Abstract
The electroencephalographic spectrum power (ESP) shows the frequency band components of the brain electrical activity. The electroencephalogram (EEG) has a characteristic ESP during different behavioural states such as waking or sleeping and also during pathological states as epilepsy or brain death. Methods of slaughtering the farm animals should prevent needless suffering; all the animals are expected to be unconscious and insensible to pain before being hoisted, but there are no systematic studies of the animal's brain activity after stunning. This study evaluated the brain activity of sheep after stunning by means of percussion or electrical shock. Brain activity after electrical shock showed an epileptiform EEG, with decreasing delta power and increasing theta and gamma band power. After percussion stunning, the EEG showed a slightly decrease of high frequency power.
1. Introduction
The electroencephalogram (EEG) is a technique to record brain electrical activity (Delamonica Citation1987; Kay Citation1998; Pastor et al. Citation2006; Vilatuña & Yanchaliquin Citation2007; Cwynar & Zawadzki Citation2010). Neuronal groups fire in different frequency rates as following: delta (0.5–4.0 Hz), theta (4.0–8.0 Hz), alpha (8.1–12.0), beta (12.1–30.0 Hz) and gamma (30.1–80.0 Hz) (Riquelme Citation1995; Subasi & Ercelebi Citation2005; Bergamasco et al. Citation2006; Angel Citation2009). Frequency band power analysis of the EEG can be performed by means of the Fourier transformed formula (Riquelme Citation1995; Vilatuña & Yanchaliquin Citation2007; Angel Citation2009). Neurological disorders such as epilepsy have being described in animal models, sheep and rat. These EEG recordings show high-amplitude and high-frequency waves (Annegers Citation1997; Guerrero et al. Citation1997; Opdam et al. Citation2002).
Slaughtering of farm animals should induce unconsciousness and avoid pain (Caraves & Gallo Citation2007). However, undesirable stress, anxiety and pain could affect the animals during slaughtering (Grandin Citation1996; Jongman et al. Citation2000; Velarde et al. Citation2003; Gregory Citation2004, Citation2008). Several stunning methods have being described for slaughtering ruminants and monogastric animals, for instance, non-penetrating captive bolt (Lopez & Vanaclocha Citation2004), penetrating captive bolt (Gregory & Shaw Citation2000), electrical shock (Velarde et al. Citation2000) and carbon dioxide exposure (Lopez & Vanaclocha Citation2004; Bercerril-Herrera et al. Citation2009).
The captive bolt pistol method causes brain injury and an irreversible cerebral commotion dependent of the pistol power and the target on the skull (Grandin Citation1999; Lambooij et al. Citation2003; Caraves & Gallo Citation2007; Ríos & Acosta Citation2008). The electrical shock stunning method works by applying current through the brain or through the whole body, and it induces an epileptiform brain status, loss of consciousness and absence of pain (Lopez & Vanaclocha Citation2004), but it might also bring the animal into cardiac arrest (Lambooy Citation1982; Solis Citation2005). Electrical stunning efficacy is dependent on the electrodes placement and electricity characteristics (Grandin Citation1999; Digre et al. Citation2010). For electrical stunning is recommended a voltage between 70–250 volts, and a minimum of 1.2 amp from 2 to 8 sec (Grandin Citation1994; Velarde et al. Citation2000, Citation2003; Brancacci et al. Citation2006; Kun Citation2008). Some of the parameters used to evaluate the effectiveness of stunning are un-coordinated movements, clonic convulsions and dilated pupils (Blackmore & Newhook Citation1983; Grandin Citation2010). Recent studies are focused on the cortical brain activity and the quantitative analysis of the EEG as a measurement of pain perception during slaughtering (Hermsworth & Mellor Citation2009). This study attempts to evaluate the electroencephalographic status of sheep after stunning by the non-penetrating captive bolt pistol method and the electrical shock.
2. Materials and methods
2.1. Animals
Sixty 10-week-old Dorper-mixed lambs were used during this study. The experimental animals were divided into 2 groups of 30 animals each. Animals from group one were stunned by electrical shock and those from group two were stunned by penetrating captive bolt.
2.2. Stunning methods
2.2.1. Electrical shock
The electro narcosis was carried out by placing bipolar electrodes on the temporal area on each side of the skull at a point between the eyes and the ears, previously moistened with saline solution (Velarde et al. Citation2000). An electrical alternating current of 250 volts and 0.9 amp was administrated during 6 sec (HSA Citation2000; Velarde et al. Citation2003). The stunning was performed in a V-shaped box where the animals were standing and immobilised.
2.2.3. Penetrating captive bolt
A penetrating captive pistol bolt was used (Cash Special 22 calibre). The heads of the animals were immobilised and the pistol shot addressed on the midline of the skull, behind the ridge between the horns, pointing to the base of the tongue (HSA Citation2006).
2.3. EEG recording
The EEG was recorded in a physiograph (Power Lab® ADInstruments), at 1 KHz sample rate, 0.5 Hz high-pass digital filter. Two needle electrodes were placed subcutaneously over the skull. The recording electrodes were placed bilaterally over the frontotemporal area, and a reference electrode was placed on the parieto occipital boundary. The recording began 30 sec before the stunning and continued until the time of hoisting the animal (approximately 30 sec after stunning).
2.4. EEG analysis
The EEG recording from each animal was divided in two segments: baseline recording (prior to the stunning) and post-stunning recording. Spectrum power was obtained from the EEG data by means of the fast Fourier transform formula. The spectrum data were divided into six frequency bands: 1–4 Hz (delta), 4–8 Hz (theta), 8–13 Hz (alpha), 13–30 Hz (beta), 30–80 Hz (gamma), 80–500 Hz (higher frequencies). Data from each frequency band were normalised and reported as percentage values (Beyssen et al. Citation2004).
2.5. Statistical analysis
Multifactorial variance analysis ANOVA was carried out for statistical analysis (Statgraphics Centurion XV, Statistical Graphics Corp., USA). A multiple minimum range (LSD test by Fischer, P<0.05) was performed.
3. Results
The baseline EEG analysis from all the experimental animals showed an electroencephalographic spectrum power (ESP) coincident with those reported in the previous studies (Ong et al. Citation1997; Jongman et al. Citation2000) which showed high absolute power for delta, (beta 1–2), alpha (1–2) and theta (1–2) waves during handling and shearing.
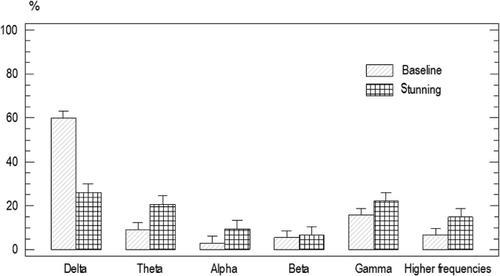
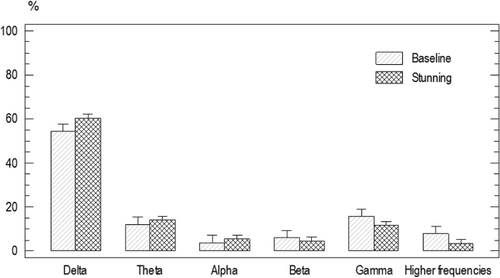
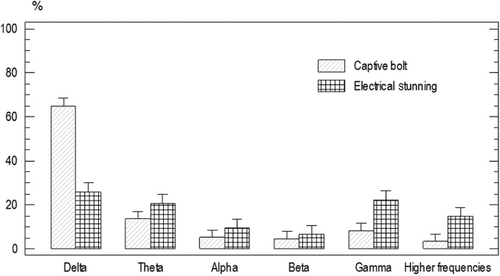
There was no significant difference for the baseline ESP of the animals from group one and two (P<0.05). Frequency band power average was as following: delta 60%±1.5, beta 5.9%±1.4, alpha 3.4%±1.4 gamma 16.15%±1.4, theta 11%±1.4, and higher frequencies band 7%±1.4 ().
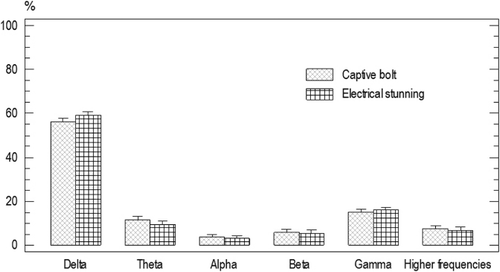
There was a significant decrease in delta power 23.2%±2.4, and a significant increase in theta power 43.22%±1.75 (P<0.05) after electrical shock stunning ().
The spectrum power after penetrating captive bolt stunning was as follows: delta 60.4%±2.7, alpha 5.4%±2.1, beta 4.7%±2.7, theta 14.2%±2.4, gamma 17.1%±2.1, and higher frequencies band 34.5%±2.3. There was no significant difference for the spectrum power before and after penetrating captive bolt stunning ().
Delta power was significantly higher for the animals stunned by a penetrating captive bolt than those animals from the electrical shock group [68.06%±2.71, 23.21%±2.54, respectively (P<0.05)]. Gamma power was significantly lower for the animals stunned by a penetrating captive bolt than those animals from the electrical shock group [8.65%±2.61, 19.93%±2.43, respectively (P<0.05); ].
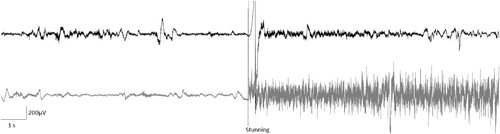
The amplitude of the EEG during baseline recordings was about 200 µV, as well as after the penetrating captive bolt stunning. Amplitude of the EEG after the electrical shock stunning was up to 600 µV. The EEG during basal recording and after the penetrating captive bolt stunning shows low-frequency waves prevalence, but very high-frequency waves after electrical shock stunning ().
4. Discussion
Perception of pain in animals is associated with its description and the physiological responses in human beings (Stubsjøen et al. Citation2009); pain intensity is associated in turn with the level of molecular biomarkers of stress (Sattari et al. Citation2009). Additionally, EEG recordings can also be associated with this type of responses to painful stimulation. In humans, a perception of pain by immersion of the hands in cold water was related to an increasing of delta and beta waves in the EEG (Chen et al. Citation1989). On the other hand, inducing stress and pain to sheep by electrical stimulation caused a decreasing of the delta and alpha waves in the EEG (Ong et al. Citation1997); these results contrasted to the response to placing the hands into water at different temperatures (Egsgaard et al. Citation2009). Non-invasive measurements of pain have been evaluated in sheep, such as infrared thermography, ocular temperature and heart rate, where responses to painful stimuli are associated with pain perception in humans (Stubsjøen et al. Citation2009). In a study it was observed that pain was related to delta, alpha, and beta waves increasing, and, consequently, a reduction in pain by carbamazepine treatment was related to a decrease in those waves (Music et al. Citation2008). Even though there are divergent findings when attempting to show electrophysiological markers for pain perception, there are also coincidences between them. Delta power increasing in some brain areas is usually described as coincident with pain perception (Chen et al. Citation1989; Ong et al. Citation1997; Chen Citation2001). As shown in , electrical shock stunning showed a decrease in delta waves, allowing us to infer that there was a reduction in pain perception.
Epilepsy could be a localised or a generalised condition (Subasi & Ercelebi Citation2005; Bennet et al. Citation2006; Nelson et al. Citation2006; Sakkalis et al. Citation2010). Pain is not perceived during generalised epilepsy crisis but could be present during a localised epilepsy crisis (Opdam et al. Citation2002; Loddenkemper & Kotagal Citation2005; Sun et al. Citation2008; Charlesworth et al. Citation2009; Machado & Solarte Citation2010). Based on these studies, we might speculate that there was no pain perception for the animals after the electrical shock stunning because an epileptic-like EEG state was induced.
By analysing the power spectrum of the EEG recordings, we evaluated the efficiency of the captive bolt method and the electric shock in sheep slaughtering. The EEG recordings after the electric shock resemble an epileptic crisis where there is no pain perception. On the other hand, the results for captive bolt stunning suggest no change in cortical activity and no alteration in the EEG spectrum power; in contrast there is a tendency to increase delta waves, which can be associated to pain perception. Although the penetrating captive bolt stunning leads to loss of mobility, our findings do not show evidence of absence of pain.
Finally, it is quite important to highlight that this study demonstrated that there are extreme differences between the stunning methods, thus, scientists must look for the best stunning method in terms of animal well-being.
Acknowledgement
The authors would like to thank Mr Erick Rodrigo Martínez for assistance in data recording.
References
- Angel RD. 2009. Simulación y análisis estadístico de una señal electroencefalográfica (EEG) [Simulation and statistical analysis of electroencephalographic signal (EEG)]. Paper presented at: LACCEI 2009. Proceedings of the seventh LACCEI Latin American and Caribbean Conference for Engineering and Technology; San cristobal, Venezuela.
- Annegers JF. 1997. United States perspective on definitions and classifications. Epilepsia. 38:9–12. 10.1111/j.1528-1157.1997.tb06137.x
- Bennet L, Roelfsema V, Pathipati P, Quaedackers JS, Gunn AJ. 2006. Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near-infrared spectroscopy in preterm fetal sheep. J.Physiol. 572:141–154.
- Bercerril-herrera M, Alonso M, Lemus C, Legarreta I, Olmos A, Ramirez R, Mota D. 2009. CO2 stunning may compromise swine welfare compared with electrical stunning. Meat Sci. 81:233–237. 10.1016/j.meatsci.2008.07.025
- Bergamasco L, Mecchi E, Facello C, Badino P, Odore R, Re G, Osella M. 2006. Electroencephalographic power spectral analysis of growing goat kids (Capra hircus). Small Ruminant Res. 66:265–272. 10.1016/j.smallrumres.2005.08.025
- Beyssen C, Babile R, Fernandez X. 2004. Electrocorticogram spectral analysis and somatosensory evoked potentials as tools to assess electrical stunning efficiency in ducks. Br Poult Sci. 45:409–415. 10.1080/00071660410001730923
- Blackmore D, Newhook J. 1983. The assessment of insensibility in sheep, calves and pigs during slaughter. In: Eikenenboom G, editor. Stunning of animals for slaughter. Boston (MA): Martinus Nijhoff Publishers; p. 13–25.
- Brancacci N, Alves de Souza P, Alves de Souza H, Garcia da Silva A, Leone E. 2006. Parâmetros de qualidade da carne de cordeiros submetida aos processos de maturação e injeção de cloreto de cálcio [Parameters of meat quality of lambs subjected to the processes of maturation and calcium chloride injection]. Ciencia Rural. 36:1558–1564 10.1590/S0103-84782006000500034
- Caraves M, Gallo C. 2007. Caracterización y evaluación de la eficacia de los sistemas de insensibilización utilizados en equinos en Chile [Characterization and evaluation of the effectiveness of stunning systems used in horses in Chile]. Arch Med Vet. 39:105–113. 10.4067/S0301-732X2007000200003
- Charlesworth G, Soryal I, Smith S, Sisodiya SM. 2009. Acute, localised paroxysmal pain as the initial manifestation of focal seizures: a case report and a brief review of the literature. Pain. 141:300–305. 10.1016/j.pain.2008.11.005
- Chen AC. 2001. New perspectives in EEGMEG brain mapping and PETfMRI neuroimaging of human pain. J Psychophysiol. 42:147–159. 10.1016/S0167-8760(01)00163-5
- Chen AC, Dworrkin SF, Hang J, Gehrig J. 1989. Topographic brain measures of human pain and pain responsivity. Pain. 37:129–141. 10.1016/0304-3959(89)90125-5
- Cwynar P, Zawadzki W. 2010. Recording of bioelectrical activity changes in sheep cerebral cortex. Arch Med Vet. 42:51–62. 10.4067/S0301-732X2010000200006
- Delamonica EA. 1987. Potenciales evocados somatosensitivos [Somatosensory evoked potentials]. In: Delamonica EA, editor. Encefalografía. 2nd ed. Buenos Aires (Argentina): El Ateneo; p. 581–598.
- Digre H, Erikson U, Misimi E, Lambooij B, Van de vis H. 2010. Electrical stunning of farmed Atlantic cod Gadus morhua L.: a comparison of an industrial and experimental method. Aquaculture Res. 41:1190–1202.
- Egsgaard LL, Wang L, Arendt L. 2009. Volunteers with high versus low alpha EEG have different pain–EEG relationship: a human experimental study. Exp Brain Res. 193:361–369. 10.1007/s00221-008-1632-1
- Grandin T. 1994. Euthanasia and slaughter of livestock. J Am Vet Med Assoc. 204:1354–1360.
- Grandin T. 1996. Animal welfare in slaughter plants. Paper presented at: 29th Annual Conference of American Association of Bovine Practitioners Proceedings; San Diego, CA.
- Grandin T. 1999. Good manufacturing practices for animal handling and stunning [Internet]. c. 1999 Fort Collins, CO; [cited 2012 Nov 6]. Available from: http://www.grandin.com/meat103097.html
- Grandin T. 2010. Auditing animal welfare at slaughter plants. Meat Sci. 86:56–65. 10.1016/j.meatsci.2010.04.022
- Gregory N. 2004. Physiology and behaviour of animal suffering. 1st ed. Oxford: Blackwell Publishing.
- Gregory NG. 2008. Animal welfare at markets and during transport and slaughter. Meat Sci. 80:2–11. 10.1016/j.meatsci.2008.05.019
- Gregory NG, Shaw F. 2000. Penetrating captive bolt stunning and exsanguination of cattle in abattoirs. J Applied Anim Welfare Sci. 3:215–230. 10.1207/S15327604JAWS0303_3
- Guerrero MF, Meneses B, Garcia C. 1997. Registro encefalografico computarizado en ratas para la obtencion de anticonvulsionantes [Rats computerized encephalographic recording for obtaining anticonvulsants]. Revista colombiana de ciencias quimicas farmaceuticas. 26:59–62.
- Hermsworth P, Mellor DJ. 2009. Scientific comment on the welfare of sheep slaughtered without stunning [Internet]. c-2009. Melbourne (Australia); Animal Welfare Science Centre (AWSC); [cited 2012 Nov 6]. Available from: www.daff.gov.au/—data/assets/pdf_file/0018/1370331/welfare-sheep-slaughter.pdf
- HSA. 2000. Electrical stunning of red meat animals. 1st ed. Wheathampstead: Humane Slaughter Association. p. 1–23.
- HSA. 2006. Captive bolt stunning of livestock. 4th ed. Wheathampstead: Humane Slaughter Association; p. 1–13.
- Jongman E, Morris J, Barnett J, Hemsworth P. 2000. EEG changes in 4-week-old lambs in response to castration, tail docking and mulesing. Autralian Vet J. 78:339–343. 10.1111/j.1751-0813.2000.tb11789.x
- Kay I. 1998. Introduction to animal physiology. 1st ed. Oxford: Bios Scientific.
- Kun C. 2008. Efecto del proceso duchado y tiempo de escaldado durante la cosecha de cerdo en las propiedades físico-químicas, microbiológicas y sensoriales de la carne [Effect of showered and scalding time on physicochemical, microbiological and sensory properties of meat harvested pork] [ thesis]. Zamorano, Honduras: Escuela agricola panamericana.
- Lambooij E, Kloosterboer RJ, Pieterse C, Gerritzen MA, Van de vis JW. 2003. Stunning of farmed African catfish (Clarias gariepinus) using a captive needle pistol; assessment of welfare aspects. Aquaculture Res. 34:1353–1358. 10.1046/j.1365-2109.2003.00966.x
- Lambooy E. 1982. Electrical stunning of sheep. Meat Sci. 6:123–135. 10.1016/0309-1740(82)90022-5
- Loddenkemper T, Kotagal P. 2005. Lateralizing signs during seizures in focal epilepsy. Epilepsy Behav. 7:1–17. 10.1016/j.yebeh.2005.04.004
- Lopez R, Vanaclocha AC. 2004. Tecnología de mataderos [Slaughterhouses technology]. 1st ed. Madrid (España): Mundiprensa.
- Machado RA, Solarte RA. 2010. Ictal extension (dorsiflexion) of the toes in a patient with temporal lobe epilepsy: a new ictal lateralizing sign. Epilepsy Behav. 18:481–484. 10.1016/j.yebeh.2010.05.011
- Music M, Babic N, Fajkic A, Sivic S, Huseinagic S, Alicajic F, Toromanovik S. 2008. Analysis of the electroencephalogram and pain characteristic in patients before and after carbamazepine treatment. Medicinski Arhiv. 62:256–258.
- Nelson MT, Todorovic SM, Perez E. 2006. The role of T-type calcium channels in epilepsy and pain. Curr Pharm Des. 12:2189–2197. 10.2174/138161206777585184
- Ong RM, Morris JP, O'dwyer JK, Barnett JL, Hemsworth PH, Clarke IJ. 1997. Behavioural and EEG changes in sheep in response to painful acute electrical stimuli. Autralian Vet J. 75:189–193. 10.1111/j.1751-0813.1997.tb10064.x
- Opdam H, Federico P, Jackson GD, buchanan J, Abbott DF, Fabinyi GC, Vosmansky AM, Archer JS, Wellard RM, Bellomo R. 2002. A sheep model for the study of focal epilepsy with concurrent intracranial EEG and functional MRI. Epilepsia. 43:779–787. 10.1046/j.1528-1157.2002.04202.x
- Pastor J, Uzcategui YG, Gal-Iglesias B, Ortega GJ, Sola R, Menendez L. 2006. The pathophysiological foundations of temporal-lobe epilepsy: studies in humans and animals. Rev Neurol. 42:663–673.
- Ríos FG, Acosta DC. 2008. Sacrificio humanitario de ganado bovino e inocuidad de la carne [Humane slaughter of cattle and meat safety]. Nacameh. 2:106–123.
- Riquelme L. 1995. Bases neurologicas de las señales bioelectricas cerebrales [Neurological bases of brain bioelectric signals]. In: Ferrero RGA, Ferrero AL, editors, Analisis computado del EEG. 1st ed. Buenos Aires, (Argentina): FADEC; p. 32–41.
- Sakkalis V, Cassar T, Zervakis M, Giurcaneanu CD, Bigan C, Micheloyannis S, Camilleri KP, Fabri SG, Karakonstantaki E, Michalopoulos K. 2010. A decision support framework for the discrimination of children with controlled epilepsy based on EEG analysis. J Neuroeng Rehabil. 7:24–38. 10.1186/1743-0003-7-24
- Sattari A, Mirzargar SS, Abrishamifar A, Lourakzadegan R, Bahonar A, Mousavi HE, Niasari A. 2009. Comparison of electroanesthesia with chemical anesthesia (MS222 and Clove oil) in rainbow trout (Oncorhynchus mykiss) using plasma cortisol and glucose responses as physiological stress indicators. Asian J Anim Vet Adv. 4:306–313. 10.3923/ajava.2009.306.313
- Solis JL. 2005. Manual de practicas tecnologia de carnes [Manual meat technology practices] [ thesis]. Huncayo, (Perú): Departamento academico de ciencia y tecnologia de alimentos.
- Stubsjøen SM, Flø AS, Moe RO, Janczak AM, Skjerve E, Valle PS, Zanella AJ. 2009. Exploring non-invasive methods to assess pain in sheep. Physiol Behav. 98:640–648.
- Subasi A, Ercelebi E. 2005. Classification of EEG signals using neural network and logistic regression. Comput Methods Programs Biomed. 78:87–99. 10.1016/j.cmpb.2004.10.009
- Sun FT, Morrell MJ, Wharen RE. 2008. Responsive cortical stimulation for the treatment of epilepsy. Neurother. 5:68–74. 10.1016/j.nurt.2007.10.069
- Velarde A, Gispert M, Diestre A, Manteca X. 2003. Effect of electrical stunning on meat and carcass quality in lambs. Meat Sci. 63:35–38.
- Velarde A, Ruiz de la Torre JR, Stub C, Diestre A, Manteca X. 2000. Factors affecting the effectiveness of head-only electrical stunning in sheep. Vet Rec. 147:40–43.
- Vilatuña BJ, Yanchaliquin VJ. 2007. Diseño y construccion de un sistema de monitorizacion del nivel de alerta humano en tiempo real basado en ondas cerebrales [Design and construction of a system for monitoring human alertness in real time based on brain waves] [ thesis]. Quito, (Ecuador): Escuela Politecnica Nacional.