Abstract
The aim of this research was to study the differences between breeds through the analyses of the intramuscular fat content and fatty acid profile of the main local hair sheep breeds of Brazil, Morada Nova (MO), Brazilian Somali (SO) and Santa Inês (SI) and the crossbred ½ Dorper×½ Morada Nova (F1). Genetic group was a significant source of variation for intramuscular fat content, individual fatty acids, polyunsaturated fatty acid (PUFA)/saturated fatty acid (SFA) ratio, total PUFA, essential fatty acids (EFA), total n-3 and n-6 PUFA and Δ9 desaturase and atherogenic index. MO breed presented the highest values of conjugated linoleic acid, PUFA (similar to F1) and PUFA/SFA (similar to F1). MO meat had the highest proportion of EFA, despite the value was similar to F1. SO breed showed highest proportion of myristc, pentadecanoic, palmitic and palmitoleic acids in muscle, and highest Δ9 desaturase indices. In conclusion, there are differences between genetic group for the profile of fatty acids and intramuscular fat. The meat from MO lambs had better attributes of fatty acid composition with higher PUFA, PUFA/SFA ratio, EFA and total n-3 PUFA.
1. Introduction
Sheep meat is an important source of protein in the diet of the population of all states of Brazil, mainly for local populations in the north-east and midwest regions. However, meat from ruminants has high content of saturated fatty acids (SFAs), which has been implicated in diseases (Wood et al. Citation2003). However, researches carried out during the last few years have revealed that not only the amount of fat but also its profile should be taken account, as other nutritional benefits of the consumption of ruminant meat which has good levels of conjugated linoleic acid (CLA). CLA participates in various metabolic processes and is beneficial to human health.
In Brazil, in general, the researches on meat quality (intramuscular fat content and fatty acid composition) of sheep have been performed with animals finished under feedlot, considering mainly the nutrition as a source of variation affecting these traits (Madruga et al. Citation2006, Citation2008; Costa et al. Citation2009; Landim et al. Citation2011). However, the base system for the sheep industry in the north-east of Brazil is the pasture, either native or cultivated, and the great diversity of animal genotypes available in the region also indicates the possibility of differences of these traits in these genotypes.
The north-eastern region of Brazil has around 54% of the population of sheep of the country (14 million, IBGE Citation2006), and these are almost entirely of hair sheep. The main genotypes available in this region are the local breeds of hair sheep: Morada Nova (MO), Santa Inês (SI) and Brazilian Somali (SO). These breeds show differences in size at birth, as well different patterns of development and muscle growth. The SI breed is commonly used in crossbreeding as maternal breed in all regions of Brazil. SO and MO are important genetic resources and are characterised by small body size and slow growth when compared with SI. Currently, the breeders have used these local breeds with Dorper breed (introduced into Brazil from South Africa) in terminal crossings with the objective to obtain lambs with rapid growth and better carcass conformation.
Considering the importance of the local breeds and the extreme conditions that the sheep breeding activity finds to develop in semi-arid regions (Costa et al. Citation2009) and the demand for a quality product by consumers, it is important to improve the aspects of production, industrialisation and commercialisation of these animals, to meet consumers’ needs in terms of health and presentation (Landim et al. Citation2011).
The aim of this study was investigate the differences in fatty acid profile and intramuscular fat content of the main genotypes of sheep raised in semi-arid of Brazil to identify the best options for the finishing of lambs on pasture.
2. Materials and methods
The experiment was carried out using the experimental flock of Embrapa Caprinos e Ovinos, Sobral, CE – Brazil. Thirty-four unrelated males lambs of MO (n =6), SO (n=7) and SI (n=13) breeds and ½ Dorper×½ Morada Nova (F1, n=8) crossbred, born in the same season, with the same birth type (single) and weaned at an average of 84 days of age were used in this study. After the weaning, the lambs were raised on irrigated pasture of Tanzania grass (Panicum maximum Jacq cv. Tanzania) with free access to water and mineral salt. The lambs were free to graze and were supplemented once a day with a concentrate (corn − 48%, cotton cake − 35%, soybean meal − 15%, limestone −1%, mineral salt −1%) at a rate of 1.5% of body weight. The pasture and concentrate chemical compositions are presented in . All animals were managed in the same flock in the same pasture, following the principle of rotational grazing, allowing appropriate conditions of grass surface. The trial followed a completely randomised design with four treatments (genotype), respecting the principles of randomisation, repetition and uniformity of animals and management. The lambs were slaughtered at Embrapa Caprinos e Ovinos facilities with the same ages with averages of 200.18±7.54 days of age (a minimum of 191 and a maximum of 208 days) and 20.62±3.46 kg of body weight. The averages of age were similar according to breeds (F1=196.71 days; MO=198.17 days; SI=199.15 days; SO=198.28 days), however, there were some differences in slaughter weight according to genetic differences of the breeds (F1=20.82 kg; MO=14.38 kg; SI=23.98 kg; SO=19.54 kg). The conditions analysed here seek to simulate the present conditions of diet/management and production system for lambs in Brazil. The purpose of this was to compare the breeds under practical conditions imposed by the market and production systems in Brazil. A 5-cm length of the Longissimus dorsi muscle, between 12th and 13th ribs, was removed from one side of the carcass. All samples were vacuum-packed, frozen rapidly and stored at −20°C for further analysis.
Table 1. Chemical composition of Tanzania grass (Panicum maximum Jacq cv. Tanzania) and concentrate (corn − 48%, cotton cake − 35%, soybean meal − 15%, limestone −1%, mineral salt − 1%).
The lipids from samples were extracted using the methodology presented by Bligh and Dyer (Citation1959). The fatty acids were transmethylated according to method described by Precht and Molkentin (Citation2000). The fat extracted was dissolved in 1 mL of hexane and mixed with 20 µL sodium methylated solution (2 N in methanol) in a sample vial. The solution was shaken vigorously for 3 min (vortex mixer) and centrifuged for 1 min (35×g). After addition of 10 mg sodium sulphate monohydrate, the vial was recapped, mixed again for 2 min and centrifuged at same speed for 1 min. The clear supernatant was used for gas chromatography analysis.
The fatty acid profile was determined by gas chromatography according to model modified from Chilliard et al. (Citation2006), under the following conditions: Column: SP 2560 (100 m×0.25 mm×0.25 µm) – Supelco; Patterns: Supelco 37 – Component FAME Mix (10,000 µg in CH2Cl2) – Supelco cat. 47885-U, linoleic conjugated acid methyl ester – SIGMA Cat. O5632; flow of gas, injection in the split mode (1:100), 1 µL of sample, carrier gas – hydrogen (30 mL/min), make up − 30 mL/min, synthetic air − 300 mL/min; temperature of injector and detector (FID) − 250°C; programming temperature of oven: initial temperature 50°C hold 3 min, rate 4°C/min to 150°C hold 1 min, rate 1°C/min to 170°C hold 1 min, rate 8°C/min to 220°C hold 30 min, total run 86.25 min. The analyses were performed in the Animal Nutrition Laboratory of Embrapa Caprinos e Ovinos. The fatty acid composition of intramuscular fat is expressed as amount of each individual fatty acid per total fatty acids present. The atherogenic index was calculated as (C12:0+(4*C14:0)+C16:0)/total unsaturated fatty acids (Chilliard et al. Citation2003). The indices used to predict the activity of desaturase enzyme (C18:1n9c/(C18:0+C18:1n9c)), (C16:1/(C16:0+C16:1)) and (C18:1n9c+C16:1)/(C18:0+C18:1n9c+C16:0+C16:1)) were also evaluated. Desaturase index is based on the relationship between substrate and product for Δ9 desaturase.
Data were analysed as a completely randomised design with a model that included genotype effects and experimental error by least-squares method using the GLM procedure of SAS Institute Inc (Citation1996). When the effect of genotype was significant, the means were compared by Tukey-Kramer test.
3. Results
The genetic group was a significant source of variation for the percentage of individual fatty acid (FA; ) and the intramuscular fat content (IMF; ). The IMF varied from 28.84 mg/g to 58.88 mg/g among the four genetic groups, and only two groups differed statistically to each other, SI and SO (P<0.05; ).
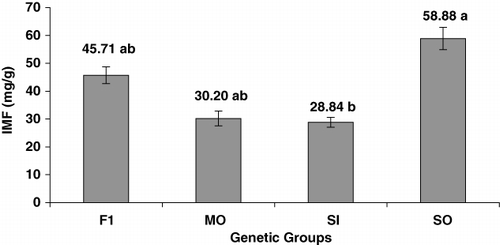
Table 2. Least square means±standard error on the fatty acid profile (% of total) in Longissimus muscle of ½ Dorper×½ Morada Nova (F1), Morada Nova (MO), Santa Inês (SI) and Brazilian Somali (SO) lambs.
The main fatty acids present in the Longissimus dorsi of lambs of the four genetic groups were C18:1n9c (oleic acid − 28%), C18:0 (estearic acid − 25%) and C16:0 (palmitic − 24%) which represented approximately 78% of the total fatty acids (). Differences among the genetic groups were observed only for capric, myristc, pentadecanoic, palmitic, palmitoleic, stearic, oleic, linoleic, conjugated linoleic, arachidonic and lignoceric acids. The highest proportion of oleic acid was observed in SO (32.15), however, this value did not differ from averages for F1 (29.14) and MO (26.01). Stearic acid proportion was the lowest in SO meat (19.8) and the highest in SI (27.3) and F1 (27.6) meats. SO breed showed highest proportion of myristc, pentadecanoic, palmitic and palmitoleic acids (P<0.05). Proportion of the CLA was higher in MO meat than in the other breeds.
Genotype also was a significant source of variation for polyunsaturated fatty acid (PUFA)/SFA ratio, total PUFA, essential fatty acids (EFA), total n-3 and n-6 PUFA, Δ9 desaturase and atherogenic index (). The meat of MO lambs had greater proportion of PUFA and EFA than that of SI and SO lambs (). F1 lambs had similar proportion of PUFA to lambs MO and SI. The SO meat showed lowest proportion of PUFA, similar to SI. MO lambs also had a greater relation of PUFA/SFA (0.30) than SI and SO but similar to F1 lambs. The highest value of total n-3 PUFA was observed in MO (0.80) despite that it did not differ from F1 (0.69) and SI (0.61) values. The lowest value for n-6 PUFA was observed in SO (4.07) that this did not differ from SI (5.89).
Table 3. Least square means±standard error on the fatty acid profile (% of total) according to classification of saturation and indices of Δ9 desaturase activity in Longissimus muscle of ½ Dorper×½ Morada Nova (F1), Morada Nova (MO), Santa Inês (SI) and Brazilian Somali (SO) lambs.
The atherogenic index was similar in F1, MO and SI lambs and superior in SO. There was no difference between genetic groups for the total of SFA, monounsaturated fatty acid (MUFA) and UFA. The unsaturated and SFAs represented around 43% and 58%, respectively, of the total fatty acid of lambs’ meat ().
In C18:1n9c/(C18:0+C18:1n9c) and C16:1/(C16:0+C16:1) desaturase indices, the highest value was observed in SO meat. For the index (C18:1n9c+C16:1)/(C18:0+C18:1n9c+C16:0+C16:1), the highest value was observed in SO meat (0.41), despite that it did not differ from the F1 (0.37) and MO (0.35) meats.
4. Discussion
The present study demonstrated significant difference between breed to IMF content of muscle tissue of the four genetic groups. The total amount of fat is the major factor affecting the fatty acid composition in the muscle of several species (Wood et al. Citation2008), and the amount of IMF in the Longissimus muscle has high phenotypic correlation with marbling and with subcutaneous fat thickness (Sainz & Paganini Citation2004; McPhee et al. Citation2006). Genetically, each breed has its own pattern of development and muscle growth and maturing rate according to the process of evolution and selection (natural and artificial) that it was submitted. So the total amount of fat in the muscle is intrinsically dependent of this rate of growth and maturing. These aspects are controlled by many physiological aspects such the hormones biosynthesis. Lobo et al. (Citation2009) determined the polymorphism C242T of the aromatase gene (Cyp19) in some of the breeds of this study that can help explain the differences in the growth and maturing rate of them. Lobo et al. (Citation2012) also reported that transcripts involved in skeletal muscle development (MyoD1 and IGFBP4), lipogenesis and adipogenesis (C/EBPδ, PPARγ and PGDS) were differentially expressed in some comparisons between the genetic groups studied here. The proliferative activity of satellite cells, which is the source of new nuclei embedded the muscle fibres, is greater in SO than in MO. The results of that study suggest that the SO lambs are more efficient with respect to tissue growth than the MO lambs during postnatal growth. The results of these studies point out that the sequence of growth rate is F1=SI>SO>MO and the sequence of mature rate is SO=F1>SI>MO. The study of the differences in these aspects among breeds is important to choose breeds more appropriated to attend the market since these characteristics are related to meat quality and preference of consumers.
The percentages of the main fatty acids predominant in the meat of ruminants (oleic, estearic and palmitic) observed in this study were similar to those reported in meat of culled woollen ewes raised in south of the Brazil (Pelegrini et al. Citation2007) and lambs grazed on pasture (Enser et al. Citation1998). Except for the SI breed, the meat of the lambs had higher proportion of oleic acid than other fatty acids. Oleic acid is a fatty acid typically found in ruminant feed and in the rumen is largely hydrogenated to estearic acid by rumen microorganisms. In tissues, the concentration of oleic acid is dependent upon the activity of Δ9 desaturase (Smith et al. Citation2006) that is the major lipogenic enzyme that catalyses the conversion of palmitic (C16:0) to palmitoleic (C16:1) and estearic (C18:0) to oleic (C18:1). Dinh et al. (Citation2010) explain that the significant presence of oleic acid is explained by Δ9 desaturase, which may convert as much as 10% of estearic to oleic acid in the enterocyte (Byers & Schelling Citation1993). Oleic acid is considered hypolipidaemic, as it reduces cholesterol and triglycerides in plasma (EFSA Citation2010; Díaz et al. Citation2011). The trend of higher oleic acid (C18:1) and lower estearic acid (C18:0) in SO animals was confirmed by the highest activity of the Δ9 desaturase in these animals (Lobo et al. Citation2012). Wood et al. (Citation2008) highlighted that oleic acid increases with fatness. In fact, the SO breed is originated from Black-Head Persian breed, which was brought to Brazil in recent years (1939). It is a breed of the fat-tail group which has the ability to accumulate fat in the tail as a reserve to critical periods of food shortages. Among the studied breeds, SO has the highest index of maturing and fatness.
Estearic acid concentrations were significantly smaller in the meat of SO lambs than in all others groups. This may be the result of lower biohydrogenation of oleic acid in the rumen or increased enzyme stearoyl-CoA desaturase (SCD) activity in muscle tissue of animals of this breed. However, no study has suggested breed-related differences in biohydrogenation in the rumen (Dinh et al. Citation2010). Bauman et al. (Citation1999) and Dinh et al. (Citation2010) suggested that the increase in MUFA concentration, especially regarding the increased concentration of oleic acid, might be an indicator of desaturase activity. Although SO lambs have presented the highest average for the concentration of MUFA, they did not differ from the other genetics groups. Despite the fact that SO lambs had the highest proportion of oleic acid, they differed only from F1. On the other hand, the Δ9 desaturase indices used as an estimator of SCD enzyme activity were significantly highest in SO muscle. Dervish et al. (Citation2010) suggested that the highest content of oleic acid in the muscle could be related to highest enzyme activity.
Higher proportion (9.5%) of the linoleic acid (C18:2n6c; omega 6) was observed in the meat of MO lambs than those of SI and SO lambs, being similar to proportion in F1. According to Wood et al. (Citation2008) linoleic is the main PUFA in sheep tissues and only a small proportion of it, around 10%, is available for incorporation into tissue lipids. Our results for MO lambs were similar to those found in Kivircik and Sakiz lambs fed with concentrates (Demirel et al. Citation2006) and those found in intramuscular depots by Cañeque et al. (Citation2005). The proportion of α-linolenic (C18:3n3; omega 3), which according to Wood et al. (Citation2008) is the second most important PUFA in sheep and cattle and did not differ among the genotypes, and its proportion represented only around of 0.45% of the fatty acids total proportion. Wood et al. (Citation2008) presented values of 1.2% and 4.6% for the fatty acid composition of longissimus muscle triacylglycerol (neutral lipid) and phospholipid, respectively, in sheep. Fisher et al. (Citation2000) reported values of 0.7% and 2.3% for the linolenic acid in the semimembranosus muscle of Suffolk animals fed with concentrates and grass, respectively. The values observed in this study are below that expected for animals fed in pasture. It is possible that the concentrate used for supplementation of animals in this study, with the participation of cotton cake (35%) and soybean meal (15%) have influenced this result. Actually, animals fed with concentrates which are rich in cereals that have high proportion of linoleic acid present a meat with an undesirable and high n-6:n-3 ratio. However, the meat of animals that eat grass (ruminant) which contains high levels of linolenic has beneficial n-6:n-3 ratio (Wood et al. Citation2003). Therefore, some authors claim that lambs raised at pasture produce meat with quality more favourable for consumer health than those raised indoors (Landim et al. Citation2011).
Today special attention has been given to the type of PUFA and the appropriate balance in the diet of n-3 and n-6 PUFA (Williams Citation2000). In this study, the meat of the lambs did not differ and showed values above that recommended (<4.0) for ratio n-6/n-3 PUFA, although meats of others species also have values superior to this limit (Wood et al. Citation2003). According to Wood et al. (Citation2008), a long rumen transit time for forage diets and the greater biohydrogenation of 18:3n3 limit its amount available for tissue uptake compared with 18:2n6 from concentrate diets. This occurs because concentrates have small particle size and a shorter rumen transit time than fibrous forage diets limiting the opportunities for microbial biohydrogenation (Wood et al. Citation2008). This can explain the better ratio n-6/n-3 PUFA in the meat of ruminants such as sheep, compared to other species of non-ruminant.
Though the meat of lambs presented an undesirable n-6/n-3 ratio, it showed a considerable percentage of CLA (c18:2c9t11), that is an important nutrient in human nutrition. The CLA proportion of MO meat represented 1.24% of total fatty acids. This value was higher than those reported for cattle (Laborde et al. Citation2001) and those presented by Dervish et al. (Citation2010) for lambs. These latter authors reported the following values: 1.17, 1.14, 0.55 and 0.43% for lambs grazing alfalfa pasture, grazing alfalfa pasture with supplementation, indoor lambs with grazing ewes and indoors lambs, respectively. Ruminant CLA isomers come from two sources: one from biohydrogenation in the rumen, produced during microbial biohydrogenation of dietary linoleic acid (LA; C18:2c9c12) and in the tissues through Δ9-desaturation (through of enzyme stearoyl-CoA desaturase; SCD) of the rumen-derived trans-vaccenic acid (Corl et al. Citation2001). Lobo et al. (Citation2012) reported greater expression of SCD gene transcripts in the muscle of SO lambs in relation to MO lambs, suggesting a higher activity of this enzyme in SO animals. These observation contrast with observed here where the highest value of CLA was observed in the meat of MO compared to the other groups. Lobo et al. (Citation2012) observed that MO lambs had higher expression of transcripts that are activated at the beginning of preadipocyte differentiation process and lower expression of those transcripts expressed at the intermediate period of this process compared to SO lambs. This indicates that differentiation of preadipocytes in MO breed occurs latter than in SO breed. As CLA could also come from microbial biohydrogenation in rumen we have extrinsic and intrinsic factors related to its levels at the animal tissue. So the reason for the differences observed in CLA among the genetic groups studied is not clear. Wood et al. (Citation2008) reported that CLA increases with fatness; however, in this study the fatness was higher in SO, but MO had highest CLA.
Genetic group influenced the concentration of PUFA and EFA with MO meat showing the highest proportions of desirable fatty acids. This breed had the better PUFA:SFA ratio, similar to F1 lambs. No significant difference between genetic groups was observed by Landim et al. (Citation2011) to PUFA/SFA ratio. In general, F1 lambs were more similar to MO lambs, due to the participation of this breed in this crossing (½ Dorper×½ Morada Nova). Although MO is an important local breed with high adaptation to the region, it is under the risk of extinction and these aspects are relevant for its conservation and improvement. Values found here for PUFA/SFA ratio are below those recommended by Department of Health (Citation1994) in the UK (above 0.4). However, the meat of MO lambs showed value above those reported to some ruminant meats (0.30 vs 0.1). Our results were similar to those reported by Cañeque et al. (Citation2005) and slightly higher than those found by Demirel et al. (Citation2006) in lambs fed with grass and concentrate.
The highest atherogenic index observed in the SO is probably due to highest concentration of C12:0, C14:0 and C16:0. The atherogenic index was proposed as a measure of the tendency of the food to influence the incidence of coronary heart disease. Therefore, from the standpoint of human health, meat with fewer short chain SFAs content is desirable.
The present work demonstrated that there is variation in fatty acid composition among the studied genetic groups. This variability offers opportunities to be explored in production systems of lambs. The influence of genotype on fatty acid composition has also been observed in goats (Santos et al. Citation2007; Peña et al. Citation2011). Emphasise that the fatty acid composition is influenced not only by genetic factors but also by environmental factors such as diet and supplementation. But, according Soyeurt et al. (Citation2006), the feed supplementation, the most popular way to improve the nutritional quality, presents certain disadvantages. It does not consider the animal genetic effect, and this improvement is not permanent.
In addition, nutritional aspects must be taken into account, although in Brazil the breeders of sheep tend to lead mass selection only for productivity (weight). However, this usually causes problems with intrinsic product quality, such as very low intramuscular fat content of lean meats, reducing the flavour and juiciness of cooked meats (OECD Citation2010).
5. Conclusion
This study revealed differences in the intramuscular fat content and fatty acid composition of meat among the studied genetic groups. This variability is important for improvement of meat quality by choice of breeds to be used in different productions systems. In the conditions of the study, the meat from MO lambs had better attributes of fatty acid composition with higher PUFA, PUFA/SFA ratio, EFA and total n-3 PUFA. These aspects are crucial to reinforce among the breeders the importance of conservation and multiplication of this breed. F1 lambs showed fatty acid profile similar to MO, being this crossing an option for the finishing of lambs on pasture. However, it is important highlight the necessity for further studies to confirm the results. Farther, a future goal should be to identify genetic markers for identifying and selecting animals with improved profiles before they express the phenotype.
Acknowledgements
The authors acknowledge Embrapa for the financial support. The first author thanks CNPq and Universidade Federal de Viçosa for the studentship.
References
- Bauman DE, Baumgard LH, Corl BA, Griinari JM. 1999. Biosynthesis of conjugated linoleic acid in ruminants. Proc Am Soc Anim Sci. 77:1–15.
- Bligh EC, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37:911–917. 10.1139/o59-099
- Byers FM, Schelling GT. 1993. Los lipidos en la nutricion de los rumiantes [The lipids in the nutrition of ruminants]. In: Church DC, editor. El ruminante fisiología digestiva y nutrición [The ruminant digestive physiology and nutrition]. Zaragoza: Acribia; p. 339–356.
- Cañeque V, Díaz MT, Álvarez I, Lauzurica S, Pérez C, De la Fuente J. 2005. The influences of carcass weight and depot on the fatty acid composition of fats of suckling Manchego lambs. Meat Sci. 70:373–379. 10.1016/j.meatsci.2005.02.003
- Chilliard Y, Ferlay A, Rouel J, Lamberet G. 2003. A review of nutritional and physiological factors affecting goat milk synthesis and lipolysis. J Dairy Sci. 86:1751–1770. 10.3168/jds.S0022-0302(03)73761-8
- Chilliard Y, Rouel J, Leroux C. 2006. Goat's alpha-s1 casein genotype influences its milk fatty acid composition and delta-9 desaturation ratios. Anim Feed Sci Tech. 131:474–487. 10.1016/j.anifeedsci.2006.05.025
- Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Phillips BS, Bauman DE. 2001. The role of 9-desaturase in the production of cis-9, trans-11 CLA. J Nutr Biochem. 12:622–630. 10.1016/S0955-2863(01)00180-2
- Costa RG, Batista ASM, Azevedo PS, Queiroga RCRE, Madruga MS, Araújo Filho JT. 2009. Lipid profile of lamb meat from different genotypes submitted to diets with different energy levels. Brazil J Anim Sci. 38:532–538.
- Demirel G, Ozpinar H, Nazli B, Keser O. 2006. Fatty acids of lamb meat from two breeds fed different forage: concentrate ratio. Meat Sci. 72:229–235. 10.1016/j.meatsci.2005.07.006
- Department of Health (DH). 1994. Nutritional aspects of cardiovascular disease. London: HMSO. p. 178, ( Report on Health and Social Subject, 46).
- Dervish E, Serrano C, Joy M, Serrano M, Rodellar C, Calvo JH. 2010. Effect of the feeding system on fatty acid composition, expression of the Δ9 desaturase, peroxisome proliferators-activated receptor alpha, gamma, and sterol regulatory element binding protein 1 genes in the semitendinous muscle of light lambs of the Rasa Aragonesa breed. BMC Vet Res. 40:3–11.
- Díaz MT, Cañeque V, Sánchez CI, Lauzurica S, Pérez C, Fernández C, Álvarez I, De la Fuente J. 2011. Nutritional and sensory aspects of light lamb meat enriched in n-3 fatty acids during refrigerated storage. Food Chem. 124:147–155. 10.1016/j.foodchem.2010.05.117
- Dinh TTN, Blanton JRJR, Riley DG, Chase CC Jr, Coleman SW, Phillips WA, Brooks JC, Miller MF, Thompson LD. 2010. Intramuscular fat and fatty acid composition of longissimus muscle from divergent pure breeds of cattle. J Anim Sci. 88:756–766. 10.2527/jas.2009-1951
- [EFSA] European Food Safety Authority. 2010. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 8:1–107.
- Enser M, Hallett KG, Hewett B, Fursey GAJ, Wood JD, Harrington G. 1998. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 49:329–341. 10.1016/S0309-1740(97)00144-7
- Fisher AV, Enser M, Richardson RI. 2000. Fatty acid composition and eating quality of lamb types derived from four diverse breed x production systems. Meat Sci. 55:141–147. 10.1016/S0309-1740(99)00136-9
- [IBGE] Instituto Brasileiro de Geografia e Estatística [Brazilian Institute of Geography and Statistics]. 2006. Censo Agropecuário [Agricultural census]. Rio de Janeiro: IBGE. p. 1–777.
- Laborde FL, Mandell IB, Tosh JJ, Wilton JW, Buchanan-Smith JG. 2001. Breed effects on growth performance, carcass characteristics, fatty acid composition and palatability attributes in finishing steers. J Anim Sci. 79:355–365.
- Landim AV, Cardoso MTM, Castanheira M, Fioravanti MC, Louvandini H, McManus C. 2011. Fatty acid profile of hair lambs and their crossbreds slaughtered at different weights. Trop Anim Health Pro. 6:1–6.
- Lobo AMBO, Guimarães SEF, Paiva SR 2012. Differentially transcribed genes in skeletal muscle of lambs. Livest Sci. 150:31–41. 10.1016/j.livsci.2012.07.027
- Lobo AMBO, Lobo RNBL, Paiva SR 2009. Aromatase gene and its effects on growth, reproductive and maternal ability traits in a multibreed sheep population from Brazil. Genet Mol Bio. 32:484–490. 10.1590/S1415-47572009005000054
- Madruga MS, Araújo WO, Sousa WH, Cézar MF, Galvão MS, Cunha MGG. 2006. Efeito do genótipo e do sexo sobre a composição química e o perfil de ácidos graxos da carne de cordeiros [Effect of genotype and sex on chemical composition and fatty acid profile of sheep meat]. Brazil J Anim Sci. 35:1838–1844.
- Madruga MS, Vieira TRL, Cunha MGG, Pereira Filho M, Queiroga RCRE, Sousa WH. 2008. Efeito de dietas com níveis crescentes de caroço de algodão integral sobre a composição química e o perfil de ácidos graxos da carne de cordeiros Santa Inês [Effect of diets with increasing levels of whole cotton seed on chemical composition and fatty acid profile of Santa Inez (Santa Inês) lamb meat]. Brazil J Anim Sci. 37:1496–1502.
- McPhee MJ, Oltjen JW, Famula TR, Sainz RD. 2006. Meta-analysis of factors affecting carcass characteristics of feedlot steers. J Anim Sci. 84:3143–3154. 10.2527/jas.2006-175
- OECD. 2010. Challenges for agricultural research. Paris: OECD Publishing, p. 304. http://dx.doi.org/10.1787/9789264090101-en
- Pelegrini LFV, Pires CC, Kozloski GV, Terra NN, Baggio SR, Campagnol PCB, Galvani DB, Chequim RM. 2007. Fatty acids profile in meat from culling ewes of two breed submitted to two managing systems. Ciênc Rural. 37:1786–1790. 10.1590/S0103-84782007000600044
- Peña F, Juárez M, Bonvillani A, Garcia P, Polvillo O, Domenech V. 2011. Muscle and genotype effects on fatty acid composition of goat kid intramuscular fat. Ital J Anim Sci. 10:212–216.
- Precht J, Molketin D. 2000. Validation of gas-chromatography method for the determination of milk fat by butyric acid analysis. Eur J Lipid Sci Technol. 102:194–201.
- Sainz RD, Paganini RFV. 2004. Effects of different grazing and feeding periods on performance and carcass traits of beef steers. J Anim Sci. 82:292–297.
- Santos VAC, Silva AO, Cardoso JVF, Silvestre AJD, Silva SR, Martins C, Azevedo JMT. 2007. Genotype and sex effects on carcass and meat quality of suckling kids protected by the PGI “Cabrito de Barroso”. Meat Sci. 75:725–736.
- SAS Institute Inc. 1996. SAS/STAT. User's guide, v. 6.11. 4th ed., v.2. Cary: SAS Institute; p. 842.
- Smith SB, Lunt DK, Chung KY, Choi CB, Tume RK, Zembayashi M. 2006. Adiposity, fatty acid composition, and delta-9 desaturase activity during growth in beef cattle. J Anim Sci. 77:478–486.
- Soyeurt H, Dardenne P, Gillon A, Croquet C, Vanderick S, Mayeres P, Bertozzi C, Gengler N. 2006. Variation in fatty acids contents of milk and milk fat within and across breeds. J Dairy Sci. 89:4858–4865.
- Williams CM. 2000. Dietary fatty acids and human health. Ann Zootech. 49:165–180.
- Wood JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, Sheard PR, Enser M. 2003. Effects of fatty acids on meat quality: a review. Meat Sci. 66:21–32.
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. 2008. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78:343–358.
