Abstract
The persistence time and transformation possibility to the rumen bacteria genome of the foreign genes (Bar and CaMV 35S) and self genes (SPS and Actin) in grain and straw of a glufosinate herbicide-tolerant transgenic rice (Bar68-1) and its transgenic hybrid generation (X125s/Bar68-1) were investigated in an in vitro fermentation system with the rumen fluid/buffer. Results showed that the fermentation characteristics of rice grain and straw were similar between Bar68-1 and X125s/Bar68-1. The Bar and CaMV 35S genes in the rice grain and straw could persist for 36 and 62 h, respectively in the incubation media, which were similar to the persistence times for the self genes. No dissociative gene fragment was detected in the supernatant of the rumen fluid/buffer media. No fragment of the Bar and CaMV 35S genes was detectable in the ruminal microbe genome at any incubation time. This study implies that the possibility of transformation of Bar and CaMV 35S genes to the ruminal microbes is scarce.
1. Introduction
The debate about the safety of genetically modified (GM) food remains in the academic community. One of the main concerns regarding the application of bacterium-originated genes or antibiotic resistance genes to transgenic crops is the potential of horizontal gene transfer to other organisms in the environment, most notably to microorganisms including pathogens in the alimentary tract of humans or animals (Bertolla et al. Citation2000; Jonas et al. Citation2001; Gay and Gillespie Citation2005). It has been reported that more than 40 soil- or water-borne bacterial species can actively take up DNA from the environment and use them as a source of genome (Lorenz and Wackernagel Citation1994). Gebhard and Smalla (Citation1998) found that under the optimised laboratory conditions, gene transfer from the transgenic sugar beet chromosome to bacteria might occur in soil if homologous sequences presented in competent bacteria. Moreover, it has been demonstrated that plasmid DNA had the ability to transform natural competent bacteria gordonii DL1 in human saliva (Mercer et al. Citation1999). This research demonstrates that gene transformation can readily occur between different phylogenetic kingdoms.
Although the possibility of transformation of plant genes to bacterial genome is relatively low, the homologous sequence of a bacterial gene can sharply elevate 105-fold of horizontal gene transfer from bacterial replicons to bacterial genome (Prudhomme et al. Citation2002). It has been demonstrated that the introduction of bacterial genes, promotor and terminator sequences into the plant genome might enhance the probability of gene transform from transgenic plants to bacteria (Bertolla and Simonet Citation1999; Sharma et al. Citation2004). Therefore, it is necessary to provide more solid data on safety evaluation of transgenic crops which have microbe-derived foreign genes. Ruminants harbour numerous bacteria, fungi and protozoa in their rumens and lower digestive tracts, and these animals consume large amounts of plant materials (De Vega et al. Citation1998). The utilisation of transgenic crops as feed for ruminants has prompted the interests of studying the possible uptake of the transgene fragments by the ruminal or intestinal micro-organisms. Duggan et al. (Citation2000) used the plasmid that contains cry1A (b) transgenic fragments to study the possibility of gene transfer to the ruminal microbes using an in vitro method. Sharma et al. (Citation2004) studied the persistence time and transformation possibility of liner foreign EPSPS and rapeseed self Rubisco gene fragments in transgenic rapeseed to the microbial genome in the rumen. The results of above-mentioned experiments suggest that liner plant gene fragments are unsteady and have a low possibility of gene transformation. However, other transgenic fragments widely applied in GM crops, such as Bar and CaMV35s promoter, have not yet been studied.
Recently transgenic technologies have been induced to improve rice productivity (Khush Citation1997). The Bar-gene transgenic rice is one of the most potentially transgenic species in the world. Bar gene is a bialaphos-resistant gene isolated from the soil micro-organism Streptomyces hygroscopicus strain HP632, which expresses enzyme phosphinothricin-N-acetyltransferase (PAT) to detoxify the herbicide by acetylation (Thompson et al. Citation1987). The Cauliflower mosaic virus (CaMV) 35S promoter is being widely used in almost all GM crops currently planted. The promoter has more homogeneity than plant DNA with the microbe genome. Some researchers thought that the Bar or CaMV 35S gene would transform to the microbe genome by homologous recombination. On the other hand, there would be a potential that hybridisation of GM plants (GMPs) with conventional crops might change the stability of a foreign gene in the genome of the hybrid generation (Lin et al. Citation2009). Therefore, this study was conducted to investigate the persistence and transformation possibility of the Bar gene and CaMV 35S gene present in grain and straw of a transgenic glufosinate herbicide-tolerant rice variety (Bar68-1) and a transgenic hybrid generation (X125S/Bar68-1) in the in vitro ruminal environment, to understand the safety issue of GM rice varieties.
2. Materials and methods
The Animal Care Committee, the Institute of Subtropical Agriculture (ISA) of Chinese Academy of Sciences, Changsha, China, approved this experiment. The grains and straws of the GM and conventional rice used in this study were produced following the general guidelines of ISA (Citation2005).
2.1. Rice grain, straw and processing
Two rice varieties, a glufosinate herbicide-tolerant GM rice (Bar68-1, derived from Bar gene transfer into its corresponding conventional line D68), and a hybrid generation (X125S/Bar68-1) of X125S (female parent, conventional rice variety) with Bar68 (male parent), were generated by the ISA in 2000 and 2002, respectively. The 35S promoter sequence, Bar ORF, and NOS terminator sequence were verified and then transferred into the Bar68-1 genome using Southern blot and PCR analysis methods (data not shown). The two rice varieties were cultivated under similar agronomic conditions in the same field at the Experimental Station of the ISA, with three replicated plots (10 m×5 m) for either variety. The plants were cultivated from April until August 2007, and managed under the same regimen (e.g. fertilisation and irrigation) as rice producing practices in southern China without using any herbicide. At harvest after rice grains were separated, about 1 kg of rice straw was randomly sampled and manually chopped to 2 cm length. Three representative grain or straw samples, about 500 g each, were taken for either variety. The samples were dried at 65°C, milled through a 1-mm screen and then kept at −700°C until analyses for chemical compositions and determination of in vitro gas production. The chemical compositions of all the samples have been reported elsewhere (Lin et al. Citation2009).
2.2. In vitro fermentation system
The in vitro fermentation system was set up using the method described by Tang et al. (Citation2008). An equal volume of rumen fluid was obtained from each of three ruminally-fistulated castrated goats before the morning feeding, and subsequently mixed, and strained through four layers of cheesecloth into a pre-warmed Erlenmeyer flask. The goats were fed a ration containing rice straw and concentrate in the ratio 60:40. The feed offered was controlled to supply 1.3×maintenance requirement for metabolisable energy according to our previous studies (Wang et al. Citation2008). All laboratory handling of the rumen fluid was carried out under a continuous flow of CO2. In vitro fermentation was carried out in 100 mL graduated glass syringes fitted with plungers (Tang et al. Citation2008). Each syringe was filled with 10 mL of the rumen fluid and 20 mL of McDougall's buffer solution, plus 200±1 mg dried rice grain or straw sample. In total, there were 126 (for rice grain, two varieties, three replicates, three repeats, and seven incubation time-points) or 144 syringes (for rice straw, two varieties, three replicates, three repeats, and eight incubation time-points) containing oven-dried rice grain or straw samples (200±1 mg) which were incubated in a shaking water bath at 39°C, respectively. Three observations per fermented sample were obtained for in vitro gas production. In addition, three syringes containing only an incubation medium were incubated as blanks to correct for gas production resulting from rumen fluid activity. Three syringes of each sample were removed after the incubation of 6, 12, 18, 24, 30, 36 and 42 h for the rice grain, and 6, 12, 24, 36, 48, 54, 62 and 70 h for the rice straw, respectively. Gas production, as an index of the fermentation process, was recorded at each incubation time point according to the movement of the plunger of the syringe.
The fermentation was terminated by injecting 1 mL of HgCl2 into the syringe, followed by the medium being filtrated through four layers of cheesecloth into a 200-mL centrifuge tube. An aliquot (2 mL) of the filtrated fluid was taken for determining volatile fatty acids (VFA) immediately. VFA were measured as another index to monitor the fermentation, and analysed using a HP5890 gas chromatographer equipped with a HP-INNOWax column (30 m in length with a 0.25 mm i.d.). The attenuation was set at nitrogen diffluent ratio 1:60, hydrogen flow 50 ml/min, airflow 500 ml/min, injector temperature at 200°C, column temperature at 150°C , and detector temperature at 200°C.
2.3. Sample process for DNA extraction
The bacteria in the fermentation media particles in the syringe were removed according to the modified method described by Martinez et al. (Citation2009). The plant debris was obtained by centrifugation at 1000 rpm for 20 min at 4°C, and frozen immediately in liquid N2 for DNA extraction. The supernatant fraction was decanted into another 200 mL centrifuge tube, then immediately re-centrifuged at 12,000 rpm for 20 min at 4°C. The pellet, which comprised primarily liquid-associated bacteria, was frozen in liquid N2 for DNA extraction. Finally, the supernatant was frozen at 20°C to extract dissociative DNA as described later.
2.4. DNA extraction methods
Plant DNA was extracted by the CTAB method as described by Ren et al. (Citation2006). Briefly, 100 mg rice or rice straw samples were ground into fine powder and frozen in liquid N2 immediately. Pre-warmed (at 65°C) 600 µl of DNA extraction buffer (100 mM Tris-HCl-pH 8.0, 20 mM Na2EDTA-pH 8.0, 1.4 M NaCl, 2% CTAB, and 0.4% (v/v) β-mercaptoethanol) was added to the ground sample, mixed thoroughly and incubated for 60 min at 65°C with intermittent mixing every 5–10 min. Chloroform extraction was conducted using an equal volume of ice-cold chloroform. Phase-separation was accomplished by centrifugation at 5000 rpm for 5 min. Upper aqueous phase was transferred to another Eppendorf tube and re-extracted with ice-cold chloroform three to four times. The DNA was precipitated with 2/3 volume of ice-cold isopropanol and kept at 4°C overnight. DNA was pelleted down by centrifugation at 4°C for 10 min at 10,000 rpm and washed with 75% ethanol and air dried for 1 h. Air-dried DNA was re-suspended in 200 µl TE buffer (10 mM Tris-HCl-pH 8.0, and 1 mM EDTA-pH 8.0), and treated with RNase A (10 µg/L) at 37°C for 30 min. RNase reaction was terminated by adding one volume of phenol: chloroform (1:1 v/v). The DNA was again precipitated with two volumes of absolute ethanol mixed with 1/10 volume of 3 M sodium acetate at 20°C for 3 h and centrifuged at 13,000 rpm for 10 min. The DNA pellet was rinsed twice with 75% and 95% ethanol, air-dried, and dissolved into 100 µl sterile double distilled water (ddH2O).
The ruminal bacterial DNA were extracted by the Axygen bacterial genomic DNA miniprep Kit (Bioscience, USA) according to the manufacturer's protocol. The supernatant was subjected to isolation of free DNA using TIANquick Mini DNA purification Kit (TIANGEN, Inc. Beijing, China) according to the manufacturer's protocol for the body fluid.
2.5. Gene primers and PCR analysis
Gene names, primers and amplification length used for PCR amplification are described in . Foreign genes (Bar and CaMV 35S), self genes (SPS and Actin) in the rice grain and straw were used to evaluate gene persistence time during in vitro rumen fermentation process. Bacterial 16S rDNA gene was used to validate the quality of fluid-associated bacterial genomic DNA, which was extracted from the supernatant of the incubation media.
Table 1. Gene primers used to detect survival ability of target gene during in vitro incubation.
Conventional PCR for amplification of the Bar genes was carried out in an Eppendorf 5333 PCR apparatus (Eppendorf China Ltd., Germany) in a final volume of 25 µL, containing 1.5 mM MgCl2, 0.2mM dNTP, 0.3µM primers, 1.25 U of Taq Polymerase and 1×PCR buffer. All of the reagents were purchased from Promega (Promega Corporation, USA) except for the primers and DNA template. The primers were synthesised by TaKaRa (TaKaRa Bio INC., Japan). Conventional PCR for amplification of sucrose phosphate synthase (SPS), CaMV 35S, Actin, and 16S rDNA genes was the same as for the Bar gene, except for the concentrations of Mg2+ were set at 1.5, 1.8, 1.5 and 1.5 mM, respectively.
Conditions for PCR amplification of the Bar gene were set as follows: denaturing of DNA 1 cycle of 5 min at 95°C, 40 cycles of 30 s at 94°C, 30 s at 59°C, 60 s at 72°C, and a final extension at 72°C for 5 min. Conditions for PCR amplification of SPS, CaMV 35S, Actin and 16S rDNA genes were the same as Bar gene, except that the annealing temperature for SPS, CaMV 35S, and Actin gene was at 58, 56, 56 and 52°C, respectively. Ten µL of amplification products was electrophoresed in 2% agarose gels for 1 h at 40 mA, and stained with EtBr for visualisation.
3. Statistical analysis
Data of in vitro gas production of rice grain and straw were analysed using the statistical model of Y ij =µ+R I +V j +e ij , where µ is overall mean, R i is effect of replicate (i=1–3), V j is effect of variety (j=1–2), e ij is the random residual error. When variety effect was significant, differences among means were tested with Duncan's multiple range tests. Statistical significances were considered to exist if P<0.05.
4. Results
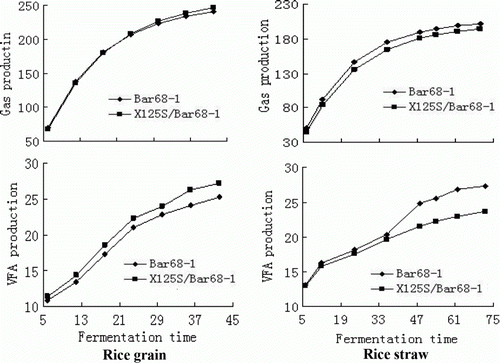
4.1. Gas and VFA production of rice grain and straw in in vitro system
Gas and VFA production of the rice grain and straw incubated in 30 mL of rumen fluid/buffer for various times are shown in . Gas production and VFA concentration of the rice grain ranged from 240 to 256 mL/g DM, and from 25.2 to 27.2 mM/L at 42 h incubation; gas production and VFA concentration of the rice straw ranged from 194 to 202 mL/g DM, and from 23.6 to 27.4 mM/L, respectively. There was no significant difference in gas production between Bar68-1 and X125S/Bar68-1 grains, whereas X125S/Bar68-1 grain produced more VFA consistently with time than Bar68-1 grain. X125S/Bar68-1 straw tended to produce less gas and VFA than Bar68-1 straw throughout the fermentation period,. In particular, VFA production of Bar68-1 straw was significantly higher than X125S/Bar68-1 straw at later stages of the fermentation.
4.2. Persistence time of the plant genes in incubation media
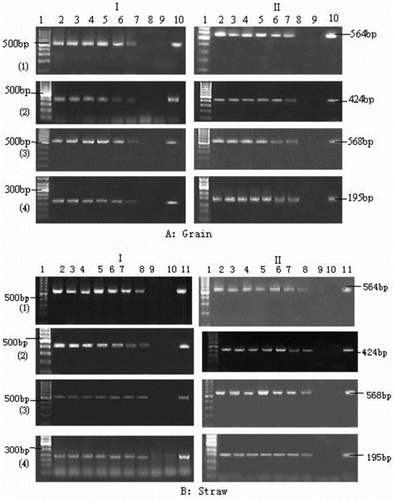
The presences of the foreign (Bar and CaMV 35S) and self (Actin and SPS) genes in the plant debris of rice grain and straw incubated for up to 42 and 70 h are shown in , respectively. The foreign Bar and CaMV 35S genes of grain could be detected at up to 36 h incubation, and were fully degraded after 42 h incubation. The self genes (Actin and SPS) of grain showed a similar degradation pattern. The foreign Bar and CaMV 35S genes, and the self genes (Actin and SPS) were detectable in the debris of Bar68-1 and X125S/Bar68-1 straw incubated up to 62 h, and degraded thoroughly at 70 h incubation. Furthermore, the same gene fragments of Bar68-1 and X125S/Bar68-1 were observed in both grain and straw.
Figure 2. Detection of foreign genes (Bar and CaMV35s) and self genes (Actin and SPS) in the debris of grain (A) and straw (B) of Bar68-1 and X125S/Bar68-1. The grain and straw were incubated in rumen fluid/buffer media for up to 42 and 70 h, respectively.
I: Bar68-1; II: X125S/Bar68-1.
(1): Amplified SPS gene fragment;
(2): Amplified Actin gene fragment;
(3): Amplified Bar gene fragment; and
(4): Amplified CaMV 35S promoter gene fragment.
A: Lan1, DNA markers; Lan2-8, target genes incubated for 6, 12, 18, 24, 30, 36 and 42 h; Lan9, negative control (no DNA template); Lan10, positive control (DNA isolated from Bar68-1 and X125S/Bar68-1 grain).
B: Lan1, DNA markers; Lan2-9, target genes incubated for 6, 12, 24, 36, 48, 60 and 70 h; Lan10, negative control (no DNA template); Lan11, positive control (DNA isolated from Bar68-1 and X125S/Bar68-1 straw).
4.3. Detection of dissociative gene fragments in supernatant of the incubation media
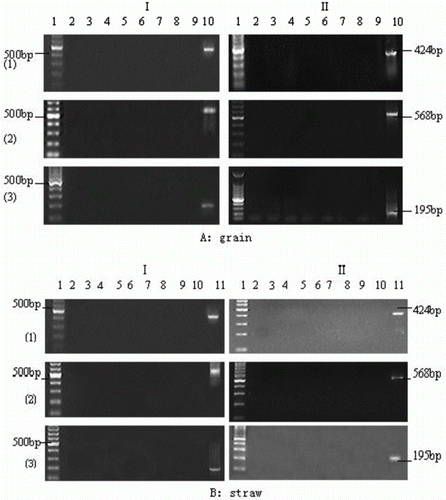
The free fragments of the self Actin gene and the foreign Bar and CaMV 35S genes in the grain and straw of Bar68-1 and X125S/Bar68-1 varieties presented in supernatants of the incubation media were determined using conventional PCR, and the results are shown in . No free gene fragment was detected in the supernatant throughout the incubation period.
Figure 3. Detection of dissociative genes (Actin, Bar and CaMV 35S) of grain and straw from Bar68-1 and X125S/Bar68-1 in supernatant of rumen fluid/buffer media.
I: Bar68-1; II: X125S/Bar68-1.
(1): Amplified Actin gene fragment;
(2): Amplified Bar gene fragment; and
(3): Amplified CaMV 35S gene fragment.
A: Lan1, DNA markers; Lan2-8, Amplified dissociative target genes incubated for 6, 12, 18, 24, 36 and 42 h; Lan9, negative control (no DNA template); Lan10, positive control (DNA isolated from Bar68-1 and X125S/Bar68-1 grain).
B: Lan1, DNA markers; Lan2-9, Amplified dissociative target genes incubated for 6, 12, 24, 36, 48, 54, 62 and 70 h; Lan10, negative control (no DNA template); Lan11, positive control (DNA isolated from Bar68-1 and X125S/Bar68-1 straw).
4.4. Detection of the foreign gene fragments in the ruminal bacteria
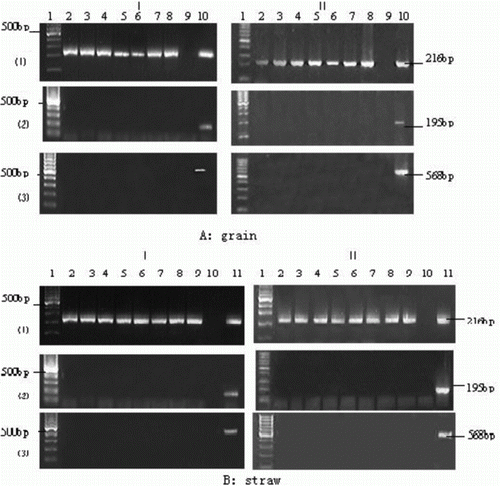
The foreign gene (Bar and CaMV 35S) fragments from the grains of straw of Bar68-1 and X125S/Bar68-1 varieties were determined in the liquid-associated bacterial genome, and the results are presented in . The 16S rDNA gene fragment of the ruminal liquid-associated bacteria could be detected at every point, demonstrating the high quality of the extracted ruminal bacteria genome, which could be used for PCR amplification. Conversely, Bar and CaMV 35S gene fragments were not detectable in the ruminal microbial genome at any time point.
Figure 4. Amplified 16s rRNA gene fragments from ruminal liquid-associated bacteria. A: Foreign Bar and CaMV 35S gene fragments were from grain of Bar68-1 and X125S/Bar68-1 in the ruminal microbe; B: Foreign Bar and CaMV 35S gene fragments were from straw of Bar68-1 and X125S/Bar68-1 in the ruminal microbe.
I: Bar68-1; II: X125S/Bar68-1.
(1): Amplified 16S rDNA gene fragment from the ruminal bacteria genome, which could be used to testify the extraction quality of bacteria genome;
(2): Amplified CaMV 35S promoter gene fragment; and
(3): Amplified Bar gene fragment.
A: Lan1, DNA markers; lan2-8, Amplified target gene fragments in the ruminal bacteria microbe genome incubated for 6, 12, 18, 24, 30, 36 and 42 h; Lan9, negative control (no DNA template); Lan10, positive control (DNA isolated from Bar68-1 and X125S/Bar68-1 grain).
B: Lan1, DNA markers; lan2-9, Amplified target gene fragments in the ruminal bacteria microbe genome incubated for 6, 12, 24, 36, 48, 54, 62 and 70; Lan10, negative control (no DNA template); Lan11, positive control (DNA isolated from Bar68-1 and X125S/Bar68-1 straw).
5. Discussion
An in vitro fermentation system was used in this research to test whether the plant genes could be transferred into the ruminal bacterial genome during fermentation. It was essential that the fermentation system performed normally, which was assessed by monitoring gas and VFA production. As shown in , gas and VAF production increased continuously with time, indicating that fermentation progressed consistently. The system was therefore, valid for the purpose of this research. It is interesting to note that gas and VFA production of the straw differed between the two rice varieties, but not in their grains. This finding is in agreement with our previous in vitro fermentation results for some conventional rice varieties (Tang et al. Citation2008; Cong et al. Citation2009). Changes in gas and VFA production could reflect variations in nutrient compositions of the grain and straw reported by Lin et al. (Citation2009), where chemical compositions (including crude protein, ether extract and gross energy) were similar between Bar68-1 and X125S/Bar68-1 grain, but the fibre contents in straw varied markedly. Lin et al. (Citation2009) reported that X125S/Bar68-1 straw contained higher NDF and ADF contents than Bar68-1 straw. The current results are supported by findings of De Boever et al. (Citation2005) and Getachew et al. (Citation2005), who suggested that gas production of feedstuffs was negatively correlated with NDF and ADF contents. Lin et al. (Citation2009) also pointed out that changes in chemical compositions of rice grain and straw might not be caused by the Bar gene but could mainly originate from the hybridisation female parent (X125S) under the same cultivation regimen. Thus, the current results suggested that Bar gene introduction might not influence in vitro fermentation characteristics of transgenic or transgenic hybridised grain and straw. Some previous studies also suggested that foreign gene transfer did not affect digestive characteristics of a transgenic plant and its native counterpart (Aulrich et al. Citation2002; Aumaitre et al. Citation2002).
The current study indicated that the foreign (Bar and CaMV 35S) and self (SPS and Actin) genes in both grain and straw of transgenic rice (Bar68-1) and transgenic hybrid (X125S/Bar68-1) had the same persistence time during in vitro fermentation process. Similar findings have been reported in literature. Sharma et al. (Citation2004) suggested that EPSPS gene in whole or cracked rapeseed could survive for 48 h in incubation with rumen fluid. Einspanier et al. (Citation2004) reported both the foreign and endogenous genes of transgenic maize followed similar degradation trends throughout the gastrointestinal tract of cattle and chicken. The longer survival time of the foreign and self genes in rice straw than in grain might be ascribed to the differences of physical structure of substrates, because rice straw is well known to be degraded slower than grain by the rumen microbes. Alternatively, the relatively long survival time of the rice foreign and self genes in the in vitro fermentation system might indicate that plant gene fragments probably escaped from rumen nuclease degradation and reached the lower digestive tract, and this would be a reason for some plant gene fragments could be detected in the animal body (Einspanier et al. Citation2001; Phipps et al. Citation2003). Therefore, the survival of these gene fragments in the lower digestive tract of animals needs further research
Although this study demonstrated that rice gene fragments could survive for up to 36 or 62 h in the in vitro fermentation, no free fragments of Bar, CaMV 35S, SPS and Actin genes were detected in supernatant of the rumen fluid/buffer media, which confirms that plant genes only existed in the fermentable substrates and were under the protection of nuclear or protein. This is consistent with the finding by Alexander et al. (Citation2002), who reported that any intact plant DNA found in the digesta was likely to be contained within intact plant cells. However, Duggan et al. (Citation2000) found that the plasmid-borne bla gene fragments could survive in the ovine rumen fluid for 30 min, and the maize-encoded bla gene could survive for 1 min. Duggan et al. also suggested that a plasmid-borne gene was a circular molecule and more stable than maize-encoded liner molecule. The above-mentioned two studies have proven that the naked free plant gene fragments may survive for a short time in the rumen fluid. However, we did not detect any free gene fragments in the in vitro system. Moreover, the current study and previous experiments of Duggan et al. (Citation2000), Alexander et al. (Citation2004) and Sharma et al. (Citation2004) were undertaken under the in vitro static conditions, whereas the ruminant's rumen is a dynamic fermentation pot with much inflow and outflow of feed stuffs (Dijkstra et al. Citation2002), which means there would be many gene fragments released from feed particles and the rumen microbes may consistently touch these gene fragments. Thus, research is still needed to study free gene fragment transformation that could occur in the dynamic conditions of the rumen.
The transformation of foreign and self genes from transgenic rice to the rumen bacteria under practical feeding situations is defined as natural transformation. The prerequisites for natural transformation include the availability of free DNA, the development of competent bacteria, and the uptake and integration of the captured DNA by bacteria (Thomas and Nielsen Citation2005). The results of the present study demonstrated that none of free gene fragments (including the foreign and self genes in rice grain and straw) could be detected in the rumen fluid/buffer, indicating that the first prerequisite for natural transformation did not exist (Duggan et al. Citation2000). Furthermore, the PCR detection results indicated that none of the foreign Bar or CaMV 35S gene fragments from the grain and straw of Bar68-1 and X125S/Bar68-1 was transformed into the rumen microbial genome, even if Bar and CaMV 35S gene fragments could survive up to 36 h for rice grain and 62 h for rice straw in in vitro incubation process. Although only one fragment of a gene was testified and found gene transfer did not occur in this study, we could not extrapolate that transformation did not exist for other genes. Sharma et al. (Citation2004) detected seven fragments of the whole sequence of foreign EPSPS gene from transgenic rapeseed by conventional PCR, and the seven fragments were all not detectable in the bacterial genome. Additionally, two copies of CaMV 35S-Bar-NOS foreign gene cassette (1.6 kp) in the transgenic rice (Bar68-1) genome accounted for only approximately 1/120000 of the rice genome (Rathore et al. Citation1993), which indicates that a low concentration of a foreign gene in a genome would be associated with a probability of the natural transformation in the rumen. Moreover, transformation of recombinant DNA into gut micro-organisms might be DNA size-dependent. The whole 1.6 kp transgenic cassette was easy to degrade into short fragments when it was incubated in the rumen and less likely to transform totally into the bacterial genome. Up until now, no experimental evidence has shown that the horizontal gene transfer of GM genes from plants to bacteria has occurred in the natural environment (Kay et al. Citation2002; Bennett et al. Citation2004; Sharma et al. Citation2004; Broothaerts et al. Citation2005).
It was reported that gene transformation could be completed within a very short time and that a plasmid DNA molecule could probably transform to rumen bacteria within 1 min (Mejean and Claverys Citation1993; Duggan et al. Citation2000). Although no free foreign gene fragment was detected in an in vitro fermentation in this study, gene fragments might exist soon before we begin to detect these gene fragments (Duggan et al. Citation2000). Thus, the transforming possibility of Bar and CaMV 35S genes in a shorter or longer culture time needs to be tested in the future.
6. Conclusion
Our results indicated that the foreign (Bar and CaMV 35S) and self (SPS and Actin) genes in transgenic rice (Bar68-1) and transgenic hybrid generation (X125S/Bar68-1) had the same digestive characteristics in the in vitro fermentation system. The foreign and self genes in the grain and straw had the same persistence time during the incubation. There were no free foreign or self gene fragments found in supernatant of the rumen fluid/buffer media, and no foreign and self gene fragments were detectable in the rumen microbe genome during the whole fermentation process. The results indicate that the transforming possibility of Bar and CaMV 35S gene fragments into the rumen microbe genome would be very low, and Bar and CaMV 35S transgenic rice and transgenic hybrid generation could be safe for the ruminal microbes under an in vitro culture system.
Acknowledgements
We wish to express our appreciation to the Chinese Academy of Sciences (No. KZCX2-YW-JS407 and No. KZCX2-YW-T07) for a financial support to this study.
References
- Alexander , TW , Sharma , R , Deng , MY , Whetsell , AJ , Jennings , JC , Wang , Y , Okine , E , Damgaard , D and McAllister , TA. 2004 . Use of quantitative real-time and conventional PCR to assess the stability of the cp4 epsps transgene from Roundup Ready® canola in the intestinal, ruminal, and fecal contents of sheep . Journal of Biotechnology , 112 : 255 – 266 . doi: 10.1016/j.jbiotec.2004.04.026
- Alexander , TW , Sharma , R , Okine , EK , Dixon , WT , Forster , RJ , Stanford , K and McAllister , TA. 2002 . Impact of feed processing and mixed ruminal culture on the fate of recombinant EPSP synthase and endogenous canola plant DNA . FEMS Microbiology Letters , 214 : 263 – 269 . doi: 10.1111/j.1574-6968.2002.tb11357.x
- Aulrich , K , Böhme , H , Daenicke , R , Halle , I and Flachowsky , G. 2002 . Novel feeds—a review of experiments at our institute . Food Research International , 35 : 285 – 293 . doi: 10.1016/S0963-9969(01)00198-3
- Aumaitre , A , Aulrich , K , Chesson , A , Flachowsky , G and Piva , G. 2002 . New feeds from genetically modified plants: substantial equivalence, nutritional equivalence, digestibility, and safety for animals and the food chain . Livestock Production Science , 74 : 223 – 238 . doi: 10.1016/S0301-6226(02)00016-7
- Bennett , PM , Livesey , CT , Nathwani , D , Reeves , DS , Saunders , JR and Wise , R. 2004 . An assessment of the risks associated with the use of antibiotic resistance genes in genetically modified plants: report of the working party of the British Society for Antimicrobial Chemotherapy . Journal of Antimicrobial Chemotherapy , 53 : 418 – 431 . doi: 10.1093/jac/dkh087
- Bertolla , F , Kay , E and Simonet , P. 2000 . Potential dissemination of antibiotic resistance genes from transgenic plants to microorganisms . Infection Control and Hospital Epidemiology , 21 : 390 – 393 . doi: 10.1086/501779
- Bertolla , F and Simonet , P. 1999 . Horizontal gene transfers in the environment: natural transformation as a putative process for gene transfers between transgenic plants and microorganisms . Research in Microbiology , 150 : 375 – 384 . doi: 10.1016/S0923-2508(99)80072-2
- Broothaerts , W , Mitchell , HJ , Weir , B , Kaines , S , Smith , LM , Yang , W , Mayer , JE , Roa-Rodriguez , C and Jefferson , RA. 2005 . Gene transfer to plants by diverse species of bacteria . Nature , 433 : 629 – 633 . doi: 10.1038/nature03309
- Cong , ZH , Tang , SX , Tan , ZL , Sun , ZH , Zhou , CS , Han , XF , Wang , M and Ren , GP. 2009 . Effects of different nonionic surfactants on in vitro fermentation characteristics of cereal straws . Jounal of Animal Science , 87 : 1085 – 1096 . doi: 10.2527/jas.2008-1316
- De Boever , JL , Aerts , JM , Vanacker , JM and De Brabander , DL. 2005 . Evaluation of the nutritive value of maize silages using a gas production technique . Animal Feed Science and Technology , 123–124 : 255 – 265 . doi: 10.1016/j.anifeedsci.2005.04.019
- De Vega , A , Gasa , J , Castrillo , C and Guada , JA. 1998 . Passage through the rumen and the large intestine of sheep estimated from faecal marker excretion curves and slaughter trials . British Journal of Nutrition , 80 : 381 – 389 . doi: 10.1079/096582198388337
- Dijkstra , J , Mills , JA and France , J. 2002 . The role of dynamic modelling in understanding the microbial contribution to rumen function . Nutrition Research Review , 15 : 67 – 90 . doi: 10.1079/NRR200237
- Duggan , PS , Chambers , PA , Heritage , J and Forbes , JM. 2000 . Survival of free DNA encoding antibiotic resistance from transgenic maize and the transformation activity of DNA in ovine saliva, ovine rumen fluid and silage effluent . FEMS Microbiology Letters , 191 : 71 – 77 . doi: 10.1111/j.1574-6968.2000.tb09321.x
- Einspanier , R , Klotz , A , Kraft , J , Aulrich , K , Poser , R , Schwägele , F , Jahreis , G and Flachowsky , G. 2001 . The fate of forage plant DNA in farm animals: a collaborative case–study investigating cattle and chicken fed recombinant plant material . Euroup Food Research Technology , 212 : 129 – 134 . doi: 10.1007/s002170000248
- Einspanier , R , Lutz , B , Rief , S , Berezina , O , Zverlov , V , Schwarz , W and Mayer , J. 2004 . Tracing residual recombinant feed molecules during digestion and rumen bacterial diversity in cattle fed transgene maize . Euroup Food Research Technology , 218 : 269 – 273 . doi: 10.1007/s00217-003-0842-9
- Gay , PB and Gillespie , SH. 2005 . Antibiotic resistance markers in genetically modified plants: a risk to human health? . The Lancet Infection Disease , 5 : 637 – 646 . doi: 10.1016/S1473-3099(05)70241-3
- Gebhard , F and Smalla , K. 1998 . Transformation of Acinetobacter sp. strain BD413 by transgenic sugar beet DNA . Applied and Environmental Microbiology , 64 : 1550 – 1554 .
- Getachew , G , DePeters , EJ , Robinson , PH and Fadel , JG. 2005 . Use of an in vitro rumen gas production technique to evaluate microbial fermentation of ruminant feeds and its impact on fermentation products . Animal Feed Science Technology , 123–124 : 547 – 559 . doi: 10.1016/j.anifeedsci.2005.04.034
- ISA , 2005 . Management strategies of breeding GMP and conducting animal studies to evaluateGMP . Changsha : Institute of Subtropical Agriculture, Chinese Academy of Sciences .
- Jonas , DA , Elmadfa , I , Engel , KH , Heller , KJ , Kozianowski , G , Konig , A , Muller , D , Narbonne , JF , Wackernagel , W and Kleiner , J. 2001 . Safety considerations of DNA in food . Annals of Nutrition and Metabolism , 45 : 235 – 254 . doi: 10.1159/000046734
- Kay , E , Vogel , TM , Bertolla , F , Nalin , R and Simonet , P. 2002 . In situ transfer of antibiotic resistance genes from transgenic (transplastomic) tobacco plants to bacteria . Applied and Environmental Microbiology , 68 : 3345 – 3351 . doi: 10.1128/AEM.68.7.3345-3351.2002
- Khush , GS. 1997 . Origin, dispersal, cultivation and variation of rice . Plant Molecular Biology , 35 : 25 – 34 . doi: 10.1023/A:1005810616885
- Lin , B , Tan , ZL , Xiao , GY , Wang , M , Cong , ZH , Wang , SP , Tang , SX , Zhou , CS , Sun , ZH and Wang , WJ. 2009 . Evaluation of compositional and nutritional equivalence of genetically modified rice to conventional rice using in situ and in vitro techniques . Journal of the Science of Food and Agriculture , 89 : 1490 – 1497 . doi: 10.1002/jsfa.3613
- Lorenz , MG and Wackernagel , W. 1994 . Bacterial gene transfer by natural genetic transformation in the environment . Microbiological Reviews , 58 : 563 – 602 .
- Martinez , ME , Ranilla , MJ , Ramos , S , Tejido , ML , Saro , C and Carro , MD. 2009 . Evaluation of procedures for detaching particle-associated microbes from forage and concentrate incubated in Rusitec fermenters: efficiency of recovery and representativeness of microbial isolates . Journal of Animal Science , 87 : 2064 – 2072 . doi: 10.2527/jas.2008-1634
- Mejean , V and Claverys , JP. 1993 . DNA processing during entry in transformation of Streptococcus pneumoniae . The Journal of Biological Chemistry , 268 : 5594 – 5599 .
- Mercer , DK , Scott , KP , Bruce-Johnson , WA , Glover , LA and Flint , HJ. 1999 . Fate of free DNA and transformation of the oral bacterium Streptococcus gordonii DL1 by plasmid DNA in human saliva . Applied and Environmental Microbiology , 65 : 6 – 10 .
- Phipps , RH , Deaville , ER and Maddison , BC. 2003 . Detection of transgenic and endogenous plant DNA in rumen fluid, duodenal digesta, milk, blood, and feces of lactating dairy cows . Journal of Dairy Science , 86 : 4070 – 4078 . doi: 10.3168/jds.S0022-0302(03)74019-3
- Prudhomme , M , Libante , V and Claverys , JP. 2002 . Homologous recombination at the border: insertion–deletions and the trapping of foreign DNA in Streptococcus pneumoniae . Proceedings of the National Academy of Sciences United States of America. , 99 : 2100 – 2105 . doi: 10.1073/pnas.032262999
- Rathore , KS , Chowdhury , VK and Hodges , TK. 1993 . Use of bar as a selectable marker gene and for the production of herbicide-resistant rice plants from protoplasts . Plant Molecular Biology , 21 : 871 – 884 . doi: 10.1007/BF00027118
- Ren , X , Zhu , X , Warndorff , M , Bucheli , P and Shu , Q. 2006 . DNA extraction and fingerprinting of commercial rice cereal products . Food Research International , 39 : 433 – 439 . doi: 10.1016/j.foodres.2005.09.006
- Sharma , R , Alexander , TW , John , SJ , Forster , RJ and McAllister , TA. 2004 . Relative stability of transgene DNA fragments from GM rapeseed in mixed ruminal cultures . British Journal of Nutrition , 91 : 673 – 681 . doi: 10.1079/BJN20041100
- Tang , SX , Tayo , GO , Tan , ZL , Sun , ZH , Shen , LX , Zhou , CS , Xiao , WJ , Ren , GP , Han , XF and Shen , SB. 2008 . Effects of yeast culture and fibrolytic enzyme supplementation on in vitro fermentation characteristics of low-quality cereal straws . Journal of Animal Science , 86 : 1164 – 1172 . doi: 10.2527/jas.2007-0438
- Thomas , CM and Nielsen , KM. 2005 . Mechanisms of, and barriers to, horizontal gene transfer between bacteria . Nature Reviews Microbiology , 3 : 711 – 721 . doi: 10.1038/nrmicro1234
- Thompson , CJ , Movva , NR , Tizard , R , Crameri , R , Davies , JE , Lauwereys , M and Botterman , J. 1987 . Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus . EMBO Journal , 6 : 2519 – 2523 .
- Wang , M , Hu , Y , Tan , ZL , Tang , SX , Sun , ZH and Han , XF. 2008 . In situ ruminal phosphorus degradation of selected three classes of feedstuffs in goats . Livestock Science , 117 : 233 – 237 . doi: 10.1016/j.livsci.2007.12.016