Abstract
The present study was undertaken to evaluate the efficacy of acellular dermal matrix (ADM) of caprine origin for the repair of abdominal wall hernias in six goats. The skin of goat was de-epithelialised using a 2 M sodium chloride solution and 0.25% trypsin, and further decellularised using 2% sodium deoxycholate, which was then used as graft material. Under xylazine sedation and local infiltration analgesia, the abdominal wall hernias were repaired with ADM graft using inlay graft technique. All animals had an uneventful recovery without clinical signs of wound dehiscence, infection or recurrence of hernia during 6 months follow-up periods.
1. Introduction
Abdominal wall defects secondary to trauma, infection, or previous surgery are typically large. Despite improved surgical techniques, repair of these defects is a clinical challenge for surgeons. Literature on human surgery emphasises the use of prosthetic materials for hernioplasty when the size of the hernial ring exceeds 3 cm in diameter (Kingsnorth and LeBlanc Citation2003; Venclauskas et al. Citation2008). Non-absorbable synthetic mesh is one of the most widely used prosthetic materials for reconstruction of abdominal wall hernias. This material allows for a tension free repair, which significantly reduces the hernia recurrence rate compared with primary suture repair (Burger et al. Citation2004). However, there are many complications in the form of mesh infection, adhesions to underlying viscera, fistula formation, mesh extrusion and foreign body reaction associated with the use of synthetic mesh material for abdominal wall reconstruction (Falagas and Kasiakou Citation2005). To overcome these synthetic mesh-related complications, biomaterials derived from animal sources may be preferred for the surgical repair of these abdominal wall defects.
Biological biomaterials are considered superior to synthetic materials for the repair of abdominal wall defects, owing to their ability to minimise adhesion formation, provide a better framework for fibroblast proliferation, and neovascularisation (Clarke et al. Citation1996). Moreover, their multidirectional fibrous structure helps in better suture retention, complete absorption and gets replaced by host tissue (Clarke et al. Citation1996). However, biological biomaterials in their native form tend to be more immunogenic and hence are decellularised to minimise their immunogenicity (Gilbert et al. Citation2006). Acellular dermal matrix (ADM) grafts have been used by several researchers for the reconstruction of abdominal wall defects in rabbits (Gangwar et al. Citation2006; Singh et al. Citation2008; Eberli et al. Citation2010; Ngo et al. Citation2011) and rats (Kaya et al. Citation2006) with excellent results. Although results of the preclinical animal studies have been promising, uses of acellular dermal grafts for the reconstruction of abdominal hernias in clinical situations are limited. Therefore, the present study was undertaken to evaluate the efficacy of ADM of caprine origin for the repair of abdominal wall hernias in goats.
2. Materials and methods
2.1. Animals
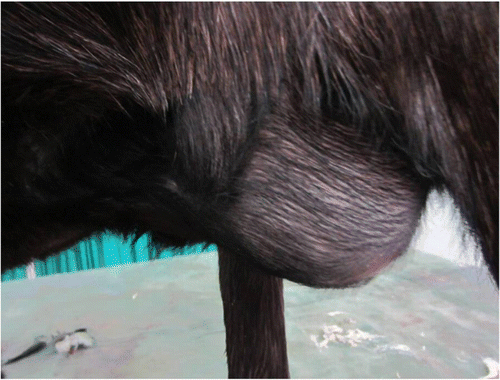
The present study was conducted on six client owned goats that were presented to the Surgery Unit of the Referral Veterinary Polyclinics, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, India from March, 2009 to September, 2011. The goats were five male and one female, and were belonged to three breeds (one Jamunapari, three Sirohi, two crossbred). The goats were 4–36 months old (mean age, 13.33 months) and weighed 10–55 kg (mean weight, 27.29 kg). In two goats, incisional ventral hernia was developed about 2 months after undergoing tube cystostomy for urethral obstruction. Three goats had history of progressive development of swelling at the ventral abdomen following gore injury and one had umbilical hernia. Palpation at the ventral abdomen revealed a painless, reducible hernia with a discernable ring (). Multiple loops of intestine could be palpated traversing the hernial ring. The intestinal loops appeared to be located within the peritoneum. The size of the hernial ring ranged from 4 to 12 cm (mean size, 6.67 cm) in diameter. Details are given in . At the time of presentation, animals had normal temperature, respiration and pulse-rates.
Table 1. Cases of abdominal wall hernias in goats.
2.2. Preparation of ADM
Caprine skin was decellularised as per the method described by Purohit (Citation2008) with some modifications. In brief, the skin of caprine origin (Capra hircus) was collected from the local abattoir and immediately preserved in ice-cold sterile phosphate buffered saline (PBS, pH 7.4) containing a broad spectrum antibiotic (Amikacin-1 mg/ml), and a proteolytic inhibitor (0.02% EDTA). In laboratory, it was shaved and washed thoroughly with sterile PBS to remove all the adherent blood and debris. The skin was de-epithelialised using 0.25% trypsin and 2 M sodium chloride solution for 8 hours. After de-epithelialisation, the dermis was decellularised using 2% sodium deoxycholate for 48 hours. The samples were subjected to continuous agitation in a horizontal orbital shaker at the rate of 180 rotations per minute during de-epithelialisation and decellularisation process to provide better contact of tissue with chemicals. To confirm the acellularity of the prepared matrix microscopic examination was conducted after Haematoxylin and Eosin (H&E) staining of the representative samples. Following decellularisation, the prepared ADM was washed six times (2 hours each) with sterile PBS to remove the residual chemicals and stored in PBS solution containing 0.1% amikacin solution at −20°C.
2.3. Preoperative treatment
In each goat tetanus toxoid (5 Lf) was administered intramuscularly 5 days prior to surgery. The goats were kept off feed for 24 hours, and deprived of water for 2 hours prior to anaesthesia. Meloxicam (0.5 mg/kg) and ceftriaxone sodium (20 mg/kg) were intravenously administered in each animal approximately 1 hour prior to surgery.
2.4. Surgical procedure
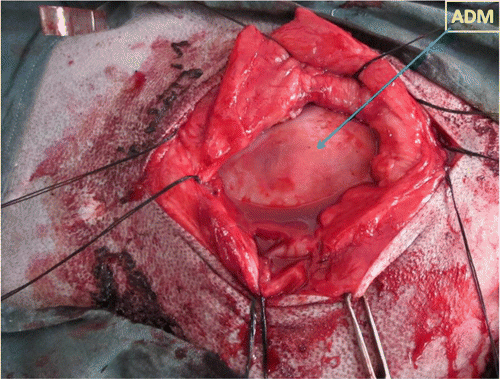
The goats were sedated with 0.1 mg/kg xylazine hydrochloride, diluted in 20 mL of normal saline, which was administered intravenously over 5 minutes. The goats were then positioned in dorsal or lateral recumbency, according to the type and position of the hernia, and the ventral abdomen was prepared for aseptic surgery. Circular infiltration analgesia was done using 2% lignocaine hydrochloride. To expose the hernial sac, an elliptical skin incision was made that spanned the length of the hernia and extended 2 cm beyond the cranial and caudal margins of the hernial ring. Forcipressure was used to control subcutaneous haemorrhage, if any. The hernial sac was dissected from overlying skin and dissection was continued laterally to expose the hernial ring and the external sheath of rectus abdominis muscle. The hernial sac was opened and contents such as small intestine, omentum, or fat were repositioned into the abdominal cavity. The ADM graft material exceeding the defect by 2 cm in all directions was adjusted for adequate closure of the hernial ring. An appropriately sized piece of ADM graft with pre-placed horizontal mattress suture of number 2 surgical silk with long ends, attached to its cranial, caudal and mid-lateral edges was introduced into the abdomen through the hernial ring. The ADM was oriented within the abdomen and the suture ends were retrieved on an external sheath of rectus abdominis muscle using a non-traumatic needle. Each suture was tied with the knots resting on the external sheath of rectus abdominis muscle, thus provisionally securing the ADM to the peritoneum (). While the graft was being implanted, the surgical site was lavaged periodically with sterile PBS containing 0.1% amikacin. The subcutaneous tissues were closed in two layers, using number 2-0 chromic catgut placed in a simple continuous suture pattern. The skin incision was then closed using number 2 surgical silk suture material in a horizontal mattress suture pattern. After surgery, the ventral abdomen of each goat was compressed with a sterile bandage to protect the surgical site from the external environment and to minimise postoperative oedema. Owners were advised to maintain the bandage for to 30 days minimum, at which time healing was assessed by a physical examination.
2.5. Postoperative care
Postoperative analgesia was provided by meloxicam (0.2 mg/kg intramuscularly, once daily) for 3 days. Ceftriaxone (10 mg/kg intramuscularly, twice daily) was administered for 7 days. The antiseptic dressing of the suture line was performed with povidone iodine solution for 10 days. The skin sutures were removed on the 10th postoperative day. To assess the integrity of the repair, clinical evaluation of goats were carried out at least up to 6 months. Clinical evaluation was repeated at 4-week interval.
3. Results and discussion
The abdominal wall hernias were successfully repaired in all six goats with ADM graft using this technique. All of the animals had an uneventful recovery without clinical signs of wound dehiscence, infection, or recurrence of hernia, except for a mild inflammatory oedema during the first week after surgery. The oedema reduced daily, until complete resolution in all cases between 7 and 10 days after surgery. In all the animals, integrity of the ADM graft was never lost nor rejected at least up to 6 months after hernioplasty. Acellular tissue matrices are biocompatible, slowly degraded upon implantation and are replaced and remodelled by the extracellular matrix proteins synthesised and secreted by ingrowing host cells (Pariente et al. Citation2001). Elce et al. (Citation2005) reported a relatively high incidence of post-operative complications associated with retroperitoneal placement of a synthetic mesh material, such as tearing of the internal abdominal oblique muscle and incisional swelling and drainage. However, in the present study, no post-operative complications were observed after retroperitoneal placement of ADM in any animals at least up to 6 months after their repair. Similar results were reported by Kumar et al. (Citation2012) following the use of acellular aortic matrix for reconstruction of abdominal hernias in buffaloes. The acellular matrix possesses the appropriate mechanical properties and induces appropriate interaction with the host cells that resulted in the regeneration of functional tissues (Voytik-Harbin et al. Citation1998).
4. Conclusion
The results of the present study demonstrated that ADM of caprine origin serves as a viable option for the repair of abdominal wall hernias in goats.
Acknowledgements
The authors acknowledge the financial assistance received from the Department of Biotechnology, Ministry of Science and Technology, New Delhi, India to carry out this research work.
References
- Burger , JWA , Luijendijk , RW , Hop , WC , Halm , JA , Verdaasdonk , EGG and Jeekel , J . 2004 . Long term follow up of a randomized controlled trial of suture verses mesh repair of incisional hernia . Annals of Surgery , 240 : 578 – 583 .
- Clarke , KM , Lantz , GC , Salisbury , K , Badylak , SF , Hiles , MC and Voytik , SL . 1996 . Intestinal submucosa and polypropylene mesh for abdominal wall repair in dogs . Journal of Surgical Research , 60 : 107 – 114 . doi: 10.1006/jsre.1996.0018
- Eberli , D , Rodriguez , S , Atala , A and Yoo , JJ . 2010 . In vivo evaluation of acellular human dermis for abdominal wall repair . Journal of Biomedical Materials Research Part A , 93A : 1527 – 1538 .
- Elce , YA , Kraus , BM and Orsini , JA . 2005 . Mesh hernioplasty for repair of incisional hernias of the ventral body wall in large horses . Equine Veterinary Education , 17 : 252 – 256 . doi: 10.1111/j.2042-3292.2005.tb00385.x
- Falagas , ME and Kasiakou , SK . 2005 . Mesh-related infections after hernia repair surgery . Clinical Microbiology and Infection , 11 : 3 – 8 . doi: 10.1111/j.1469-0691.2004.01014.x
- Gangwar , AK , Sharma , AK , Kumar , N , Kumar , N , Maiti , SK , Gupta , OP , Goswami , TK and Singh , R . 2006 . Acellular dermal graft for repair of abdominal wall defects in rabbits . Journal of South African Veterinary Association , 77 : 79 – 85 .
- Gilbert , TW , Sellaroa , TL and Badylak , SF . 2006 . Decellularization of tissues and organs . Biomaterials , 27 : 3675 – 3683 .
- Kaya , M , Baba , F , Bolukbas , F , Boleken , ME , Kanmaz , T and Yucesan , S . 2006 . Use of homologous acellular dermal matrix for abdominal wall reconstruction in rats . Journal of Investigative Surgery , 19 : 11 – 17 . doi: 10.1080/08941930500444370
- Kingsnorth , A and LeBlanc , K . 2003 . Hernias: inguinal and incisional . Lancet , 302 : 1561 – 1571 . doi: 10.1016/S0140-6736(03)14746-0
- Kumar , V , Devarathnam , J , Gangwar , AK , Kumar , N , Sharma , A K , Pawde , AM and Singh , H . 2012 . Use of acellular aortic matrix for reconstruction of abdominal hernias in buffaloes . Veterinary Record , 170 : 392 doi: 10.1136/vr.100594
- Ngo , MD , Aberman , HM , Hawes , ML , Choi , B and Gertzman , AA . 2011 . Evaluation of human acellular dermis versus porcine acellular dermis in an in vivo model for incisional hernia repair . Cell Tissue Bank , 12 : 135 – 145 . doi: 10.1007/s10561-011-9245-5
- Pariente , JL , Kim , BS and Atala , A . 2001 . In vitro and in-vivo biocompatibility assessment of natural-derived and polymeric biomaterials using normal human urothelial cells . Journal of Biomededical and Matererial Research , 55 : 33 – 39 . doi: 10.1002/1097-4636(200104)55:1%3C33::AID-JBM50%3E3.0.CO;2-7
- Purohit S. 2008 . Biocompatibility testing of acellular dermal grafts in a rabbit model: An in vitro and in vivo study [dissertation] . [Izatnagar] : IVRI .
- Singh , J , Kumar , N , Sharma , AK , Maiti , SK , Goswami , TK and Sharma , AK . 2008 . Acellular biomaterials of porcine origin for the reconstruction of abdominal defects in rabbits . Trends in Biomaterial and Artificial Organs , 22 : 33 – 44 .
- Venclauskas , L , Silanskaite , J and Kiudelis , M . 2008 . Umbilical hernia: factors indicative of recurrence . Medicina (Kaunas) , 44 : 855 – 859 .
- Voytik-Harbin , SL , Brightman , AO , Waisner , BZ , Robinson , JP and Lamar , CH . 1998 . A tissue-derived extracellular matrix that promote tissue growth and differentiation of cells in-vitro . Tissue Engineering , 4 : 157 – 174 . doi: 10.1089/ten.1998.4.157