Abstract
The cDNA encoding T cell receptor-zeta (TCR-ζ; CD247) molecule of water buffalo (Bubalus bubalis) was isolated, cloned and sequenced in the present study. The CD247 cDNA comprised 1078 nucleotides including a 30 nucleotide 5′-untranslated region (UTR), 495 nucleotide single open reading frame (ORF) and 553 nucleotide 3′-UTR. Deduced amino acid of buffalo CD247 sequence was two residues shorter than the corresponding cattle and sheep sequences. However, ruminant-specific insertions and substitutions in intra-cytoplasmic (IC) domain were present in buffalo. Immunoreceptor tyrosine-based activation motifs (ITAMs), the important motifs for TCR signalling, were totally conserved among ruminants including buffalo. The 3′-UTR region of the buffalo CD247 was highly homologous to the corresponding region in the cattle sequence and showed lack of polymorphism after polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis using HaeIII and MseI restriction enzymes in buffalo population. Phylogenetically, buffalo sequence was closer to cattle sequence under the ruminant's lineage. The conserved nature of this gene ensures TCR integrity which is vital for induction of optimal and efficient immune response.
Introduction
T cell receptors (TCR) are important for the initiation of antigen-specific immune response through antigen recognition and signal transduction (Weiss Citation1991). TCR molecules are a complex of two major subunits: a variable antigen binding Ti and relatively invariant CD3 subunit. The CD3 subunit, a dimer of different ε, γ, δ and ζ chains, has an important role in signal transduction (Sussman et al. Citation1988). Among different chains, ζ–ζ homodimer is most essential for efficient transport of assembled TCR complexes to the cell surface under signal transduction (Sussman et al. Citation1988). Failure of the ζ chain to associate with the pre-TCR leads to its lysosomal degradation (Klausner et al. Citation1989). The ζ chain is encoded by CD247 (CD3Z) gene. This gene is located in the distal regions of human and mouse chromosomes 1 (Modi et al. Citation1989; Seldin et al. Citation1989), whereas it has been localised to chromosome 3q11-q14 in cattle (Amarante et al. Citation1996). Murine CD247 spans at least 31 kilobases and divides into eight exons (Baniyash et al. Citation1989). Three highly conserved immunoreceptor tyrosine-based activation motifs (ITAMs; Kersh et al. Citation1998) in ζ molecule are vital for the initiation of signalling. Deletion of the ITAMs or mutation of the tyrosine residues inside the motifs causes loss of function (Underhill & Goodridge Citation2007). Similarly, mutations in upstream sequence, aberrant transcription and alternate splicing at 3′-untranslated region (UTR) of the CD247 gene have been found to be associated with autoimmune diseases including celiac disease, juvenile idiopathic arthritis, juvenile autoimmune diabetes, multiple myeloma, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus (SLE) and infectious diseases in humans (Takeuchi et al. Citation1998; Nambiar et al. Citation2001; Tsuzaka et al. Citation2005; Dieudé et al. Citation2011; Li et al. Citation2011; Zhernakova et al. Citation2011).
Water buffalo (Bubalus bubalis) is an important dairy animal in South Asian countries. They are generally known to have good adaptation to hot and humid agro-climatic conditions and tolerance to many infectious diseases. However, the genetic basis of these important traits is yet to be explored extensively in buffalo. In this study, we cloned and characterised buffalo TCR-zeta (TCR-ζ) cDNA, which will further help to understand disease resistance in animals.
Materials and methods
cDNA amplification and sequence analysis
Venous blood was collected for cDNA amplification from a healthy Murrah buffalo. Lymphocytes isolated from the blood were cultured through incubating with Roswell Park Memorial Institute (RPMI) media (107 cells/ml) enriched with foetal calf serum (10%) and Con A (10 mg/107cells) at 37 °C temperature and 5% CO2 level for four hours. Total RNA was isolated from cultured lymphocytes by using the total RNA minipreps super kit (Biogene, USA). cDNA molecules were synthesised by using RevertAid first strand cDNA synthesis kit (MBI Fermentas) according to the manufacturer's instructions. Primers for PCR amplification were (Forward: 5′-GCTCCGGGCACCATCCTG-3′, Reverse: 5′-CTACCTACCCCACCTTCCCCTCTG-3′) designed on the basis of cattle sequence (GenBank Acc. no. U25688). PCR amplification was carried out in 25 µl reaction mixture containing 1×PCR buffer, 1.5 mM MgCl2, 200 µM dNTPs, 30 ng of each primer, 1 U Taq DNA polymerase and cDNA template. Amplification was carried out using 35 amplification cycles (94°C/1 min, 60°C/1 min and 72°C/1 min 30 sec). The PCR product was purified by agarose gel electrophoresis using the QIAquick gel extraction kit (Qiagen). The purified product was cloned into pGEM-T Easy cloning vector (Promega) and transformed into DH5∝ competent Escherichia coli cells according to manufacturer's instructions. Three positive clones selected randomly were sequenced on both strands by automated DNA sequencer.
The obtained sequence was annotated and analysed by using public database (www.ncbi.nlm.nih.gov) and submitted to NCBI GenBank (Acc. no. DQ057984). Nucleotide and deduced amino acid sequences of buffalo CD247 were aligned and compared with those of other species available in the database. Phylogenetic tree was derived by neighbor-joining method using MEGA 5 programme (Tamura et al. Citation2011) based on CD247 cDNA sequences of buffalo and other species. Bootstrap values were obtained using 500 replicates (Felsenstein Citation1985). The evolutionary distances were computed using the Kimura 2-parameter method based on the nucleotide model (Kimura Citation1980). Nucleotide and amino acid distances were also computed to estimate evolutionary divergence between sequences by using p-distance model from MEGA 4 programme (Tamura et al. Citation2007).
Genomic DNA amplification and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis
For polymorphism study of the buffalo CD247 3′-UTR region, venous blood was collected from 90 unrelated Murrah buffaloes selected from two different herds. Genomic DNA was isolated by phenol–chloroform extraction method (Sambrook & Russell Citation2001). Primers (Forward: 5′-ACCTATGACGCCCTCCACA-3′, Reverse: 5′-ACATGCCGCGTTCACAGT-3′) were designed using the obtained buffalo sequence (Acc. no. DQ057984) encompassing 42 bases of the coding region and remaining bases of 3′-UTR. A 298 bp long product was amplified using 35 amplification cycles (94°C/45 sec, 65°C/45 sec and 72°C/1 min). RFLP analysis of the amplified PCR products was carried out using HaeIII and MseI restriction enzymes.
Results and discussion
In the present study, we amplified and characterised a 1078-nucleotide-long cDNA sequence of buffalo CD247 gene. The obtained nucleotide sequence showed highest homologies with cattle (96–98%), followed by sheep (95%) and pig (83%) CD247 sequences. A number of nucleotide indels (insertions/deletions) were observed in buffalo compared to cattle. Open reading frame (ORF) of buffalo CD247 sequence was found to be six nucleotides shorter than that of cattle and sheep sequences. The deduced amino acid sequences of buffalo CD247 cDNA showed highest homology with cattle (97.0%) followed by sheep (93.3%), pig (84.1%), human (83.5%), rat (75.8%) and mouse (71.5%) sequences (). Predicted topologies for exon and domain boundaries were found to be similar with other ruminant species (Hagens et al. Citation1996; Weissman et al. Citation1988; Baniyash et al. Citation1989) (A). However, the intra-cytoplasmic (IC) domain was shorter by two amino acid residues in buffalo than other ruminants. As expected, signal peptide (SP) domain showed highest variability among different domains, whereas extra cellular (EC) domain which is responsible for setting the structural framework for surface expression of assembled receptor was found to be completely conserved across all species (Baniyash et al. Citation1989). Some of the important amino acid residues in the hydrophobic trans-membrane (TM) domain, essential for dimer formation and lipid raft association (cysteine, 32), mediating disulphide homodimerisation and association to CD16 (aspartic acid, 36) (Ravetech & Kinet Citation1991; Itoh et al. Citation1993) and interaction with TCR α−β chain (tyrosine, 42) (Johansson et al. Citation1999) are highly conserved in buffalo. Similarly, glycine (Gln) residue at position 43 of dimerisation motif (positions 43–46) was found to be invariant in buffalo similar to other species. Noticeably, mutations at these positions disrupt the key functions of TCR-ζ chain. Substitution of residue 46 (Leu→Val/Ile) in consensus motif L31 CY x LD x ILF x YG xx LT x LF x51 (x represents any residue) of TM domain, which is essential for dimer/tetramer formation (Torres et al. Citation2002), was also found in buffalo similar to other ruminant species. The IC domain, functionally independent for signalling was found to be conserved. However, some changes especially in the antigen recognition activation motifs (ARAMs), ITAMs and guanosine di-/tri-phosphate (GDP/GTP) binding site were observed in ruminants. In ARAM1 region of bubaline CD247 amino acid sequence, deletion of one amino acid at position 60 was observed compared to that of cattle sequence. Similarly, in ARAM2 region, another residue at position 101 was found to be absent in buffalo CD247 compared to cattle. The same amino acid deletion was also found in pig and human. Amino acid substitutions at positions 70 and 71 of ARAM1 and 103, 107 and 109 of ARAM2 (Tsuzaka et al. Citation2005) in buffalo CD247 were also found in cattle and sheep sequences, which supports the fact that these substitutions are specific to ruminant species. Similarly, buffalo sequence exhibited the ruminant-specific insertions of Asparagine (Asn) and Gln at 132 and 133 positions, respectively, at the region encompassing critical GDP/GTP binding motif, ARAM3 (Hagens et al. Citation1996) and SNID2 (Schaefer et al. Citation2000) compared to non-ruminant species. Such additions also increased the length of GDP/GTP binding domain with the sequence G130ERRRGKGHDGLYQG in the loop of helix–loop–helix (Peter et al. Citation1992). ITAMs (Kersh et al. Citation1998), the important motifs for TCR signalling, particularly ITAM1 and ITAM2, are conserved among ruminants. However, no variation was observed in ITAM3 across the species. Highly conserved Tyr/Ile/Leu residues of consensus motif Yxx I/L x6–8 YxxI/L (Schaefer et al. Citation2000) for TCR signalling along with YxxL for CD26 mediated signalling indicates their paramount importance in different species. Similarly, residues like lysine at position 129 (GTP oxi-modification site) and Gln at position 137 in the GDP/GTP binding domain motif including putative nucleotide binding (Peter et al. Citation1992; Itoh et al. Citation1993) were also found to be conserved in buffalo as in other species. Highly conserved GDP/GTP binding domain, ARAM3 and ITAM3 across the species were also notable features. Substitutions at position 77 (Leu→Val) of SNID1 (72–77) and J5 Nef/BD binding area 1 (72–81), where the simian immunodeficiency virus (SIV) and HIV bind resulting down-modulation of TCR from T cell surface, 130 (Gly→Ser) and 131 (Glu→Asp) along with insertions at 132, 133 (Asn/Gln) in SNID2 (123–136, Schaefer et al. Citation2000) were found to be ruminant-specific.
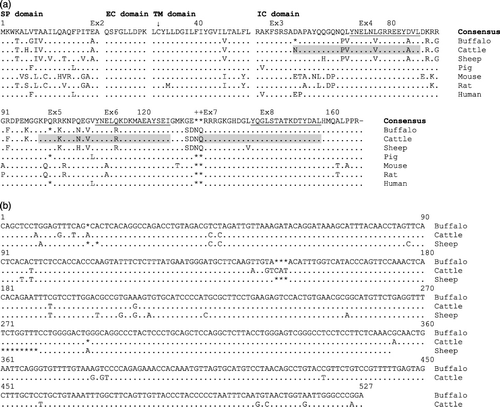
Table 1. Nucleotide and amino acid similarity and divergence of buffalo CD247 cDNA sequence with other species.
Phylogenetic analysis
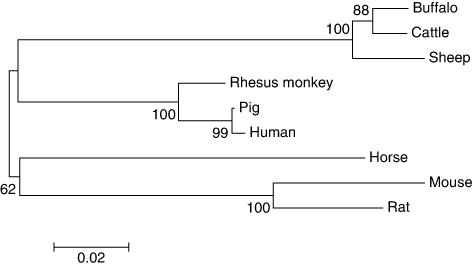
The phylogenetic tree () based on cDNA sequences of CD247 gene from different species revealed higher resemblance among different ruminant species. As expected, buffalo was found to be nearer to cattle than sheep. Analyses also indicated recent diversification of the CD247 gene among ruminants. Ruminants were found to be closer to rhesus monkey, pig and human than horse, rat and mouse at this locus. Evolutionary divergence for buffalo estimated by calculating nucleotide and amino acid difference per site was lowest with cattle followed by sheep, pig, human, rat and mouse () which also indicated the closeness of buffalo to cattle compared to other ruminants. There was remarkable interspecies conservation between cattle and buffalo at 3′-UTR of the CD247 sequences (B).
Polymorphism study
For polymorphism study, a 298-bp-long genomic fragment corresponding to 42 bases of coding region and 256 bases of 3′-UTR was amplified from 90 buffalo samples. Furthermore, digestion of amplified product with HaeIII and MseI restriction enzymes revealed monomorphic patterns for all the individuals with 228 and 70 bp, and 203 and 95 bp restriction fragments, respectively. However in cattle, two single-strand conformation polymorphism (SSCP) variants for the same region of CD247 gene have been reported (Agaba et al. Citation1997). This region is reported to be associated with SLE disease in humans through alternate splicing of exon-7 and 3′-UTR of CD247 gene (Takeuchi et al. Citation1998; Nambiar et al. Citation2001; Chowdhury et al. Citation2005; Tsuzaka et al. Citation2005). Moreover, variation in TCR-ζ expression has been associated with polymorphisms in the CD247 3′-UTR in SLE patients (Gorman et al. Citation2008). The non-polymorphic 3′-UTR in buffalo pointed to its conserved nature.
Results of this study indicated that the cDNA of TCR-ζ (CD247) molecule which plays a decisive role in coupling cell surface receptors to intracellular signalling pathways was invariant in critical residues notably dimerisation motif, GTP oxi-modification site and the very heart of signalling – tyrosine residues in ITAMs for phosphorylation in buffalo. The conserved functionally important motifs and residues ensure the functional integrity of TCR which is vital for the induction of optimal and efficient immune response.
Acknowledgements
The authors wish to acknowledge the Director, Indian Veterinary Research Institute, Izatnagar, India and Department of Biotechnology (DBT), Government of India, New Delhi for providing all the facilities and funds for this study.
References
- Agaba M, Kemp SJ, Barendse W, Teale A. 1997. Genetic mapping of bovine T-cell receptor complex loci. Anim Genet. 28:235–237. 10.1111/j.1365-2052.1997.00117.x
- Amarante MRV, Ansari HA, Maher DW, Pearce PD, Broad TE. 1996. Localization of the antigen CD3, zeta polypeptide (CD3Z) to cattle chromosome 3qll-ql4. Mamm Genome. 7:397–398. 10.1007/BF03035317
- Baniyash M, Hsu VW, Seldin MF, Klausner RD. 1989. The isolation and characterization of the murine T-cell antigen receptor zeta chain gene. J Biol Chem. 264:13252–13257.
- Chowdhury B, Tsokos CG, Krishnan S, Robertson J, Fisher CU, Warke RG, Warke VG, Nambiar MP, Tsokos GC. 2005. Decreased stability and translation of T cell zeta mRNA with an alternatively spliced 3' untranslated region contribute to zeta chain down regulation in patients with systemic lupus erythematosus. J Biol Chem. 280:18959–18966. 10.1074/jbc.M501048200
- Dieudé P, Boileau C, Guedj M, Avouac J, Ruiz B, Hachulla E, Diot E, Cracowski JL, Tiev K, Sibilia J, et al. 2011. Independent replication establishes the CD247 gene as a genetic systemic sclerosis susceptibility factor. Ann Rheum Dis.70:1695–96.
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 39:783–791. 10.2307/2408678
- Gorman CL, Russell AI, Zhang Z, Graham DC, Cope APVyse TJ. 2008. Polymorphisms in the CD3Z gene influence TCRζ expression in systemic lupus erythematosus patients and healthy controls. J Immunol. 180:1060–1070.
- Hagens G, Galley Y, Glaser I, Davis WC, Baldwin CL, Clevers H, Dobbelaere DAE. 1996. Cloning, sequencing and expression of the bovine CD3 epsilon and TCR-zeta chains, two invariant components of the T-cell receptor complex. Gene. 169:165–171. 10.1016/0378-1119(95)00769-5
- Itoh Y, Matsurra A, Kinebuchi M, Honda R, Takayama S, Kon S, Kikuchi K. 1993. Structural analysis of the CD3 zeta/eta locus of the rat: expression of zeta but not eta transcripts by rat T cells. J Immunol. 151:4705–4717.
- Johansson B, Palmer E, Bolliger L. 1999. The extracellular domain of the TCR zeta chain is essential for TCR function. J Immunol. 162:878–885.
- Kersh EN, Shaw AS, Allen PM. 1998. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 281:572–575. 10.1126/science.281.5376.572
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16:111–120. 10.1007/BF01731581
- Klausner RD, Weissman AM, Baniyash M, Bonifacino JS, Samelson LE. 1989. The role of zeta chain in the expression, structure and function of the T cell receptor. Adv Exp Med Biol. 254:21–24.
- Li Y, Chen S, Yang L, Chen S, Lin C, Wang L, Lu Y, Geng S, Du X, Schmidt CA. 2011. Change in expression pattern of TCR-CD3 complex in patients with multiple myeloma. Hematology16:143–50. 10.1179/102453311X12953015767491
- Modi WS, Weissman AM, Seuanez H, Klausner RD, O'Brien SJ. 1989. Chromosomal localisation of the T-cell receptor zeta chain. Cytogenet Cell Genet. 51:1047.
- Nambiar MP, Enyedy EJ, Warke VG, Krishnan S, Dennis G, Kammer GM, Tsokos GC. 2001. Polymorphisms/mutations of TCR-zeta chain promoter and 3' untranslated region and selective expression of TCR-zeta chain with an alternatively spliced 3' untranslated region in patients with systemic lupus erythematosus. J Autoimmun. 16:133–142. 10.1006/jaut.2000.0475
- Peter ME, Hall C, Ruhlmann A, Sancho J, Terhorst C. 1992. The T-cell receptor ζ chain contains a GTP/GDP binding site. EMBO J. 11:933–941.
- Ravetech J, Kinet JP. 1991. Fc receptors. Annu Rev Immunol. 9:457–492. 10.1146/annurev.iy.09.040191.002325
- Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual . 3rd ed New York: Cold Spring Harbor Laboratory Press.
- Schaefer TM, Bell I, Fallert BA, Reinhart TA. 2000. The T cell receptor ζ chain contains two homologous domains with which simian immunodeficiency virus Nef interacts and mediates down-modulation. J Virol. 74:3273–3283. 10.1128/JVI.74.7.3273-3283.2000
- Seldin MF, Kingsmore SF, Moseley WS. 1989. Analyses of genetic linkage relationship in the mouse using an interspecific cross: comparative mapping of genes localised to human chromosome 1. Cytogenet Cell Genet. 51:1077.
- Sussman JJ, Bonifacino JS, Lippincott-Schwartz J, Weissman AM, Saito T, Klausner RD, Ashwell JD. 1988. Failure to synthesize the T cell CD3 chain: structure and function of a partial T cell receptor complex. Cell. 52:85–95. 10.1016/0092-8674(88)90533-8
- Takeuchi T, Tsuzaka K, Pang M, Amano K, Koide J, Abe T. 1998. TCR ζ chain lacking exon 7 in two patients with systemic lupus erythematosus. Int Immunol. 10:911–921. 10.1093/intimm/10.7.911
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599. 10.1093/molbev/msm092
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28:2731–2739. 10.1093/molbev/msr121
- Torres J, Briggs JAG, Arkin IT. 2002. Convergence of experimental, computational and evolutionary approaches predicts the presence of a tetrameric form of CD3-ζ. J Mol Biol. 316:375–384. 10.1006/jmbi.2001.5268
- Tsuzaka K, Setoyama Y, Yoshimoto K, Shiraishi K, Suzuki K, Abe T, Takeuchi T. 2005. A splice variant of TCR zeta mRNA lacking exon 7 leads to the downregulation of TCR zeta, the TCR/CD3 complex and IL-2 production in systemic lupus erythematosus T cells. J Immunol. 174:3518–3525.
- Underhill DM, Goodridge HS. 2007. The many faces of ITAMs. Trends Immunol. 28:66–73. 10.1016/j.it.2006.12.004
- Weiss A. 1991. Molecular and genetic insights into T cell antigen receptor: structure and functions. Annu Rev Genet. 25:487–510. 10.1146/annurev.ge.25.120191.002415
- Weissman AM, Hou D, Orloff DG, Modi WS, Seuanez H, O'Brien SJ, Klausner RD. 1988. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinct from the molecular CD3 complex. Proc Nat Acad Sci USA. 85:9709–9713. 10.1073/pnas.85.24.9709
- Zhernakova A, Stahl EA, Trynka G, Raychaudhuri S, et al. 2011. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 7: e1002004. 10.1371/journal.pgen.1002004
