Abstract
The host responses to disease agents differ between age and sex as is evidenced from epidemiological studies which have been ascribed to the effects of steroid hormones. The present study was carried out in 20 Murrah buffaloes of either sex at pre- and post-pubertal age having discrete sex steroid background to explore the variation, if any, in physiological and immune responses upon intravenous endotoxin challenge (E. coli 055:B5 @ 0.6µg/kg body weight). The rectal temperature, heart rate and respiration rate differed significantly from base values at only 1 h post challenge and reached peak at 4 h. The magnitude of responses determined as area under concentration × time curve (AUC) for these parameters was significantly low in post-pubertal animals of either sex. Plasma tumour necrosis factor alpha (TNFα, a pro-inflammatory cytokine) registered peak at 1 h post challenge which returned to base level value by 8 h post challenge. Responses determined as AUC of TNFα were significantly higher in post-pubertal males. Plasma NOx (an estimate of nitric oxide production) and XO (mediator of superoxide production) significantly differed from base value at 4 h and peaked at 8 h post challenge. Integrated AUC response of NOx was significantly higher in pre-pubertal males and that of XO in pre-pubertal males as well as females. Plasma cortisol (an anti-inflammatory hormone) was significantly different from base value at 2 h and peaked between 4 h and 8 h post challenge; its integrated AUC response was significantly greater in post-pubertal females. Endotoxin challenge resulted into neutrophilia (a stress marker), and the initial hyperglycemia was followed by hypoglycemia in all experimental animals irrespective of age and sex. Pre-pubertal buffaloes had no dominance of gonadal sex steroids in plasma. Post-pubertal males and females had plasma testosterone and oestrogen, respectively in significant quantity. The present findings in Murrah buffaloes recognise the age and sex as a source of variability in the magnitude of immune responses to endotoxin challenge like lipopolysaccharide (LPS).
1. Introduction
The effects of sex steroids extend far beyond their principal role in sexual differentiation and reproduction. Sex steroids have been demonstrated to participate in immune modulation at antigenic challenge (Marco et al. Citation2009). Olsen and Kovacs (Citation1996) verified the variability in host response to some diseases due to the immunomodulating effects of oestrogens, androgens and progestins. Sex steroids were found to have substantial effect on both humoral and cellular immune responses (Lee & Chiang Citation2012; Pampori & Sujata Citation2012). Sartin et al. (Citation2003) have shown oestrogen plus progesterone implants to favourably alter the time course and disease severity of many of clinical manifestations associated with coccidiosis and endotoxemia in calves. In domestic animals, the sexual dimorphism was reported in disease incidence (Haider et al. Citation2008; Ramzan et al. Citation2009) that provides an indirect evidence of role of sex hormones in immune modulation. Horadagoda et al. (Citation2002) and Kahl et al. (Citation2009) reported variation in buffalo and cattle, respectively, in their immune responses to endotoxin challenge during oestrus and dioestrus phases of reproductive cycle.
Cytokine reaction to stress conditions modelled by endotoxin administration like lipopolysaccharide (LPS) have attracted a lot of attention as biomarkers for and mediators of both homeostatic and pathophysiological processes in vivo. Many studies suggest that the outcome of some infections may be associated with, and may be predicted by, plasma levels of cytokines during disease, particularly tumour necrosis factor alpha (TNFα; Cavaillon et al. Citation2003). The pro-inflammatory cytokine TNFα is one of the first inflammatory mediators to appear in response to infection, after which it induces a number of different biological effects in various cells, thus initiating and regulating the immune response (Bemelmans et al. Citation1996). Nitric oxide (NO), xanthine oxidase (XO) and cortisol are also closely associated with infections and stress conditions, influencing the host immune responses. NO, a gaseous molecule, is a highly reactive free radical with multiple and complex roles within many biological systems, generated by macrophages in response to invading microbes, and acts as a killer molecule in immune system (Rosselli et al. Citation1998). There is a wide participation of NO in the crosstalk between neuroendocrine and immune systems; Ebru et al. (Citation2006) demonstrated profuse release of NO in splenocyte cultures from oestrogen-treated as compared to non-treated mice. Similarly XO exclusively uses O2, yielding reactive oxygen species O2− and H2O2 as reaction by-products that may initiate oxidative cell damage and organ failure (Wang et al. Citation2002). It is also involved in the generation of uric acid (a general antioxidant), generation and metabolic fate of NO (Godber et al. Citation2000). Cortisol, an adrenal steroid hormone, generally referred to as stress hormone activates anti-inflammatory and anti-stress pathways. It has a direct bearing on the host immunity regulating proliferation of T cells and responsiveness to interleukins (Palacios and Sugawara Citation1982; Besedovsky et al. Citation1986).
In view of the probable role played by sex steroids in immune responses, evaluation of plasma levels of immune biomarkers like TNFα, NO, XO and cortisol was taken up in Murrah buffaloes of different age and sex challenged with LPS. The knowledge of their immune competence at different stages of life, physiology and reproduction with distinctive sex steroid environment will probably help in improving clinical output while delivering prophylactic and therapeutic interventions.
2. Materials and methods
Buffalo being economically important livestock species in Asian and Mediterranean countries and their failure of immune responses could result in temporary or permanent infertility or sterility (Hafez Citation2002) that could adversely affect the national economy and rural food security in these regions. Present research work was conducted at National Dairy Research Institute (NDRI), Karnal, Haryana in North India that was acceptable to the ethical standards of the Institute vide IAEC No. 23/09.
2.1. Animals and sampling
Twenty apparently healthy Murrah buffaloes selected from NDRI cattle research farm were grouped into pre- and post-pubertal. Group pre-pubertal had 5 male and 5 female buffaloes of age ranging between 11 and 13 months. Group post-pubertal contained 36- to 40-month-old 5 males and 5 cyclic buffaloes of first parity at day 10 of estrous cycle. Oestrus in the buffaloes was confirmed through rectal examination by the concerned expert veterinarian at the farm and the selected buffaloes were not inseminated but challenged with LPS at day 10 of estrous cycle. All these buffaloes were maintained under routine management and nutritional practices as followed in the herd at the farm. Eight animals (4 males and 4 females) from each group with equal representation of male and female sex were given single intravenous bolus of LPS (E. coli 055:B5 from Sigma Chemical Co., USA.) in jugular vein at 0.6 µg/kg body weight, reconstituted in 10-ml sterile normal saline. Two animals serving as control from each group were administered 10 ml of sterile normal saline intravenously as placebo. The experiment was conducted during autumn season, with mean maximum temperature 30.4°C and minimum 20.9°C. All the animals, including controls, were injected in the morning at 8:30. Collection of blood as well as recording of physiological parameters was done just before challenge (0 h) and subsequently at 1, 2, 4, 8 and 24 h interval after LPS challenge. Changes in the physical activities of animals were observed and recorded after endotoxin injection for 12 h. Physiological parameters like rectal temperature, heart rate and respiration rate were recorded, with the help of digital thermometer and stethoscope, respectively. Seven millilitre of blood was drawn in heparinised vacutainers from jugular vein of all animals including placebo treated after taking all necessary aseptic measures.
2.2. Experimental procedure
Total leukocyte count (TLC) and differential leukocyte count (DLC) were determined immediately after blood collection and plasma separated by centrifugation was stored at −20°C in aliquots of 1 ml until the analysis of immune competence markers.
Plasma NO was estimated as total nitrite (NOx) using modified Griess reaction (Miranda et al. Citation2001). Acetonitrile was used for deproteinisation of plasma proteins (Ghasemi et al. Citation2007). Plasma TNFα, XO and cortisol were evaluated by using Bovine TNFα enzyme linked immunosorbant assay (ELISA) kit (Cusabio Biotech Co.), XO assay kit (Bio Vision Research Products, USA) and cortisol enzyme immune assay (EIA) kit (Cayman Chemical Company, Ann Arbor, MI), respectively. Plasma glucose was estimated using GOD-POD kit (Span Diagnostics Ltd., India).
Oestradiol and testosterone were extracted from the plasma with benzene and diethyl ether extraction method, respectively, and progesterone was estimated using plasma directly (Kamboj & Prakash Citation1993). All the three sex steroids were determined by radio-immune assay (RIA) using 3H-labelled tracers and antisera from Sigma Chemical Co., USA as per the standard procedures followed routinely in our laboratory and the Beckman beta counter, USA was used for counting β radiation.
Area under concentration × time curve (AUC) was calculated by commonly approached numerical approximation method, the trapezoidal rule, for calculating the total change in concentrations over a period of time. Baseline control values were subtracted to get net increase in AUC over a given period of time.
2.3. Statistical analysis
The data analysis was performed using Systat 12 software package 2007 (Systat Software Inc. 1735 Technology Dr. Ste. 430, San Jose, CA 95110, USA). Analysis of variance (ANOVA) with variables such as age, sex and time included in the model as fixed effects and Tukey's honestly significant difference test was employed.
3. Results
In post-pubertal female buffaloes, the mean levels of plasma oestradiol-17β and progesterone were 7.16 ± 0.70 pg/ml and 2.21 ± 0.13 ng/ml, respectively. All other animals under study did not present detectable amount of plasma oestrogen and progesterone with our RIA test. The average plasma testosterone level was significantly (P < 0.001) lower in pre-pubertal (66.66 ± 6.97 pg/ml) than post-pubertal male buffaloes (883.13 ± 43.94 pg/ml) whereas females of either group did not present any detectable level of plasma testosterone.
The LPS challenge resulted into changes in physical activities of pre-pubertal buffaloes of either sex. They became lethargic, sat down or remained confused for a long duration even up to 8 h post challenge. Mucus dribbling from mouth or nostrils and protrusion of tongue were also reported in pre-pubertal buffaloes whereas no such severe symptoms were observed in post-pubertal buffaloes of either sex. However, post-pubertal buffaloes of either sex remained isolated and puzzled for an hour or two after LPS challenge.
Rectal temperature, heart rate and respiration rate response to LPS challenge determined as AUC for first 8 h was significantly (P < 0.05) higher in pre-pubertal buffaloes as compared to other animals under study (). Rectal temperature, heart rate and respiration rate when compared to 0-h base values significantly (P < 0.001) increased at 1 h and peaked at 4 h post challenge in all animals irrespective of age and sex. However, heart rate in post-pubertal females did change least (). Rectal temperature in both the age groups differed significantly (P < 0.05) between sexes at 2- and 4-h time intervals ( and ). The heart rate and respiration rate differed significantly (P < 0.05) between the sexes within the same age group at different time intervals (–).
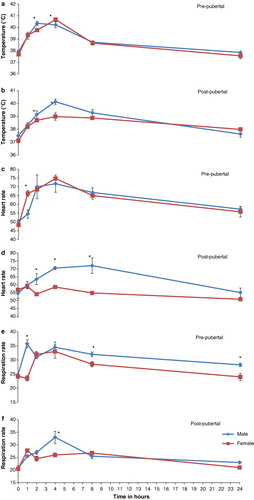
Table 1. Integrated responses to LPS challenge calculated as AUC with base line subtracted in Murrah buffaloes of different age and sex.
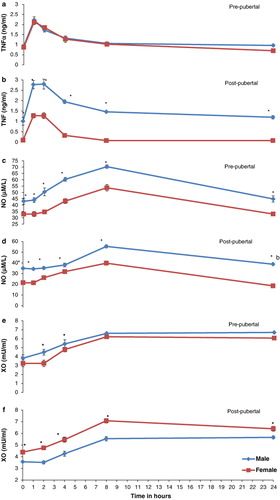
Integrated responses determined as AUC were significantly (P < 0.05) elevated for TNFα, NOx, XO and cortisol in post-pubertal males, pre-pubertal males, pre-pubertal males as well as females and post-pubertal females, respectively, as compared to other respective groups (). Plasma TNFα registered peak at 1 h that was significantly (P < 0.001) different from 0-h control value and returned to base level by 8 h post challenge in all buffaloes irrespective of age and sex. In post-pubertal group, males had higher plasma levels of TNFα than females at all points of time interval( and ). When compared to 0-h control values, NOx and XO levels were significantly (P < 0.01) higher at 4 h and reached peak at 8 h post challenge. However, NOx declined to base value by 24 h whereas as XO remained elevated through 24 h post challenge in all buffaloes irrespective of age and sex (–). Pre-pubertal as well as post-pubertal male buffaloes and post-pubertal female buffaloes had respectively plasma NOx and XO higher (P < 0.05) at all points of time interval including the 0-h base value (–). Cortisol was significantly (P < 0.01) higher at 2 h post challenge as compared to 0-h base level, peaked at 4 h and declined thereafter in all animals except post-pubertal female buffaloes where it peaked at 8 h and remained significantly (P < 0.05) elevated through 24 h post challenge ( and ).
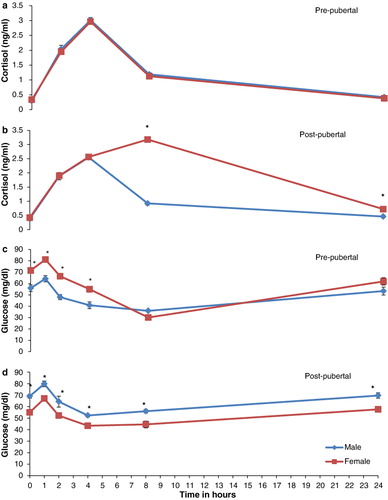
Buffaloes irrespective of age and sex exhibited hyperglycaemia within 1 h of LPS challenge but shortly entered into hypoglycaemia that remained severe between 4 h and 8 h. Plasma glucose returned to base level by 24 h post challenge. Glucose responses to LPS determined as AUC for first 2 h post challenge was significantly (P < 0.01) higher in post-pubertal buffaloes as compared to pre-pubertal buffaloes of either sex (). Hypoglycaemia followed by initial hyperglycaemia determined as AUC for 2–8 h post challenge was registered significantly (P < 0.05) severe in male buffaloes as compared to female buffaloes of either age group (). The variability trend in blood glucose was similar in all the animals irrespective of age or sex, but the levels were significantly (P < 0.05) higher in pre-pubertal females and post-pubertal males at all time points including 0 h as compared to respective counterparts ( and ).
TLC did not differ significantly in any age group of either sex at LPS challenge, however, TLC was significantly (P < 0.05) least in post-pubertal males (8.97 ± 0.36 × 103). DLC did exhibit changes at LPS challenge and neutrophilia was a common finding in all animals irrespective of age or sex. Neutrophil percentage analysed as overall mean increased significantly (P < 0.001) from 24.406 ± 0.67 at 0 h to 31.406 ± 0.67 at 24 h post challenge. Post-pubertal male buffaloes registered maximum change (27 ± 1.34 at 0 h to 38.875 ± 1.34 at 24 h) and post-pubertal females the least (25.00 ± 0.70 at 0 h to 28.00 ± 1.08 at 24 h) in neutrophil percentage upon LPS challenge.
4. Discussion
The post-pubertal females taken in present study were in dioestrous stage of reproductive cycle (day 10), therefore, had higher plasma progesterone level that was comparable to the levels reported by Rao and Pandey (Citation1982) and Sharma et al. (Citation1984). Appreciable amount of oestrogen besides progesterone recorded in dioestrous phase was in agreement with the observations of Shafie et al. (Citation1982) in buffaloes. The testosterone levels reported in male buffaloes in present study revealed a positive correlation of testosterone with age that is supported by the findings of Sharma et al. (Citation1984) in buffaloes. Plasma testosterone in post-pubertal Murrah bulls reported in present study was low as compared to the levels reported by Gupta et al. (Citation1984). This could be due to the autumn season of sampling and separate rearing of bulls from females which is substantiated by the findings of Malfatti et al. (Citation2006).
The changes in the physical activities after LPS challenge at 0.6 µg/kg body weight, in the first instance, signified the toxicity of LPS. The severity of LPS-induced endotoxicity in pre-pubertal buffaloes with little of sex steroid hormone differentiation indicated their vulnerability to infections as compared to adult ones.
The integrated responses calculated as AUC for rectal temperature, heart rate and respiration rate were higher in pre-pubertal buffaloes than post-pubertal that suggest higher sensitivity of young buffaloes to endotoxin challenge than the adults. The higher magnitude of immune reactivity in pre-pubertal buffaloes probably render this group of animals more vulnerable to antigenic insult and could be the reason of high mortality in young calves after infections, particularly gram-negative bacterial infections. The weak or attenuated physical and physiological responses to endotoxin insult in post-pubertal group with distinctive sex steroid milieu show the possibility of sex steroids playing a role in these responses. It could be a result of mature immune system or exposure of adults to an array of antigenic agents with resultant resistance in them. Responses to LPS reported by Jacobsen et al. (Citation2005) in cattle are in agreement with the present findings.
Recognised as significant markers of immunity TNFα, a pro-inflammatory cytokine essential for initiating cascade of other cytokines produced by immune cells (Cavaillon et al. Citation2003), NO a product of L-arginine metabolism with a powerful cytotoxic effect for a variety of target cells (Stuehr & Nathan Citation1989), XO a complex molybdoflavoenzyme, with antioxidant (Harrison Citation2002) as well as pro-inflammatory properties (Lance et al. Citation1997), cortisol a stress hormone with strong anti-inflammatory property and TLC, DLC important cells of host defence (Litman et al. Citation2005) were evaluated in the present study. These immune response mediators significantly elevated after LPS challenge but the magnitude of responses varied with the age and sex in buffaloes. The magnitude of response in most pro-inflammatory immune mediators was higher in male sex that signifies perceptive immune reactivity an inherent characteristic of males. The integrated responses determined as AUC recorded for plasma TNFα were higher in males of either age group which is in conformity with the findings of Jorg et al. (Citation1998) in humans. In post-pubertal males, the higher responses of TNFα could be attributed to appreciable levels of testosterone in these animals that have been seconded earlier by Cutolo and Wilder (Citation2000) and Marco et al. (Citation2009) in humans. Since plasma levels of TNFα are aligned with the outcome of disease (Cavaillon et al. Citation2003), therefore, a different therapeutic and prophylactic approach is to be exercised in mature males with antigenic insult. In the present study, males of either age group had significantly (P < 0.05) higher plasma NOx level at all points of time interval and its integrated responses to LPS (AUC) for 24 h as compared to females of either group. These findings were in agreement with that of Tokumitsu et al. (Citation2000) who reported NOx age and sex biased with high in adult men as compared to women. Low levels of NO in females have been attributed to progesterone (Li et al. Citation2009), however, higher levels of NO in males could not probably be assigned to testosterone as was significantly high in pre-pubertal males with little testosterone environment. Probably increased plasma levels of NOx in males are an inherent character of males as male lymphocytes cultured in in vitro expressed greater NO in culture medium than female lymphocytes (Pampori & Sujata Citation2012). Integrated responses of plasma XO calculated as AUC were higher in pre-pubertal group of animals as compared to post-pubertal group which, however, differed from the findings of Kuldip et al. (Citation2009) in humans who reported positive correlation of XO with age. LPS challenge resulted into increased circulatory levels of XO as well as NO in pre-pubertal buffaloes which could play an important role in immune defence and homeostasis. XO promotes systemic inflammatory responses which if not balanced can lead to deleterious effects, but elevated NO on the other hand counteracts XO functions and thus helps in maintaining homeostasis (Lance et al. Citation1997). Cortisol responses recorded as AUC were highest in post-pubertal females and least in post-pubertal males. The high and sustained levels of a strong anti-inflammatory cortisol in post-pubertal female buffaloes contrary to males probably serve as a safety factor to combat embryo rejection. Progesterone is the candidate hormone in the maintenance of pregnancy and likely to be responsible for weak immune responses in post-pubertal females which had high plasma progesterone level. Progesterone has been entailed in transmitting immunosuppressive signals during pregnancy by decreasing the production of inflammatory cytokines, particularly TNFα (Olsen & Kovacs Citation1996) which may make these post-pubertal females more vulnerable to infections. High cortisol plasma levels in progesterone-dominated female buffaloes support the findings of Ramadan et al. (Citation1997) in sheep, Wulster-Radcliffe et al. (Citation2003) in gilts and Kahl et al. (Citation2009) in cattle which ascribed the suppression of immune reactivity and increased risk of infections during luteal phase of cycle to progesterone.
Since the LPS challenge puts the animal under stress which is an energy consuming state (Pampori et al. Citation2010), therefore, plasma glucose levels were also monitored during challenge. Initial hyperglycaemia recorded as a metabolic adaptation to maintain homeostasis was followed by hypoglycaemia which was significantly (P < 0.05) least in post-pubertal females as compared to other animals under study. Least changes in the circulatory glucose level in post-pubertal female buffaloes could be tagged to the higher cortisol responses in these animals which is a known gluconeogenic hormone. Present trend in the plasma glucose at LPS challenge was in agreement with the findings of Giri et al. (Citation1990) and Kahl et al. (Citation2009) in cattle subjected to LPS challenge.
The cellular elements of immune system, neutrophils and lymphocytes act independently and provide first-hand information about the infections. They are observed as dominant system of host defence in most organisms (Litman et al. Citation2005). Neutrophilia was a common finding during endotoxin insult in the present study. The shift in neutrophil percentage was severe in post-pubertal males as compared to other animal groups under study and testosterone could be the candidate hormone responsible for it which needs further study.
The response of pro-inflammatory (TNFα) and anti-inflammatory (cortisol) immune mediators to LPS challenge was higher and lower, respectively, in post-pubertal males with plasma testosterone dominance besides coupled with severe hypoglycaemia render these animals to an increased risk of pathophysiological changes after endotoxin insult. On the other hand post-pubertal female buffaloes with plasma progesterone supremacy recorded frail or attenuated responses for TNFα, XO and NO but strong responses for anti-inflammatory mediator, cortisol, probably spare these animals from severe immune reactivity and undesirable consequences of endotoxin challenge. Reports suggest that anti-inflammatory properties of progesterone in rodents and humans are mainly mediated through inhibition of production and the release of a number of pro-inflammatory cytokines and inhibition of NO production (Miller et al. Citation1996). The present study indicates that resistance to LPS changes over time with change in age or physiological status. Therefore, sex hormone balance could be a key reason in the regulation of immune and inflammatory responses. Hence, therapeutical modulation of sex hormone balance should represent a part of advanced biological treatments or prophylactics in animal ailments.
5. Conclusion
The present study in Murrah buffaloes of different age and sex with distinctive sex steroid hormone environment was undertaken to investigate any source of variation in immune competence when challenged. The present study recognised the age- and sex-related variability in immune competence in buffaloes. The study reflected higher immune reaction in male buffaloes and least in adult cyclic buffaloes at dioestrous when challenged. The information gathered in buffaloes during present study could be utilised to modify the disease outcome in economically important domestic species. It also warrants consideration of sex hormone balance while approaching for any therapeutical or prophylactic interventions for animal diseases.
References
- Bemelmans MHA, Van Tits LJH, Buurman WA. 1996. Tumor necrosis factor: function, release and clearance. Crit Rev Immunol. 16:1–11. 10.1615/CritRevImmunol.v16.i1.10
- Besedovsky HO, Del Rey A, Sorkin E, Dinarello CA. 1986. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 233:652.
- Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. 2003. Cytokine cascade in sepsis. Scand J Infect Dis. 35:535–444. 10.1080/00365540310015935
- Cutolo M, Wilder RL. 2000. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 26:825–839. 10.1016/S0889-857X(05)70171-X
- Ebru K, Jillian BF, Rebecc AP, Andrea JL, Francois E, Ansar SA. 2006. Estrogen up-regulates inducible nitric oxide synthase, nitric oxide and cyclooxygenase-2 in splenocytes activated with T cell stimulants: role of interferon. Endocrinol. 147:662–671. 10.1210/en.2005-0829
- Ghasemi A, Hedayati M, Biabani H. 2007. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. J Med Sci Res. 15:29–32.
- Giri SN, Emau P, Cullor JS, Stabenfeldt GH, Bruss ML, Bondurant RH, Osburn BI. 1990. Effects of endotoxin infusion on circulating levels of eicosanoids, progesterone, cortisol, glucose and lactic acid, and abortion in pregnant cows. Vet Microbiol. 21:211–231. 10.1016/0378-1135(90)90033-R
- Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R. 2000. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 275:7757–7763. 10.1074/jbc.275.11.7757
- Gupta RC, Sharma AK, Khurana NK. 1984. Testosterone levels and libido in buffalo bull. 10th Int. Cong. Anim. Reprod. Artif. Insem. Univ. Illinois, Urbana-Champaign, Chicago, USA. 282.
- Hafez ESE. 2002. Reproduction in farm animals. 12th ed. Philadelphia: Lea & Febiger.
- Haider AK, Muhammad S, Muhammad A, Muhammad JA, Manzoor H. 2008. Prevalence and distribution of peste des petits ruminants virus infection in small ruminants. Small Ruminant Res. 79:152–157. 10.1016/j.smallrumres.2008.07.021
- Harrison R. 2002. Structure and function of xanthine oxidoreductase: where we are now. Free Radical Biol Med. 33:774–779. 10.1016/S0891-5849(02)00956-5
- Horadagoda NU, Hodgsony JC, Moony GM, Wijewardanaz TG, Eckersall PD. 2002. Development of a clinical syndrome resembling haemorrhagic septicaemia in the buffalo following intravenous inoculation of Pasteurella multocida serotype B:2 endotoxin and the role of tumour necrosis factor-α. Res Vet Sci. 72:194–200. 10.1053/rvsc.2001.0538
- Jacobsen S, Toelboell T, Andersen PH. 2005. Dose dependency and individual variability in selected clinical, haematological and blood biochemical responses after systemic lipopolysaccharide challenge in cattle. Vet Res. 36:167–178. 10.1051/vetres:2004062
- Jorg S, Volker K, Karl-Hermann S, Peter Z, Frank S. 1998. Gender differences in human sepsis. Arch Surg. 133:1200–1205. 10.1001/archsurg.133.11.1200
- Kahl S, Elsasser TH, Li CJ. 2009. Variability in TNF-α, nitric oxide, and xanthine oxidase responses to endotoxin challenge in heifers: effect of estrous cycle stage. Domest Anim Endocrinol. 36:82–88. 10.1016/j.domaniend.2008.10.006
- Kamboj M, Prakash BS. 1993. Relationship of progesterone in plasma and whole milk of buffaloes during cyclicity and early pregnancy. Trop Anim Health Prod. 25:185–192. 10.1007/BF02236239
- Kuldip S, Sandeep K, Kanta K, Gurpreet S, Amrit K. 2009. Alterations in lipid peroxidation and certain antioxidant enzymes in different age groups under physiological conditions. J Hum Ecol. 27:143–147.
- Lance ST, John E R, Dale P, Brooks MH. 1997. Endogenous nitric oxide decreases xanthine oxidase-mediated neutrophil adherence: role of P-selectin. J Appl Physiol. 82:913–917.
- Lee T.-P, Chiang B. 2012. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmune Rev. 11:422–429. 10.1016/j.autrev.2011.11.020
- Li S, Yixi S, Feng M, Pingping L, Hefeng H, Jun Z. 2009. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-κB activation and enhancing SOCS1 expression. Immunol Lett. 125:151–155. 10.1016/j.imlet.2009.07.001
- Litman GW, Cannon JP, Dishaw LJ. 2005. Reconstructing immune phylogeny: new perspectives. Nat Rev Immunol. 5:866–879. 10.1038/nri1712
- Malfatti A, Barbato O, Todini L, Terzano GM, Debenedetti A, Borghese A. 2006. Blood testosterone levels in Italian Mediterranean buffalo bulls managed in two different breeding conditions. Theriogenol. 65:1137–1144. 10.1016/j.theriogenology.2005.07.012
- Marco AD, Karen N, Gloria S, Lorena L, Jesús R, José AV, Jorge M. 2009. Immune sexual dimorphism: effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J Steroid Biochem Mol Biol. 113:57–64. 10.1016/j.jsbmb.2008.11.003
- Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. 1996. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 59:442–450.
- Miranda KM, Espey MG, Wink DA. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5:62–71. 10.1006/niox.2000.0319
- Olsen NJ, Kovacs WJ. 1996. Gonadal steroids and immunity. Endocr Rev. 17:369–384.
- Palacios R, Sugawara I. 1982. Hydrocortisone abrogates proliferation of T cells in autologous mixed lymphocyte reaction by rendering the interleukin-2 Producer T cells unresponsive to interleukin-1 and unable to synthesize the T-cell growth factor. Scand J Immunol. 15:25–31. 10.1111/j.1365-3083.1982.tb00618.x
- Pampori ZA, Huozha R, Shah KA, Andrabi SA, Tauseef A. 2010. Stress versus reproduction in animals. Vet Scan. 5:61.
- Pampori ZA, Sujata P. 2012. Analysis of invitro effects of sex steroids on lymphocyte responsiveness in Murrah buffaloes (Bubalus bubalis). Vet Med Int. 2012:9. doi: 10.1155/2012/139589
- Ramadan AA, Johnson GL, Lewis GS. 1997. Regulation of uterine immune function during the estrous cycle and in response to infectious bacteria in sheep. J Anim Sci. 75:1621–1632.
- Ramzan M, Akhtar M, Muhammad F, Hussain I, Hiszczyńska-Sawicka E, Haq AU, Mahmood MS, Hafeez MA. 2009. Seroprevalence of Toxoplasma gondii in sheep and goats in Rahim Yar Khan (Punjab), Pakistan. Trop Anim Health Prod. 41:1225–1129. 10.1007/s11250-009-9304-0
- Rao LV, Pandey RS. 1982. Seasonal changes in plasma progesterone concentrations in buffalo cows (Bubalus bubalis). J Reprod Fertil. 66:57–61. 10.1530/jrf.0.0660057
- Rosselli M, Paul JK, Raghvendra KD. 1998. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 4:3–24. 10.1093/humupd/4.1.3
- Sartin JL, Elsasser TH, Kahl S, Baker J, Daniel JA, Schwartz DD, Steele B, Whitlock BK. 2003. Estradiol plus progesterone treatment modulates select elements of the proinflammatory cytokine cascade in steers: attenuated nitric oxide and thromboxane B2 production in endotoxemia. J Anim Sci. 81:1546–1551.
- Shafie MM, Mourad H, Barkawi AH, Aboul-Ela MB, Mekawy Y. 1982. Serum progesterone and oestradiol concentration in the cyclic buffalo. Anim Prod. 7:283–289.
- Sharma IJ, Agarwal SP, Agarwal VK, Dwaraknath PK. 1984. Changes in profile of serum sex steroids of male buffaloes from birth to maturity. Theriogenol. 22:175–187. 10.1016/0093-691X(84)90430-8
- Stuehr DJ, Nathan CF. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumour target cells. J Exp Med. 169:1543. 10.1084/jem.169.5.1543
- Tokumitsu W, Masahiro A, Kenji T, Koichi K, Masato E, Naota S, Takahiro K, Masayoshi H, Wataru S, Yasuyoshi O. 2000. Influence of sex and age on serum nitrite/nitrate concentration in healthy subjects. Clin Chim Acta. 301:169–179. 10.1016/S0009-8981(00)00340-5
- Wang J, Van Praagh A, Hamilton E, Wang Q, Zou B, Muranjan M. 2002. Serum xanthine oxidase: origin, regulation, and contribution to control of trypanosome parasitemia. Antioxid Redox Sign. 4:161–178. 10.1089/152308602753625933
- Wulster-Radcliffe MC, Seals RC, Lewis GS. 2003. Progesterone increases susceptibility of gilts to uterine infections after intrauterine inoculation with infectious bacteria. J Anim Sci. 81:1242–1252.
