Abstract
The sex steroid profile of Caspian brown trout was investigated after gonadotropin-releasing hormone analogue (GnRHa) injection throughout the spawning season, i.e. December and January. The values of testosterone (T), 11-Ketotestosterone (11-KT), progesterone (P), cortisol (C) and 17α, hydroxyprogesterone (OHP) were measured for GnRHa treated (the treatment group) and untreated groups (the control group) during the spawning season. In treated males, the values of 11-KT, OHP and C increased 14 days after GnRHa treatment and only showed a significant decrease at the end of the season. Also, the T and P levels showed increases 14 days after GnRHa treatment and then decreased continuously towards the end of the season. In untreated males, sex steroid peaks were observed 27 days after first stripping. In conclusion, our results showed that the GnRHa treatment of Caspian brown trout promotes the final maturation by clear effects on the sex steroid profile.
1. Introduction
Final gamete maturation is a key stage in the reproductive cycle of fish being under control of the pituitary gonadotropin that stimulates the production of sex steroids (Kime Citation1993). The final maturation of fish spermatozoa comprises the releasing of spermatozoa from testis (spermiation) to sperm duct and the obtaining the ability to fertilise (reviewed by Miura & Miura Citation2003), which could be initiated naturally in wild or induced by hormonal treatment in fish hatchery. The Caspian brown trout, Salmo trutta caspius, is a critically endangered anadromous species of the Caspian Sea (Kiabi et al. Citation1999). Due to this, numerous studies have been done in order to increase the efficiency of artificial reproduction specially spermatozoa in this species. For example, in a previous study, we found that gonadotropin-releasing hormone analogue (GnRHa) improves the milt production and sperm motility of Caspian brown trout, over the course of a spawning season (Hajirezaee et al. Citation2011a). At present, it is well recognised that sex steroids play an important role in hormonal control of the reproduction in fish (Kime Citation1993; Barannikova et al. Citation2002). Several studies showed that hormonal injection induces gamete maturation with their impacts on sex steroid profile. Investigation of sex steroid changes in response to artificial induction of reproduction is useful to recognise involving steroids in final maturation. Thus, in the present study, we investigated the changes of some sex steroids after GnRHa treatments during the spawning season.
2. Materials and methods
The experiment was carried out at the Kalar dasht Salmonids Reproduction Center (KSRC), Iran, during the spawning season of Caspian brown trout. In KSRC, males (total weight: 2200 ± 108 g) were held in a concrete pond (9 × 3 × 1.5 m, under a natural photoperiod) and were fed ad libitum daily by artificial food (BioMar). The holding condition was: water flow 100 L/min; temperature range: 21–15°C; dissolved oxygen (mg/L): 9.29.5. With the onset of spermiation, 11 spermiated males were considered for the experiment and divided into two groups. One group received a GnRHa [GnRHa (OvaFact I), SAMEN Company, Mashhad, Iran, GnRHa was injected into the dorsal muscle at a dose of 60 µg kg−1 body weight] and the other was an untreated control.
2.1. Blood collection
Four blood samples were taken from the caudal vein of males using heparinised syringe: 2 December (at the time of the GnRHa treatment); 16 December, 29 December and 10 January. The blood samples were centrifuged (5000 rpm for 10 min) to separate the serum. Then, the serum samples were stored at −20°C until hormonal analysis.
2.2. Steroid assays
Radio Immuno Assay (RIA) method was used for steroid assay. Assay kits were obtained from IMMUNOTECH Company, France. Each Commercial assay kit has two necessary reagents, i.e. antibody and tracer (labelled antigen). The kit properties were described in detail by Hajirezaee et al. (Citation2011b).
2.3. Statistical analysis
The SPSS software (version 16) was used for data analysis. The values of sex steroids were normal according to the Kolmogorov–Smirnov test. One-way analysis of variance (ANOVA) was used to compare the means. When significant F-ratios were calculated using ANOVA, Tukey's test was applied to identify which means were different. The differences were investigated at P < 0.05 level.
3. Results
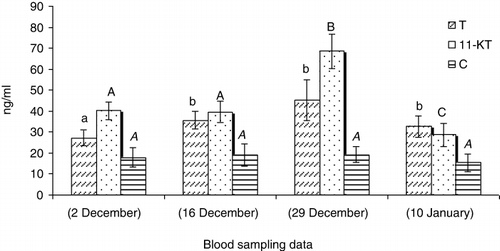
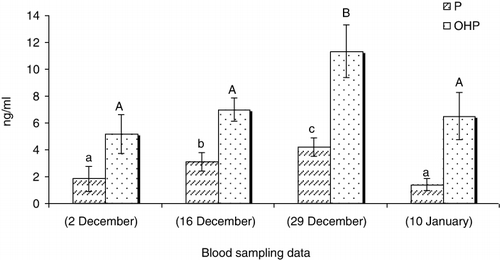
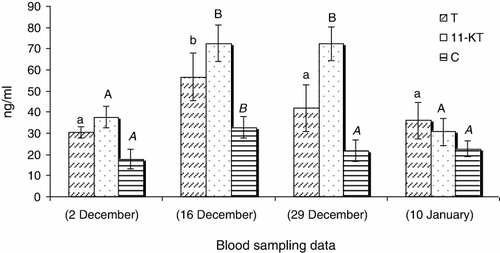
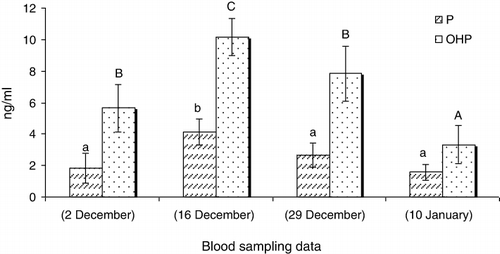
In GnRHa treated males, the values of 11-Ketotestosterone (11-KT) (), hydroxyprogesterone (OHP) () and cortisol (C) () increased 14 days after GnRHa treatment and only showed a significant decrease at the end of the season (P < 0.05). The testosterone (T) () and progesterone (P) () levels showed increases 14 days after GnRHa treatment and then decreased continuously towards the end of the season (P < 0.05). In untreated males, sex steroid peaks were observed 27 days after first blood sampling ( and ; P < 0.05).
4. Discussion
Our study showed that a single injection of GnRHa (60 mg kg−1 body weight) changes the sex steroid profile of Caspian brown trout after the beginning of spermiation. In this regard, the levels of all measured steroids increased 14 days after GnRHa injection compared to untreated fish. In untreated fish, the maximum levels of sex steroids were observed in the middle of the spawning season. But, in injected fish, the sex steroids were always high and only decreased at the last blood sampling. Several studies showed the changes of sex steroid profile of fish in response to GnRHa, although these profiles are different in terms of change levels and nature of steroids. In teleosts, it is well recognised that the effect of GnRHa on reproduction is mediated by the stimulation of the pituitary to release gonadotropin hormone (GtH), which then stimulates the testes to produce steroids (Crim et al. Citation1983; Takashima et al. Citation1984). In a previous study by Hajirezaee et al. (Citation2011a), the sperm motility and sperm production increased after GnRHa treatment of Caspian brown trout while in untreated males these parameters did not show clear changes. In the males of teleosts fish, T and 11-KT have been identified to be the main steroids involved in spermatogenesis and initiation of spermiation (Nagahama Citation1983; Yaron Citation1995). In our study, the elevated levels of androgens after GnRHa treatment may be responsible for increasing sperm production. Cortisol is the most commonly measured indicator of stress in fish that usually provides a good reflection of the severity and duration of the stress response (Barton & Iwama Citation1991; Wendelaar Bonga Citation1997; Fevolden et al. Citation2002). Cortisol has also been considered as an endocrine component of the reproductive system in teleosts fish (Billard et al. Citation1981). The milt collection involves the handling of fish that mostly is accompanied with elevations in serum C levels. But, there was no elevation in C levels after first milt collection practice from Caspian brown trout in this study. This probable may be due to the long intervals (12–14 days) between blood samplings so that probably the C levels have increased and returned to resting levels before each blood sampling; thus, we could not observe any elevation in serum C at the different times of blood sampling. On the other hand, the considerable increases of C after a long gap between the GnRHa injection and the blood sampling emphasis the probable role of this steroid in maturation. Several studies concluded that progestogens have key role on final maturation of fish spermatozoa including: acquisition of sperm motility and testis hydration (Miura et al. Citation1992). The increase of P and OHP levels after GnRHa treatments may be responsible for final maturation of Caspian brown trout spermatozoa. In conclusion, our results showed that the GnRHa treatment of Caspian brown trout promotes the final maturation by clear effects on sex steroid profile.
Acknowledgement
The authors express their sincere appreciation to the people who gave their time, advice and support to this study.
References
- Barannikova IA, Dyubin VP, Bayunova LV, Semenkova TB. 2002. Steroids in the control of reproductive function of fish. Neurosci Behav Physiol. 32:141–148. 10.1023/A:1013923308125
- Barton BA, Iwama GK. 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis. 1:3–26. 10.1016/0959-8030(91)90019-G
- Billard R, Bry C, Gillet C. 1981. Stress, environment and reproduction in teleost fish. In: Pickering AD, editor. Stress and fish. London: Academic Press; p. 185–208.
- Crim LW, Evans DM, Vickery BH. 1983. Manipulation of the seasonal reproductive cycle of land locked Atlantic salmon (Salmo salar) by LHRH analogues administered at various stages of gonadal development. Can J Fish Aquat Sci. 40:61–67. 10.1139/f83-009
- Fevolden SE, Roed KH, Fjalestad KT. 2002. Selection response of cortisol and lysozyme in rainbow trout and correlation to growth. Aquaculture. 205:61–75. 10.1016/S0044-8486(01)00660-3
- Hajirezaee S, Mojazi Amiri B, Mehrpoosh M, Nazeri S, Niksirat H. 2011a. Gonadotropin releasing hormone-analogue (GnRHa) treatment improves the milt production and sperm motility of endangered Caspian brown trout, Salmo trutta caspius, over the course of a spawning season. Aquaculture Res. 42:1789–1795. 10.1111/j.1365-2109.2010.02776.x
- Hajirezaee S, Rafiee GHR, Hushangi R. 2011b. Comparative analysis of milt quality and steroid levels in blood and seminal fluid of Persian sturgeon males, Acipenser persicus during final maturation induced by hormonal treatment. Biologia. 66:160–169. 10.2478/s11756-010-0140-5
- Kiabi BH, Abdoli A, Naderi M. 1999. Status of fish fauna in the south Caspian basin of Iran. Zool Middle East. 18:57–65. 10.1080/09397140.1999.10637782
- Kime DE. 1993. “Classical” and “non-classical” reproductive steroids in fish. Rev Fish Biol Fish. 3:160–180. 10.1007/BF00045230
- Miura T, Miura CI. 2003. Molecular control mechanisms of fish spermatogenesis. Fish Physiol Biochem. 28:181–186. 10.1023/B:FISH.0000030522.71779.47
- Miura T, Yamauchi K, Takahashi H, Nagahama Y. 1992. The role of hormones in the acquisition of sperm motility in salmonid fish. J Exp Zool. 261:359–363. 10.1002/jez.1402610316
- Nagahama Y. 1983. The functional morphology of teleosts gonads. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish Physiology. New York, NY: Academic Press; p. 223–275.
- Takashima F, Weil C, Billard R, Crim LW, Fostier A. 1984. Stimulation of spermiation by LHRH-analogue in carp. Bull Jpn Soc Sci Fish. 50:1323–1329. 10.2331/suisan.50.1323
- Wendelaar Bonga SE. 1997. The stress response in fish. Physiol Rev. 77:591–625.
- Yaron Z. 1995. Endocrine control of gametogenesis and spawning induction in the carp. Aquaculture. 129:49–73. 10.1016/0044-8486(94)00229-H