Abstract
This study was conducted to determine the effects of supplementing threonine (Thr) at different levels in the diets on growth performance and immune responses of broiler chickens challenged with infectious bursal disease (IBD) and also to estimate Thr requirement based on different response criterion. A total of 300 one-day-old male broiler chicks were assigned to one of the six dietary treatments. Chickens were fed eight graded levels of Thr: 0.60, 0.67, 0.74, 0.81, 0.88 or 0.95% from day 21 to 42 of age. On day 28, all birds were challenged with a commercial live-IBDV vaccine. Body weight gain (BWG), feed conversion ratio (FCR) and carcass characterisation (breast, thigh and fat pad percentage) were significantly influenced by Thr levels. Increasing advisable level up to 0.81% of the diet resulted in significant improvement in the FCR and BWG. Thr supplementation had great effects on the antibody titer against IBD; the broilers receiving the Thr levels of higher than National Research Council (NRC) recommendation have a higher IBD antibody titer than those that received lower levels of Thr. The highest (0.8224±0.0211) and the lowest (0.7240±0.0140) were estimated with straight broken-line analysis for immune response (14 days post-challenge) and breast weight, respectively. The results obtained in the present study indicated that Thr requirements of broiler based on the recommendation of NRC are not sufficient to meet the requirement of the new commercial broiler companies under stress and non-hygienic conditions. The best level of Thr based on the current study was 0.81% for support growth performance and immune function.
1. Introduction
Dietary amino acid concentration should closely meet maintenance and tissue accretion needs of commercial broilers, especially towards the middle and end of the growing period. Inadequate formulation of dietary amino acids impairs protein utilisation and increases total nitrogen excretion. Threonine (Thr) may be considered as the third limiting amino acid in poultry diets based on corn, wheat and soybean meal, following Met and Lys (Dozier et al. Citation2000; Rosa & Pesti Citation2001). Kidd (Citation2000) reported that Thr deficiency resulted in decreasing the utilisation of total sulphur AA and Lys. Optimal levels of these amino acids are needed to support optimum growth and health of broilers (Rehman et al. Citation2012). Nutrient requirement standards have been reported by the National Research Council (NRC Citation1994) and through more than 28 years due to genetic selection, management practices and feed formulation changes birds have more rapid growth and better performance than previous years (Williams et al. Citation2000). On the other hand, NRC recommendations are usually based on the needs of healthy birds under ideal conditions, but birds in commercial systems are normally exposed to different kinds of stresses, diseases and also combinations of environmental conditions. The Lys and sulphur amino acid requirements have been evaluated extensively, but less information is available on the Thr requirements of chicks (Webel et al. Citation1996). Evaluated Thr requirement is based on the performance for exam. Rosa and Pesti (Citation2001) suggested 0.69% Thr for growth and 0.68% for feed conversion ratio (FCR) of growing chickens (classic strains) in the starter period, whereas, in poultry production, it is very important to improve immunity so as to prevent infectious diseases. Minimising immunosuppression and its impact is an important strategy for success in the broiler industry. However, strategies to control immunosuppression are largely based on vaccination programmes for broiler breeders and broiler progeny and management to minimise stress during rearing (Fussell Citation1998). Utilisation of immunostimulants is one solution to improve the immunity of animals and to decrease their susceptibility to infectious diseases. There are extremely important interactions, synergisms and antagonisms between nutrition and immunity that markedly affect productivity of poultry. Experiments in chickens and mammalian species have shown that protein or amino acid deficiencies may reduce the circulating antibodies available to challenge organisms. Minimum requirements for a given nutrient for maximum production are fully established (NRC Citation1994). On the other hand, the National Research Council (NRC) recommendation are usually based on the needs of healthy birds under ideal management, but birds in commercial systems are normally exposed to different kinds of stresses that it may be necessary for new research to conduct multiple focus and attention to the actual broiler requirements. However, in many cases, it is not known whether the requirement values that maximise productivity in healthy, unchallenged birds are optimal for immunocompetence and disease resistance. There is some evidence that essential amino acids levels in the feed higher than NRC specifications are needed to achieve optimal growth performance, immunocompetence and disease resistance (Kidd et al. Citation2001; Quentin et al. Citation2005; Taghinejad-Roudbaneh et al. Citation2011). Therefore, it may be necessary for new research to conduct multiple focus and attention to the actual broiler requirements. Therefore, the present experiment was conducted to determine Thr requirement based on immune responses of broiler chickens challenged with infectious bursal disease (IBD).
2. Materials and methods
A total of 500 day-old male broiler chicks (Ross 308) were obtained from a local hatchery. Chicks were raised from 1 to 21 d of age before commencement of the trial. During this period, the birds were submitted to conventional broiler chicken management and housed in floor pens (with 1.5 m2 area per each pen) in an environmentally controlled broiler house with litter floors with wood shavings as litter material. The relative humidity was between 75–85%. Feed and water were provided ad libitium, and birds were maintained on 23 light:1 dark schedule lighting. They received a commercial broiler starter diet, formulated to meet or exceed the nutritional requirements of broilers, as recommended by the NRC (Citation1994). After an overnight fast, at 21-d of age, 300 chicks of similar weight were randomly assigned to 30 clean pens in the same broiler house for the starter period. Average body weights (means±standard error) at d 21 were 730±30 g. The ambient temperature was gradually decreased from 32°C when the birds were 1 d old to 22°C when 28 d old. The protein and AA levels of the corn, soybean meal and corn gluten meal were analysed before diets formulation. Experimental diet was formulated to meet or exceed the NRC (Citation1994) nutrition recommendations from 21 to 42 d of age for all nutrients except Thr (). Dietary nitrogen (N×6.25) was measured by the Kjeldahl procedure. Dietary amino acid content was determined by ion-exchange chromatography on an autoanalyzer. The analysed values of protein and amino acid contents were agree with calculated values ().
Table 1. Ingredients and nutrient composition of starter and grower diets.
Treatments were achieved by the addition of crystalline L-Thr (98.5% Thr) at the expense of inert filler in the test diet containing 0.6% Thr () to give 0.60, 0.67, 0.74, 0.81, 0.88 or 0.95% Thr from day 21–42 of age. No antimicrobial, anticoccidial drugs or feed enzymes were included in the basal diets. The chicks were vaccinated against Newcastle disease (Animal Health, Fort Dodge, Iowa, USA) on day 7 (Intraocular) and on day 21(intranasal).
The chickens were weighed individually weekly. Feed intake was recorded weekly and FCR was calculated. Mortality was recorded daily in each subgroup. On d 42, three randomly chosen chickens from each pen were wing-banded and moved to separate floor pens for an overnight fast (approximately 12 h); water was provided ad libitum. The following morning, chickens were randomly crated and transported to the processing room where each was weighed, euthanised by cervical dislocation and then scalded, defeathered and eviscerated. Abdominal fat pads and livers were collected from the birds after they were partially eviscerated. The weights of thymus, spleen and bursa of Fabricius were recorded. Organ weights were expressed on a relative body weight basis. Carcasses were weighed and chilled on ice in a walk-in cooler at 5°C overnight. The following day, breast (without skin) and thigh were excised, and weights were recorded. The abdominal fat pad percentage was calculated based on chilled carcass weight.
2.1. Challenge protocol
On day 28, all birds were challenged by oral route with a commercial live-IBD vaccine. The strain was characterised as an intermediate virulent classical strain. The content of the 1000 dose of IBD vaccine vial was reconstituted in 100 mL distilled water, and each bird was inoculated with 1 mL IBD virus into the lumen of the crop by oral gavage, and finally each bird received a dose ten times greater than the standard IBD vaccine.
2.2. Serology and immune responses
On the 28th, 35th and 42nd day ten birds from each group were chosen at random, and blood samples were collected from the brachial vein. Serum was separated by centrifugation (3000 g, 15 min), and antibody titre against IBD was performed using commercially available ELISA kits (IDEXX, Labs Inc., Westbrook, Maine, USA) according to the manufacturer's instructions. ELISA absorbency was measured at 650 nm using an ELISA reader (Bio-Tek Instruments Inc. ELX 800; Winooski, Vermont) by standard procedures. Serum albumin, globulin, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured by specific commercial kits (Roche Diagnostica, Basel, Switzerland) using an autoanalyzer (HITACHI 902 automatic autoanalyzer).
2.3. Statistical analysis
The experimental design was a completely randomised design. The experimental unit was the pen mean. All data were analysed by two-way ANOVA, using the GLM procedure of SAS (SAS Institute Citation2004). The requirement based on BWG, FCR, carcass breast, thigh weight and immune response to IBD challenge was determined applying a non-linear procedure of SAS. The iterative procedure makes repeated estimates for coefficients and minimises residual error until the best-fit lines are achieved: one line with a slope of zero and the other with a marked slope. The two lines are fitted to the values using the following equations:
3. Results
Responses to dietary Thr level were significant (P<0.05) for BWG, FCR, feed intake, percentage of fat pad carcass efficiency body, breast and thigh weight (). The BWG, BW, carcass efficiency, breast and thigh weight and feed intake increased with increasing Thr level in diet. The highest BWG (1756.6 g), BW (2484.7 g) and carcass efficiency (68.6%) were in diet containing 0.88% Thr, and the highest breast (427 g) and feed intake (3500 g) were in diet containing 0.95% Thr, and the highest thigh weight (433.0 g) was in diet containing 0.81% Thr. FCR and percentage of fat pad decreased as Thr increased in the diet, and lowest level of FCR was in diet containing 0.81% Thr and the lowest percentage of fat pad was in diet containing 0.74% Thr. However, spleen percentage was not affected by Thr level in diet. The BWG, BW and FCR in diet containing 0.81% Thr did not significantly differ with higher level of Thr in diet but significantly (P<0.05) differ with diet containing lower levels of Thr. The feed intake, breast and thigh weight in diet containing 0.74% Thr did not significantly differ (P<0.05) with higher level of Thr in diet but significantly differ with diet containing lower levels of Thr ().
Table 2. The effects of dietary Thr on BWG, feed intake, FCR, breast meat, thigh weight, carcass efficiency and abdominal fat pat percentage of male broiler chicks (21–42 d).
The effect of Thr level on liver function enzymes, blood parameters and serum antibody titer were significant (P<0.05) (). The highest AST, LDH and ALP, were observed in the chicken fed with diet containing 0.81% Thr. However, the highest ALT was found in the group with 0.88% Thr supplementation, and the chickens with 0.74% Thr supplemented diet had the highest serum total protein. Immune response to live-IBDV vaccine significantly increased with increasing Thr in the diets (P<0.001). The diet containing 0.81% Thr had the highest serum antibody titer (log2) against IBDV in a pre-challenge stage (3.00), 7 days post-challenge (3.11) and 14 days post-challenge (3.80). On the other hand, the lowest level of antibody in these stages were found in a group with the diet containing 0.6% Thr (81% of NRC recommendation).
Table 3. The effects of dietary Thr on liver function enzymes, blood parameters and serum antibody titer (log2) of broiler chickens challenged with IBD.
Regression analyses showed significant linear effects (P<0.05) due to dietary Thr concentrations for all response parameters except for ALP and serum globulin. A quadratic effect (P<0.05) due to dietary Thr concentration was noted for all parameters except for carcass efficiency, ALT, LDH, ALP and serum globulin ( and ).
The Thr requirement was determined as a percentage of the diets using linear and quadratic broken-line analysis with different response criterion ( and –). The quadratic broken-line model estimated higher requirements than the straight broken-line model for all response criterions (). The highest (0.8224±0.0211) and the lowest (0.7240±0.0140). Thr requirements with straight broken-line analysis were estimated for immune response (14 days post-challenge) and breast weight, respectively. And the highest (0.9636±0.0742) and the lowest (0.7964±0.0216). Thr requirements with quadratic broken-line analysis were estimated for immune response 14 day post-challenge and breast weight, respectively. All estimated requirements except estimated based on FCR and breast weight were higher than NRC (1998) recommendation.
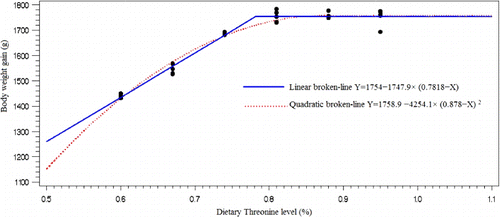
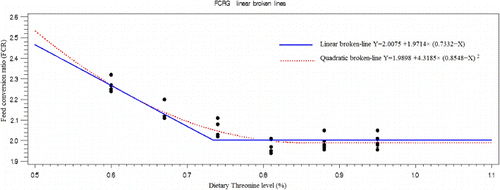
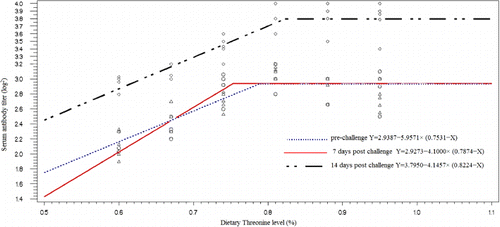
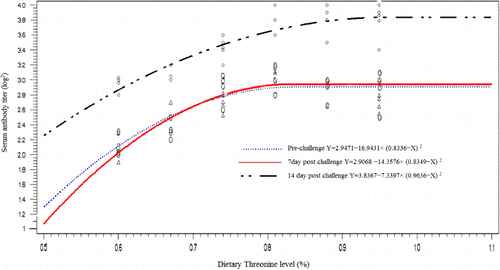
Table 4. Dietary Thr requirement based on broken-line model analyses.
4. Discussion
Increasing Thr level in the diet improved breast meat weight (from 360.8 to 427 g), BWG (from 1441.8 to 1756.6 g), thigh weight (from 392.6 to 433 g) and decreased FCR (from 2.27 to 1.98). Similarly Mack et al. (Citation1999) and Dozier et al. (Citation2000, Citation2001) indicated that supplemental Thr improved breast percentage. In contrast to our results, Dozier et al. (Citation2000) reported that Thr supplementation had no significant effect on the proportion of drumsticks and thighs. Also Dozier et al. (Citation2000, Citation2001) indicated that total carcass meat yield was not affected by Thr concentration. Lemme (Citation2003) reported that FCR of male broilers optimised as dietary Thr was increased from 0.55 to 1.05%. Tugay et al. (Citation2009) showed that the highest body weight gain was at 0.75%, and better feed efficiency was in 0.85% Thr for broilers occurred. In the current study, increasing the Thr concentration of the diet from 0.6 to 0.81% improved the growth rate, body weight and FCR of broilers. In addition, increasing the Thr concentration of the diet from 0.6 to 0.74% improved the feed intake, carcass efficiency and breast and thigh weight of broilers.
Thr supplementation did not significantly alter the abdominal fat proportion, as reported in earlier studies (Dozier et al. Citation2001; Rosa & Pesti Citation2001). The effect of dietary Thr on absolute and relative abdominal fat pad weight in broilers has typically been inconsistent (Kidd & Kerr Citation1997; Corzo et al. Citation2003; Kidd et al. Citation2004). No dietary Thr effect was observed for relative weights of spleen (). Results are in agreement with those presented by Kidd et al. (Citation2001).
The estimated Thr requirement based on BWG (0.7818±0.00681) was higher than that of breast meat (0.7240±0.0140), FCR (0.7332±0.0216), feed intake (0.7607±0.0244), thigh (0.7595±0.0122) and body weight (0.7745±0.00696). Dietary Thr needs observed for live performance measurements are in agreement with those previously published for birds during the same age period (Webel et al. Citation1996; Penz et al. Citation1997; Mack et al. Citation1999). Webel et al. (Citation1996) fed graduations of Thr to Ross×Hubbard broilers from d 21 to 42 in a corn–peanut meal-based diet and reported that optimal feed conversion occurred when dietary Thr was 0.70%. In addition, Kidd and Kerr (Citation1997) fed graduations of Thr to Ross ×Ross 308 male broilers in a corn–peanut meal-based diet and determined that the live performance needs during 30 to 42 d are satisfied with 0.70% total Thr. These live performance needs for Thr (Kidd & Kerr Citation1997) are in agreement with the former estimates (Webel et al. Citation1996; Penz et al. Citation1997), since they were obtained in the last half of the 21- to 42-d period. Similar to the result of the current study, Kidd and Kerr (Citation1997) evaluated carcass traits and found that a higher level of Thr (0.78% total of diet) was needed to yield good breast meat accretion. Amino acid requirements in growing animals depend on several factors like genotype, age and sex (Samadi & Liebert Citation2007; Muhl & Liebert Citation2008; Rehman et al. Citation2012). The NRC (Citation1994) suggest that 21- to 42-d-old broilers fed diets containing 3200 kcal/kg should receive a total dietary Thr level of 0.74% of diet. However, Ojano-Dirain and Waldroup (Citation2002) suggested that the modern rapidly growing broiler may have Thr requirements greater (0.78%) than those generally recommended (0.74%) by NRC (Citation1994). Lemme (Citation2001) reported that depending on the performance criteria chosen, the optimum dietary Thr levels for 20- to 42-d-old broilers to achieve 95% of the asymptotic response for BWG, FCR and breast meat yield were 0.66, 0.68 and 0.70%, respectively, compared to the 0.74% suggested by NRC (Citation1994).
The method of statistical evaluation can influence the estimation of an amino acid and requirement (Taghinejad-Roudbaneh et al. Citation2011). The most common statistical models used to determine amino acid requirements are the broken-line (one-slope), two-slope, quadratic, and ascending quadratic with plateau models (Vedenov & Pesti Citation2008). Estimated Thr requirement values with quadratic broken-line model in all response criterions in this experiment were higher than estimated values with straight broken-line model (). The result of this experiment closed with the results of Barbour et al. (Citation1993) and Taghinejad-Roudbaneh et al. (Citation2011) that found statistical evaluation influences the estimate of an amino acid requirement and the quadratic procedure estimates were higher for all protein sources when compared with straight broken-line model. In addition, Vazquez and Pesti (Citation1997) concluded that ‘ascending quadratic with plateau’ model may theoretically be more realistic and adequate than the ‘ascending line with plateau’ model for predicting the amino acid requirement for broiler chicks.
Additionally, environmental conditions and graded stimulation of the immune system may also affect requirement studies (Kidd et al. Citation2003).Thr participates in protein synthesis and its catabolism generates many products important in metabolism (i.e., glycine, acetyl-Coa and pyruvate). Thr serves as a component of body protein and a precursor of glysine and serine, is involved in immune responses and needed in gastrointestinal mucin production (Lemme Citation2003). Thr is a major component of intestinal mucin and plasma α-globulin in animals (Kim et al. Citation2007). By way of protein synthesis and cellular signalling mechanisms, Peng et al. (Citation2007) show that addition of 2 mM-Thr to the culture medium prevented apoptosis, stimulated cell growth and promoted antibody production in lymphocytes. Bhargava et al. (Citation1971) found that Thr need in chickens for antibody production to Newcastle disease virus was higher than that for growth. Thr is the most abundant essential amino acid in immunoglobulin protein, and Defa et al. (Citation1999) show that growing pigs fed with Thr deficient diets have a significant lower plasma concentration of total or specific IgG titre following bovine serum albumin injection and swine fever vaccination. Wang et al. (Citation2006) demonstrated that the requirement to optimize immunity was higher than to maximize weight gain and FCR in pig, as the intake of true ileal digestible Thr increased from 4.1 to 6.6g per day, serum IgG concentrations increased in response to increased intake of true ileal digestible Thr. Thr is a major component of plasma γ-globulin in poultry (Tenenhouse & Deutsch Citation1966). Bhargava et al. (Citation1971) reported that antibody titers of chicks increased as dietary Thr increased. With increasing Thr level in diet, immune responses against IBVD were improved (). NRC requirements for amino acids and protein are designed to support maximum growth and production under ideal condition and good maintenance. The estimated Thr requirements vary with response criterion, and when requirement was estimated based on immune response 14 days post-challenge with IBVD, this value was higher than other response criterions ( and and ). The control of immunosuppressive diseases is of prime importance for the nascent poultry industry in developing countries. In this regard, the improvement of feed formulation and enhancement of genetic resistance to economically important diseases should be considered (Bumstead et al. Citation1991). The resistance against infectious challenges requires an intense response orchestrated by the immune system. From the nutritional standpoint, feed substrates (amino acids, energy and enzymes) are needed to activate such a response (Rubin et al. Citation2007). Minimum requirements for a given nutrient for maximum production are fully established (NRC Citation1994). As a result of the current study, nutrition recommended by NRC are adequate for healthy birds under ideal management, but the combination of environment condition and disease increase the requirement for Thr ( 0.73% vs. 0.82%).
From the nutritional standpoint, amino acids are needed for proliferation of lymphocytes, establishment of germinative centres in the bursa of Fabricius to refine immunoglobulin affinity, recruitment of new bone marrow monocytes and heterocytes and synthesis of effector molecules (immunoglobulins, nitric oxide, lysozyme and complement) and communication molecules (cytokines and eicosanoids, for instance). Methionine plays an important role in humoral and cellular immune responses (Swain & Johri Citation2000; Shini et al. Citation2005). Additionally, there is evidence that Thr modulates immune function in livestock (Li et al. Citation2007), and immune system is sensitive to dietary Thr intake (Li et al. Citation1999). It has been reported that Thr is a major component of intestinal mucin and plasma γ-globulin in animals (Kim et al. Citation2007). Indeed, serum antibody titers increased with increasing dietary intake of Thr in chickens infected with the Newcastle disease virus (Bhargava et al. Citation1971). Also, dietary supplementation with Thr increased serum levels of IgG in sows (Cuaron et al. Citation1984). Further, increasing dietary Thr intake increased antibody production, serum IgG levels and jejunal mucosal concentrations of IgG and IgA (Wang et al. Citation2006). Furthermore, it should be notified that dietary methionine can influence the metabolism of other amino acids (Moritoki & Yoshida Citation1970; Sanchez et al. Citation1972).
It has been demonstrated that the activities of many liver enzymes involved in the catabolism of amino acids decrease when a low protein diet is fed, but increase with high protein intakes (Muramatsu et al. Citation1971). In our experiment, liver enzymes were affected by Thr level of diet, whereas in the group receiving 0.81% Thr, we found a significant raise in the concentration of ALT, AST and LDH. The findings may also explain that the current NRC recommendations for thr in broiler are probably not appropriate for broiler health under stress and diseases condition. Although, it should be noted that the concentration of ALT, AST and LDH in the group receiving 0.6% Thr also was in the normal range (Marjanovic et al. Citation1975; Chattopadhyay et al. Citation2006).
5. Conclusion
In conclusion, the results obtained on the present study indicated that Thr requirements of broiler based on recommendation of the NRC (Citation1994) are sufficient to meet the requirements of the new commercial poultry and commercial broiler companies under hygienic condition. However, it may reduce profitability under conditions of disease or if the broilers encounter the stress conditions. Therefore, in order to overcome this problem, Thr level during non-hygienic should be increased. The best level of Thr based on current study is 0.81% for support growth performance and immune function.
Acknowledgement
This work is supported by the Tabriz Branch, Islamic Azad University, and by an endowment from the Animal Science Department of agricultural faculty. The author would like to thank Mr. Mehdi Alizade for his collaborations.
References
- Barbour G, Latshaw JD, Bishop B. 1993. Lysine requirement of broiler chicks as affected by protein source and method of statistical evaluation. Br Poult Sci. 34:747–756. 10.1080/00071669308417633
- Bhargava KK, Hanson RP, Sunde ML. 1971. Effects of threonine on growth and antibody production in chickens infected with Newcastle disease virus. Poult Sci. 50:710–713. 10.3382/ps.0500710
- Bumstead N, Millard BJ, Barrow BA, Cook JKA. 1991. The genetic basis of disease resistance in chickens. In: Axford editor, Breeding for disease resistance in farm animals. England, Wallingford: CAB International; p. 10–23.
- Chattopadhyay K, Mondal MK, Roy B. 2006. Comparative efficacy of Dl-methionine and herbal methionine on performance of broiler chicken. Int J Poult Sci. 5:1034–1039. 10.3923/ijps.2006.1034.1039
- Corzo A, Kidd MT, Kerr BJ. 2003. Threonine need of growing female broilers. Int J Poul Sci. 2:367–371. 10.3923/ijps.2003.367.371
- Cuaron JA, Chapple RP, Easter RA. 1984. Effect of lysine and threonine supplementation of sorghum gestation diets on nitrogen balance and plasma constituents in first-litter gilts. J Anim Sci. 58:631–637.
- Defa L, Changting X, Shiyan Q, Jinhui Z, Johnson EW, Thacker PA. 1999. Effects of dietary threonine on performance, plasma parameters and immune function of growing pigs. Anim Feed Sci Technol. 78:179–188. 10.1016/S0377-8401(99)00005-X
- Dozier WA, Moran ET, Kidd MT. 2000. Threonine requirement of broiler males from 42 to 56 d in a summer environment. J Appl Poul Res. 9:496–500.
- Dozier WA, Moran ET, Kidd MT. 2001. Comparisons of male and female broiler responses to dietary threonine from 42–56 days of age. J Appl Poul Res. 10:53–59.
- Fussell LW. 1998. Poultry industry strategies for control of immunosuppressive diseases. Poult Sci. 77:1193–1196.
- Kidd MT. 2000. Nutritional considerations concerning threonine in broilers. World's Poult Sci J. 56:139–151. 10.1079/WPS20000011
- Kidd MT, Barber SJ, Virden WS, Dozier WA, Chamblee DW, Wiernusz C. 2003. Threonine responses of Cobb male finishing broilers in differing environmental conditions. Anim Feed Sci Technol. 12:115–123.
- Kidd MT, Corzo A, Hoehler D, Kerr BJ, Barber SJ, Branton SL. 2004. Threonine needs of broiler chickens with different growth rates. Poul Sci. 83:1368–1375.
- Kidd MT, Gerard PD, Heger J, Kerr BJ, Rowe D, Sistani K, Burnham DJ. 2001. Threonine and crude protein responses in broiler chicks. Anim Feed Sci Technol. 94:57–64. 10.1016/S0377-8401(01)00301-7
- Kidd MT, Kerr BJ. 1997. Threonine responses in commercial broilers at 30 to 42 days. J Appl Poult Res. 6:362–367.
- Kim SW, Mateo RD, Yin YL, Wu G. 2007. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Aust J Anim Sci. 20:295–306.
- Lemme A. 2001. Responses of broilers to dietary threonine: a survey of the international literature. Amino News02 (01):1–6, Degussa Corporation.
- Lemme A. 2003. Reassessing amino acid levels for Pekin ducks. Poult Int. 42:18–24.
- Li DF, Xiao CT, Qiao SY, Zhang JH, Johnson EW. 1999. Effects of dietary threonine on performance, plasma parameters and immune function of growing pigs. Anim Feed Sci Technol. 78:179–188. 10.1016/S0377-8401(99)00005-X
- Li PY, Yin D, Li S, Kim W, Wu G. 2007. Amino acids and immune function. Br J Nutr. 98:237–252. 10.1017/S000711450769936X
- Mack S, Bercovici D, De Groote G, Leclercq B, Lip-pens M, Pack M, Schutte JB, Van Cauwenberghe S. 1999. Ideal amino acid profile and dietary lysine specification for broiler chickens of 20 to 40 days of age. Br Poul Sci. 40:257–265. 10.1080/00071669987683
- Marjanovic D, Kozic L, Petrovic M, Palic T, Litricin V. 1975. Serum transaminase activity in fowls. Vet Bull. 45:2890–2896.
- Moritoki K, Yoshida A. 1970. Effect of methionine supplementation to a low casein diet on plasma level of threonine and other amino acids of rats. J Jpn Soc Food Nutr. 23:351–355.
- Muhl A, Liebert F. 2008. Growth, nutrient utilization and threonine requirement of growing chicken fed threonine limiting diets with commercial blends of phytogenic feed additives. J Poult Sci. 44:297–304. 10.2141/jpsa.44.297
- Muramatsu K, Odagiri H, Morishita S, Takeuchi H. 1971. Effect of excess levels of individual amino acids on growth of rats fed casein diets. J Nutr. 101:1117–1125.
- National Research Council (NRC). 1994. Nutrient requirements of poultry. 9th ed. Washington, DC: National Academic Press.
- Ojano-Dirain CP, Waldroup PW. 2002. Evaluation of lysine, methionine and threonine. Needs of broilers three to six week of age under moderate temperature stress. Int J Poul Sci. 1:16–21. 10.3923/ijps.2002.16.21
- Peng L, Yu-Long Y, Defa L, Kim WS, Guoyao W. 2007. Amino acids and immune function. Br J Nutr. 23:579–611.
- Penz AM, Colnago GL, Jensen LS. 1997. Threonine supplementation of practical diets for 3 to 6-wk-old broilers. J Appl Poult Res. 6:355–361.
- Quentin M, Bouvarel I, Picard M. 2005. Effects of starter diet, light intensity and essential amino acids level on growth and carcass composition of broilers. J Appl Poult Res. 14:69–76.
- Rehman HR, Ghumro PB, Dino G, Khan SH, Hussain Z, Ahmed S, Hameed A. 2012. Substitution of crystalline l-lysine with l-lysine enriched fermentation broth in feed and effect on the performance of broiler chicks. J Appl Anim Res. 40:118–123. 10.1080/09712119.2011.627138
- Rosa A, Pesti GM. 2001. Estimation of the tryptophan requirement of chickens for maximum body weight gain and feed efficiency. J Appl Poult Res. 10:135–140.
- Rubin LL, Ribeiro ALM, Canal CW, Silva IC, Trevizan L. 2007. Influence of sulfur amino acid levels in diets of broiler chickens submitted to immune stress. Braz J Poul Sci. 9:305–311.
- Samadi F, Liebert F. 2007. Threonine requirement of slow growing male chickens depending on age and dietary efficiency of threonine utilization. Poult Sci. 86:1140–1148.
- Sanchez A, Swendseid ME, Clark AJ, Umezawa C. 1972. Amino acid pools and hepatic enzyme activities in rats fed a meal of high or low methionine content. Am J Clin Nutr. 25:550–554.
- SAS Institute. 2004. SAS(r) user's guide. StatisticsVersion 9.1 ed. Cary, NC: SAS Institute Inc.
- Shini S, Li X, Bryden WL. 2005, Methionine requirement and cell-mediated immunity in chicks. In:Proceedings of 29th Annual Meeting. Melbourne, Australia: Nutrition Society of Australia; p. 123.
- Swain BK, Johri TS. 2000. Effect of supplemental methionine, choline and their combinations on the performance and immune response of broilers. Br Poult Sci. 41:83–88. 10.1080/00071660086457
- Taghinejad-Roudbaneh M, Nazeradl K, Taghavi MA, Afrooziyeh M, Zakerii A. 2011. Estimation of dietary lysine requirement based on broken line and quadratic model analyses with the use performance and immune responses criterion. J Appl Anim Res. 39:367–374. 10.1080/09712119.2011.621535
- Tenenhouse HS, Deutsch HF. 1966. Some physical–chemical properties of chicken γ-globulins and their pepsin and papain digestion product. Immunochemistry. 3:11–20. 10.1016/0019-2791(66)90277-1
- Tugay A, Ferda O, Hatice H. 2009. Threonine requirement of broiler from 22–42 days. Int. J Poult Sci. 8:862–865. 10.3923/ijps.2009.862.865
- Vazquez M, Pesti GM. 1997. Estimation of lysine requirement of broiler chicks for maximum body gain and feed efficiency. J Appl Poult Res. 6:241–246.
- Vedenov D, Pesti GM. 2008. A comparison of methods of fitting several models to nutritional response data. J Anim Sci. 86:500–507. 10.2527/jas.2007-0536
- Wang X, Qiao SY, Liu M, Ma YX. 2006. Effects of graded levels of true ileal digestible threonine on performance, serum parameters and immune function of 10–25 kg pigs. Anim Feed Sci Technol. 129:264–278. 10.1016/j.anifeedsci.2006.01.003
- Webel DM, Fernandez SR, Parsons CM, Baker DH. 1996. Digestible threonine requirement of broiler chickens during the period three to six and six to eight weeks post-hatching. Poult Sci. 75:1253–1257. 10.3382/ps.0751253
- Williams B, Solomon S, Waddington D, Thorp B, Farquharson C. 2000. Skeletal development in the meat-type chicken. Br Poult Sci. 41:141–149. 10.1080/713654918