 1992 . Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) . Journal of Veterinary Diagnostic Investigation , 4 : 127 – 133 . doi: 10.1177/104063879200400202
- Corzo , CA , Mondaca , E , Wayne , S , Torremorell , M , Dee , S , Davies , P and Morrison , RB . 2010 . Control and elimination of porcine reproductive and respiratory syndrome virus . Virus Research , 154 : 185 – 192 . doi: 10.1016/j.virusres.2010.08.016
- D'Allaire , S and Drolet , R . 1999 . “ Culling and mortality in breeding animals ” . In Diseases of swine , 8th ed , Edited by: Straw , BE , D'Allaire , S , Mengeling , WL and Taylor , DJ . 1002 – 1016 . Ames , IA : Iowa State University Press .
- D'Allaire , S and Drolet , R . 2006 . “ Longevity in breeding animals ” . In Diseases of swine , 9th ed , Edited by: Straw , BE , Zimmerman , JJ , D'Allaire , S and Taylor , DJ . 1011 – 1026 . Ames , IA : Blackwell .
- Dee , S and Joo , HS . 1994a . Clinical investigation of recurrent reproductive failure with PRRS virus in a swine herd . Journal of the American Veterinary Medical Association , 205 : 1017 – 1018 .
- Dee , S and Joo , HS . 1994b . Prevention of the spread of PRRS virus in endemically infected pig herds by nursery depopulation . Veterinary Record , 135 : 6 – 9 . doi: 10.1136/vr.135.1.6
- Dial GD , Fitzsimmons M , BeVier GW , Wiseman B. 1994 . Systems approaches for improving the productivity of the breeding herd . Proceedings of AD Leman Swine Conference ; 1994 Sep ; St. Paul MN : AD Leman editor . 84 – 93 .
- Dijkhuizen , AA , Krabbenborg , RMM , Morrow , M and Morris , RS . 1990 . Sow replacement economics: an interactive model for microcomputers . Compendium on Continuing Education for the Practicing Veterinarian , 12 : 575 – 579 .
- Dijkhuizen , AA , Morris , RS and Morrow , M . 1986 . Economic optimization of culling strategies in swine breeding herds, using the PorkCHOP computer program . Preventive Veterinary Medicine , 4 : 341 – 353 . doi: 10.1016/0167-5877(86)90015-2
- Dotti , S , Villa , R , Sossi , E , Guadagnini , G , Salvini , F , Ferrari , M and Amadori , M . 2011 . Comparative evaluation of PRRS virus infection in vaccinated and naïve pigs . Research in Veterinary Science , 90 ( 2 ) : 218 – 225 . doi: 10.1016/j.rvsc.2010.06.011
- Fablet , C , Marois , C , Kuntz-Simon , G , Rose , N , Dorenlor , V , Eono , F , Eveno , E , Jolly , JP , Le Devendec , L Tocqueville , V . 2011 . Longitudinal study of respiratory infection patterns of breeding sows in five farrow-to-finish herds . Veterinary Microbiology , 147 ( 3–4 ) : 329 – 339 . doi: 10.1016/j.vetmic.2010.07.005
- Joisel , F , Reynaud , G , Charreyre , C and Herin , JB . 2001 . PRRS: vaccination with a killed vaccine: field experience . Pig Journal , 48 : 120 – 137 .
- Kim , H , Kim , HK , Jung , JH , Choi , YJ , Kim , J , Um , CG , Hyun , SB , Shin , S , Lee , B Jang , G . 2011 . The assessment of efficacy of porcine reproductive respiratory syndrome virus inactivated vaccine based on the viral quantity and inactivation methods . Virology Journal , 8 : 323 doi: 10.1186/1743-422X-8-323
- Kimman , TG , Cornelissen , LA , Moormann , RJ , Rebel , JMJ and Stockhofe-Zurwieden , N . 2009 . Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology . Vaccine , 27 ( 28 ) : 3704 – 3718 . doi: 10.1016/j.vaccine.2009.04.022
- King , VL , Koketsu , Y , Reeves , D , Xue , J and Dial , GD . 1998 . Management factors associated with swine breeding-herd productivity in the United States . Preventive Veterinary Medicine , 35 : 255 – 264 . doi: 10.1016/S0167-5877(98)00068-3
- Kroes , Y and van Male , JP . 1979 . Reproductive lifetime of sows in relation to economy of production . Livestock Production Science , 6 : 179 – 183 . doi: 10.1016/0301-6226(79)90019-8
- Labarque , G , Van Reeth , V , Nauwynck , H , Drexler , C , Van Gucht , S and Pensaert , M . 2004 . Impact of genetic diversity of European-type porcine reproductive and respiratory syndrome virus strains on vaccine efficacy . Vaccine , 22 ( 31–32 ) : 4183 – 4190 . doi: 10.1016/j.vaccine.2004.05.008
- Lowe , JF , Zuckermann , FA , Firkins , LD , Schnitzlein , WM and Goldberg , TL . 2006 . Immunologic responses and reproductive outcomes following exposure to wild-type or attenuated porcine reproductive and respiratory syndrome virus in swine under field conditions . Journal of the American Veterinary Medical Association , 228 ( 7 ) : 1082 – 1088 . doi: 10.2460/javma.228.7.1082
- Martelli , P , Cordioli , P , Alborali , LG , Gozio , S , De Angelis , E , Ferrari , L , Lombardi , G and Borghetti , P . 2007 . Protection and immune response in pigs intradermally vaccinated against porcine reproductive and respiratory syndrome (PRRS) and subsequently exposed to a heterologous European (Italian cluster) field strain . Vaccine , 25 ( 17 ) : 3400 – 3408 . doi: 10.1016/j.vaccine.2006.12.050
- Martelli , P , Gozio , S , Ferrari , L , Rosina , S , De Angelis , E , Quintavalla , C , Bottarelli , E and Borghetti , P . 2009 . Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: clinical protection and cell-mediated immunity . Vaccine , 27 ( 28 ) : 3788 – 3799 . doi: 10.1016/j.vaccine.2009.03.028
- Mengeling , WL , Lager , KM and Vorwald , AC . 2000 . The effect of porcine parvovirus and porcine reproductive and respiratory syndrome virus on porcine reproductive performance . Animal Reproduction Science , 60–61 : 199 – 210 . doi: 10.1016/S0378-4320(00)00135-4
- Meredith , MJ . 1995 . “ Pig breeding and infertility ” . In Animal breeding and infertility , 1st ed , Edited by: Meredith , MJ . 278 – 353 . Oxford : Blackwell Science .
- Neumann , EJ , Kliebenstein , JB , Johnson , CD , Mabry , JW , Bush , EJ , Seitzinger , AH , Green , AL and Zimmerman , JJ . 2005 . Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States . Journal of the American Veterinary Medical Association , 227 : 385 – 392 . doi: 10.2460/javma.2005.227.385
- Nilubol , D , Platt , KB , Halbur , PG , Torremorell , M and Harris , DL . 2004 . The effect of a killed porcine reproductive and respiratory syndrome virus (PRRSV) vaccine treatment on virus shedding in previously PRRSV infected pigs . Veterinary Microbiology , 102 ( 1–2 ) : 11 – 18 . doi: 10.1016/j.vetmic.2004.05.006
- Papatsiros , VG , Alexopoulos , C , Boscos , C and Kyriakis , SC . 2011 . Impact of a porcine reproductive and respiratory syndrome inactivated vaccine on the health and semen traits of boars . Journal of Hellenic Veterinary Medical Society , 62 ( 3 ) : 221 – 228 .
- Papatsiros , VG , Alexopoulos , C , Kritas , SK , Koptopoulos , G , Nauwynck , HJ , Pensaert , MB and Kyriakis , SC . 2006 . Long-term administration of a commercial porcine reproductive and respiratory syndrome virus (PRRSV)-inactivated vaccine in PRRSV-endemically infected sows. Journal of Veterinary Medicine B . Infectious Diseases and Veterinary Public Health , 53 : 266 – 272 . doi: 10.1111/j.1439-0450.2006.00965.x
- Paterson R , Cargill C , Pointon A. 1996 . Investigations into deaths and excessive culling of sows in Australian pig herds . Proceedings of Nordiska Jordbruksforskares Forening Seminar No. 265 – Longevity of Sows ; 1996; Research Centre Foulum, Tjele (Denmark): Danielsen V editor . 34 – 45 .
- Paterson R , Cargill C , Pointon A. 1997 . Epidemiology of reproductive failure and urogenital disease . Proceedings of Pig Production-The A.T. Reid Course for Veterinarians; 1997; Post-Grad Foundation of Veterinary Science , University of Sydney, Sydney (Australia). University of Sydney editor . 223 – 244 .
- Piras , F , Bollard , S , Laval , F , Joisel , F , Reynaud , G , Charreyre , C , Andreoni , C and Juillard , V . 2005 . Porcine reproductive and respiratory syndrome (PRRS) virus-specific interferon-c + T-cell responses after PRRS virus infection or vaccination with an inactivated PRRS vaccine . Viral Immunology , 18 : 381 – 389 . doi: 10.1089/vim.2005.18.381
- Plana-Duran , J , Bastons , M , Urniza , A , Vayreda , M , Vila , X and Mane , H . 1997 . Efficacy of an inactivated vaccine for prevention of reproductive and respiratory failure induced by porcine reproductive and respiratory syndrome virus . Veterinary Microbiology , 55 : 361 – 370 . doi: 10.1016/S0378-1135(96)01317-X
- Prieto , C , Álvarez , E , Martínez-Lobo , FJ , Simarro , I and Castro , JM . 2008 . Similarity of European porcine reproductive and respiratory syndrome virus to vaccine strain is not necessarily predictive of the degree of protective immunity conferred . Veterinary Journal , 175 ( 3 ) : 356 – 363 . doi: 10.1016/j.tvjl.2007.01.021
- Schultz R , Dau , D , Cast W , Hoefling D , Duran O , Carson T , Becton L , Woodward C , Pollard K , Busker K , Kaster D , Steidinger M. 2001 . A sow mortality study – the real reasons sows die; identifying causes and implementing action . Proceedings of the 32nd Annual Meeting of the American Association of Swine Practitioners ; 24–27 February 2001 ; Nashville (TN, USA) : AASP Publisher , 387 – 395 .
- Scortti , M , Prieto , C , Alvarez , E , Simarro , I and Castro , JM . 2007 . Failure of an inactivated vaccine against porcine reproductive, respiratory syndrome to protect gilts against a heterologous challenge with PRRSV . Veterinary Research , 161 ( 24 ) : 809 – 813 .
- Scortti , M , Prieto , C , Simarro , I and Castro , JM . 2006 . Reproductive performance of gilts following vaccination and subsequent heterologous challenge with European strains of porcine reproductive and respiratory syndrome virus . Theriogenology , 66 ( 8 ) : 1884 – 1893 . doi: 10.1016/j.theriogenology.2006.04.043
- Serenius , T and Stalder , KJ . 2006 . Selection for sow longevity . Journal of Animal Science , 84 : 166 – 171 . doi: 10.2527/jas.2005-499
- Tummaruk , P , Lundeheim , N and Einarsson , S . 2001 . Dalin repeat breeding and subsequent reproductive performance in Swedish Landrace and Swedish Yorkshire sows . Animal Reproduction Science , 67 : 267 – 280 . doi: 10.1016/S0378-4320(01)00129-4
- Wensvoort , G , Terpstra , C , Pol , JM , ter Laak , EA , Bloemraad , M , de Kluyver , EP , Kragten , C , van Buiten , L , den Besten , A Wagenaar , F . 1991 . Mystery swine disease in the Netherlands: the isolation of Lelystad virus . Veterinary Quarterly , 13 : 121 – 130 . doi: 10.1080/01652176.1991.9694296
- Zhao , Z , Qin , Y , Lai , Z , Peng , L , Cai , X , Wang , L , Guo , X and Yang , H . 2012 . Microbial ecology of swine farms and PRRS vaccine vaccination strategies . Veterinary Microbiology , 155 ( 2–4 ) : 247 – 256 . doi: 10.1016/j.vetmic.2011.09.028
- Zimmerman , JJ , Benfield , DA , Murtaugh , MP , Osorio , FA , Stevenson , GW and Torremorell , M . 2006 . “ Porcine reproductive and respiratory syndrome virus (porcine arterivirus) ” . In Diseases of swine , 9th ed , Edited by: Straw , BE , Zimmerman , JJ , D'Allaire , S and Taylor , DJ . 387 – 417 . Ames , IA : Blackwell .
- Zuckermann , FA , Garcia , EA , Luque , ID , Christopher-Hennings , J , Doster , A , Brito , M and Osorio , F . 2007 . Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge . Veterinary Microbiology , 12 : 69 – 85 . doi: 10.1016/j.vetmic.2007.02.009