Abstract
Experiments were conducted with the aim of determining effects of melatonin on fluctuations in cloacal temperature (CT) and erythrocyte osmotic fragility (EOF) of layer hens. The CT of 14 melatonin-treated and 12 control laying hens were taken for 2 days, 1 week apart, at 06:00 h, 14:00 h and 18:00 h, using a standard digital thermometer inserted into the cloaca. The control birds were given water only, while the treated layers were individually administered at 18:00 h with melatonin orally. On day 12 of the administration, venous blood samples were collected from the layer hens to determine EOF. The overall mean CT values in melatonin-treated and control birds were 41.0 ± 0.05°C and 40.9 ± 0.04°C, respectively (P < 0.05). CT of the melatonin-treated group was lowest at 06:00 h (40.9 ± 0.10°C), but highest at 18:00 h (41.2 ± 0.05°C; P < 0.05). At 0.1%, EOF of 62.01 ± 3.75% obtained in melatonin-treated layer hens was lower (P < 0.001) than the corresponding value of 73.86 ± 2.59%, recorded in control layer hens. In conclusion, melatonin administration to layer hens sustained thermal homeostasis and decreased EOF in layer hens during the hot-dry season.
1. Introduction
Heat stress, induced by high ambient temperature (AT) and high relative humidity (RH), during the hot-dry season increases the values of body temperature and haematological parameters in birds (Sinkalu et al. Citation2008, Citation2010). It causes some changes in metabolism and increases oxidative damage to cells as evidenced by decrease in live weight, feed intake, egg production, feed efficiency and egg quality (Sahin et al. Citation2002; Yardibi & Turkay Citation2006). It also disrupts the blood acid–base balance (Franco-Jimenez et al. Citation2007). Under heat stress conditions, free radicals are generated in the body in such a large quantity that the natural antioxidant defence systems of the body are overwhelmed. This results into lipoperoxidation of cytomembranes and, consequently, cell damage and destruction (Lin et al. Citation2006; Minka & Ayo Citation2013). Lipid peroxidation, in turn, results in the loss of membrane fluidity and cellular lysis (Brzezinska-Slebodzinska Citation2003). As the heat load increases, the resulting elevation in body temperature leads to tissue damage and release of intracellular components into the circulation (Kadim et al. Citation2008).
Stress factors have been shown to increase the requirement for energy, minerals and vitamins in the body (Nardone et al. Citation2006; Yardibi & Turkay Citation2006; Kadim et al. Citation2008). Hens rely on different mechanisms to regulate their body temperature, and the thermoneutral zone (Khan & Sardar Citation2005) of 18.0–28.5°C has been established for chickens reared in the tropics (Oluyemi & Roberts Citation2000). Outside this zone, the hen adopts homeostatic mechanisms to maintain homeothermy. This requires extra energy to thermoregulate, so that less energy is available for egg production process (Bianca Citation1976; Nardone et al. Citation2006). Thus, heat tolerance has to be increased in order to alleviate the deleterious effects of heat stress (Ramnath et al. Citation2008). The body oxygen uptake increases during heat stress, resulting into an oxygen flux and, by extension, the conversion of oxygen into intermediate products; referred to as reactive oxygen species (ROS), which are toxic to the body (Lin et al. Citation2006; Ajakaiye et al. Citation2010). Antioxidants play the major role in protecting cells from the deleterious effects of ROS, achieved by reducing chemical radicals and preventing the process of lipid peroxidation (Ramnath et al. Citation2008). During heat stress, natural antioxidants involved in the elimination of excess ROS from the body are either exhausted or overwhelmed (Lin et al. Citation2006). Earlier reports suggested that environmental stress depletes in vivo antioxidants (Ramnath et al. Citation2008). The supplementation of antioxidant vitamin C (Khan & Sardar Citation2005; Surai et al. Citation2006; Sinkalu et al. Citation2008), zinc (Sahin & Kucuk Citation2003) and melatonin (Sahin et al. Citation2004) attenuates the adverse effects of extreme meteorological stress. Optimal dietary supplementation of laying hens with antioxidants enhances immune response (Pandi-Perumal et al. Citation2006), and may improve performance and egg quality (Surai et al. Citation2006; El-Sheikh & Salama Citation2010).
Melatonin, N-acetyl-5-methoxytryptamine, is a neurohormone. As the principal secretory product of the pineal gland, it is produced during the dark phase of circadian cycle (Reiter Citation2000; Cable et al. Citation2007). It is a highly conserved molecule that plays important roles in bio-rhythmicity in avian and mammalian species (Reiter Citation2000). Besides the thermoregulatory role of melatonin in humans, its exact mechanism of action on body temperature has not been elucidated (Deacon & Arendt Citation1995).
Erythrocyte osmotic fragility (EOF) is an important biomarker of oxidative stress (Minka & Ayo Citation2010b; Asala et al. Citation2011; Olaifa et al. Citation2012). The EOF test is used to assess the stability of erythrocytes in hypotonic solutions in birds (Minka & Ayo Citation2010b, Citation2013). Erythrocytes in poultry are highly fragile during the hot-dry season (Oladele et al. Citation2003). Supplementation with antioxidant vitamin reduces the intensity of oxidative stress and it enhances the antioxidant defence mechanisms; thus, greatly reduces the destruction of the erythrocytes (Brzezinska-Slebodzinska Citation2003; Adenkola & Ayo Citation2009; Adenkola et al. Citation2011).
The aim of the study was to determine the effects of melatonin administration on fluctuations in body temperature and changes occurring in the EOF of laying hens during the hot-dry season.
2. Materials and methods
The experiment was performed in a standard poultry house at the Department of Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria (11°4′N, 7°42′E), located in the Northern Guinea Savannah zone of Nigeria, where the birds were raised. The dry-bulb temperature (DBT) and wet-bulb temperature (WBT) at the experimental site were measured, using dry- and wet-bulb thermometers (Brannan, England), at 06:00 h, 14:00 h and 18:00 h daily for 12 consecutive days. From the data, RH and temperature-humidity index (THI) of each hour of measurement were calculated. The THI was calculated using the following equation of Tao and Xin (Citation2003):
2.1. Experimental design
Simple random sampling was used to assign the birds into two groups; melatonin-treated (n = 14) and control (n = 12) groups. On each experimental day at 18:00 h, 14 laying hens which served as melatonin-treated birds were administered orally and individually with melatonin (ISI Brands, Inc. Utah, USA) at the dose of 1.5 mg/kg (Nisbet et al. Citation2008) dissolved in sterile water through a gavage. The remaining 12 laying hens which served as control birds were given only sterile water without melatonin through a gavage. Thereafter, all the treated and control birds were given access to water ad libitum.
2.2. Flock management husbandry and housing
Twenty-six, apparently, healthy Isa Brown laying hens served as subjects. The birds were managed intensively on deep-litter system in a fenced poultry house in order to reduce the stress of confinement or restricted movement. The management system was also in compliance with the new European Union (EU) council directive 99/74/EC to face out the conventional layer cages by the year 2012. The fenced house was demarcated into two compartments, each housed 14 and 12 birds for melatonin-treated and control groups, respectively. The house had one main outer door and two inner doors, one for each compartment, and it was made of cement blocks and plastered floor with zinc roofing. Wood shavings were used as beddings. The house measured 6.5 m × 6.2 m wide and 2.0 m high, with wire mesh on top of the cement blocks and at 0.80 m to the roof to allow for adequate ventilation. The laying hens were fed commercially compounded, pelletised layers' mash (Vital Feeds, Jos, Nigeria) and given access to feed and water ad libitum. Proximate analysis of the feed () was conducted in the biochemical laboratory of the Department of Animal Science, Ahmadu Bello University, Zaria, Nigeria. All necessary vaccinations, medications and other related management practices were carried out on the birds as recommended in the Nigerian Veterinary Formulary (Citation2007).
Table 1. Proximate analysis of layer feed.
2.3. Experimental procedures
2.3.1. Measurement of cloacal temperature
The body temperature of each melatonin-treated and control layer hen was determined by measuring the cloacal temperature (CT) for 2 days, 1 week apart at 06:00 h, 14:00, and 18:00 h using a digital clinical thermometer (Hartmann digital thermometer, Paul Hartman AG, Heidenheim, Germany), inserted approximately 2 cm into the cloaca and in direct contact with the mucosal wall. The value was recorded after the thermometer gave an alarm sound, indicating that the reading had been stabilised (Ayo & Sinkalu Citation2007; Minka & Ayo Citation2010a).
2.3.2. Erythrocyte osmotic fragility
On day 12 of melatonin administration, venous blood samples were collected from 10 melatonin-treated and 10 control layer hens. EOF test was carried out according to the method of Oyewale (Citation1991). Briefly, EOF test was determined in vitro by measuring the release of haemoglobin from blood added to test tubes, containing serially diluted phosphate-buffered saline (PBS). Two microlitre of blood was added to six test tubes containing 5 ml of PBS (pH 7.4) of serial concentrations of 0.0%, 0.1%, 0.3%, 0.5%, 0.7% and 0.9%. The mixtures were allowed to stand for 30 minutes at room temperature of 26–28°C and then centrifuged at 252×g for 5 minutes. The supernatant of each tube was transferred into a glass cuvette and its haemoglobin concentration was determined spectrophotometrically (Spectronic-20, Philip Harris Limited, Shenstone, England) at 540 nm, using distilled water as a blank. The percentage of haemolysis in each concentration of PBS was calculated, assuming 100% haemolysis in the concentration with 0.0% PBS.
2.4. Statistical analyses
Data were analysed using the Student's t-test to compare between melatonin-treated and control hens, while one-way analysis of variance was used to compare between diurnal fluctuations in CT between both melatonin-treated and control groups at 06:00 h, 14:00 h and 18:00 h. Pearson's correlation analysis was used to compare between CT and meteorological parameters, including DBT, RH and THI. Multiple means were compared by Tukey's post hoc test. Data were expressed as mean ± standard error of mean (SEM). Values of P < 0.05 were considered significant. Analyses were performed using a Graphpad prism software package version 4.0 for windows (San Diego, California, USA, www.graphpad.com).
3. Results
3.1. Meteorological parameters
The mean daily meteorological data outside the poultry house during the study period were obtained from the meteorological unit of the Institute for Agricultural Research of Ahmadu Bello University, Zaria. The overall mean value of RH was high (71.2 ± 3.13%). It fluctuated during the hot-dry season between a minimum of 55.0% obtained on the 6th day, and a maximum of 94.0% recorded on the 10th day of the recordings. The mean values of AT fluctuated between a minimum of 21.4 ± 0.61°C and a maximum of 33.2 ± 1.13°C, obtained with a range of 11.8 ± 1.08°C. The overall sunshine duration, rainfall, evaporation and wind speed were 7.3 ± 0.72 h/d, 6.0 ± 2.69 mm, 10.5 ± 0.84 mm/d and 2.7 ± 0.21 m/s, respectively. The prevailing wind directions were South-West (SW) and North-West (NW). The hourly fluctuations in RH, DBT and THI inside the poultry house are shown in . During the hot-dry season, the DBT and THI values were different at different hours of the day from 06:00 h and attained peak values at 18:00 h (P < 0.05). The highest RH value of 69.08 ± 3.91% was recorded at 06:00 h, while the lowest value of 50.83 ± 4.08% was obtained at 14:00 h (P < 0.05). The maximum THI was recorded at 14:00 h, while the lowest was obtained at 06:00 h (P < 0.05).
Table 2. Thermal environmental conditions inside the poultry house during the study period.
3.2. Cloacal temperature
shows the fluctuations in diurnal CT of laying hens during the hot-dry season. On day 1, CT values obtained in the melatonin-treated group at 06:00 h (40.5 ± 0.10°C) and 14:00 h (40.8 ± 0.14°C) did not differ significantly (P > 0.05), but were lower (P < 0.05) than the value of 41.3 ± 0.05°C recorded at 18:00 h. Melatonin-treated group had a significantly higher CT value than that of the control (P < 0.05). On day 2, CT values of the melatonin-treated layer hens, unlike in the control birds, fluctuated significantly as the hours of the day increased (r = 0.439; P < 0.05). The overall mean CT for the melatonin-treated and control birds were 41.0 ± 0.05°C and 40.9 ± 0.04°C, respectively (P < 0.05). The recorded overall mean value of CT of the melatonin-treated group was lowest at 06:00 h (40.9 ± 0.10°C) and 14:00 h (40.9 ± 0.08°C), but highest at 18:00 h (41.2 ± 0.05°C, P < 0.05). In the control layers, the CT values from 06:00 h to 18:00 h were not different (P > 0.05) from one another.
Table 3. Fluctuations in hourly CT of layer hens during the hot-dry season.
and show the extent of between-layer variations in CT values of both melatonin-treated and control hens. The individual fluctuations in CT of melatonin-treated laying hens varied from the minimum value of 40.0°C to the maximum value of 41.8°C, recorded in bird numbers E2, E3, E6, E11 and E12. The range value of the CT was 1.8°C. The mean values of CT varied between 40.68 ± 0.16°C and 41.25 ± 0.22°C, and the values were recorded in bird numbers E6 and E2. The values recorded in control layer hens fluctuated between the extreme minimum and extreme maximum values of 40.2°C and 41.7°C, respectively with a range value of 1.5°C. The mean range value of CT was 0.97 ± 0.07°C. The extreme maximum value was recorded in bird numbers C1, C2, C4 and C10, while the extreme minimum value of 40.2°C was recorded in bird number C5. The mean values of extreme CT fluctuated between the maximum and minimum of 41.22 ± 0.16°C recorded in bird number C2, and 40.02 ± 0.18°C recorded in bird number C3. The values were not significantly different from the corresponding values, recorded in the laying hens administered with melatonin.
Table 4. Between-layer variations in CT of layer hens administered with melatonin during the hot-dry season (°C, n = 14).
Table 5. Between-layer variations in cloacal of control layer hens (not administered with melatonin) during the hot-dry season (°C, n = 12).
3.3. Correlation between CT and meteorological parameters
The hour of the day was significantly correlated with the CT values of melatonin-treated hens (r = 0.439; P < 0.01), but not with those of the control layers (r = 0.307; P > 0.05). The RH was negatively and significantly correlated with the CT in both melatonin-treated (r = −0.650; P < 0.001) and control layers (r = −0.424; P < 0.05; ). The THI was correlated directly with CT in both melatonin-treated (r = 0.668; P < 0.01) and control (r = 0.468; P < 0.01) layer hens.
Table 6. Relationships between thermal micro-environmental parameters and CT of laying hens during the experimental study period.
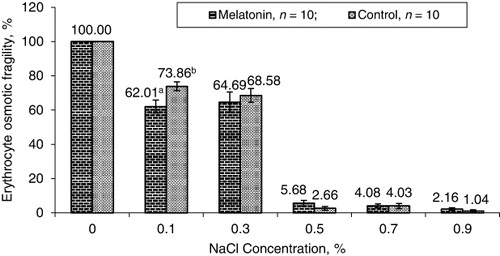
3.4. Erythrocyte osmotic fragility
At 0.1%, EOF percentage of 62.01 ± 3.75% obtained in melatonin-treated layer hens and the value was significantly (P < 0.001) lower than the corresponding value of 73.86 ± 2.59%, recorded in control layer hens ().
4. Discussion
The values of the thermal micro-environment parameters of AT, RH and THI fluctuated as the hours of the day increased. The findings agree with the results obtained by Ayo et al. (Citation2005), Sinkalu et al. (Citation2008) and Moller (Citation2010), who reported that marked fluctuations in thermal environmental parameters are responsible predominantly for production losses during the hot-dry season. This is because large amount of energy is utilised to maintain the CT within the physiological limits under such marked RT fluctuations. Therefore, measures aimed at reducing the risk of adverse effects of the high range of meteorological factors during the season may enhance the production of layer hens in the zone.
High AT and high RH recorded during the study period showed that the laying hens were subjected to heat stress. The values obtained were outside the thermoneutral zone of 18.0–28.5°C, established for hens reared in the tropics (Oluyemi & Roberts Citation2000) and lower optimal limit of 20°C reported by Pis (Citation2010). Birds are homeotherms, lacking sweat glands (Sandhu et al. Citation2012) and they depend primarily on evaporative cooling to combat the adverse effects of heat stress. The high RH and high DBT, resulting in high THI, recorded in the present study, rendered evaporative cooling mechanism ineffective. The results agree with the finding of Purswell et al. (Citation2012), who showed that as THI exceeds approximately 21°C, the performance of birds significantly declined and their body temperature increased. Also, Marais et al. (Citation2011) reported that high AT is physiologically deleterious to Pekin ducks' survival. The finding of the present study demonstrates that the thermal micro-environment conditions prevailing during the hot-dry season are not conducive for the production of laying hens.
The higher values of CT recorded in melatonin-treated laying hens may be attributed to greater metabolic and production processes, associated with high laying performance. The finding agrees with the results obtained by Osei et al. (Citation1989) that melatonin administration increased weight gain and energy retention by an average of 19% in male broiler chickens. The result of the present study shows that melatonin administration did not significantly affect the individual fluctuations in CT values in the laying hens during the hot-dry season. Although the fluctuations were, apparently, higher in melatonin-treated hens, compared to those of the control laying hens, the differences in the value were not significant. Melatonin may exert considerable modulatory effect on CT values in layer hens, if administered over a long period of time, but not short period, during the hot-dry season. This requires further investigation.
The results of the present study further demonstrate that CT variation in layer hens was predominantly due to diurnal and thermal micro-environmental factors, rather than individual variations of CT in the layer hens. Thus, thermal micro-environmental factors exerted greater influence on the CT values in layer hens than the individual variations in CT values. Melatonin administration also exerted greater influence on diurnal variation in CT values of the treated broiler chickens. This finding is in agreement with Bentley et al. (Citation2013), who elucidated the established physiological role of melatonin in the regulation of light–dark cycle (photoperiods). The role of melatonin in the modulation of CT values in layer hens subjected to varying lighting regimes also requires further investigation. Overall, the findings of the present study are in agreement with the results obtained by Ajakaiye et al. (Citation2010), who demonstrated that exotic layer hens have successfully adapted to the hot-dry season in the Northern Guinea Savannah zone of Nigeria by maintaining thermal equilibrium (homeothermy) during the season. Therefore, the breed may be successfully reared during the hot-dry season because of its successful adaptation to the adverse thermal environmental stress factors, prevailing during the season in the zone. This is because the breed may be able to conserve the energy required in the maintenance of the thermal equilibrium and use it for production, rather than thermoregulatory adjustments to heat stress.
Furthermore, melatonin circadian rhythmicity entrainment of CT observed in the present study is suggestive of its innate ability to enhance productive activities in layer hens during the day. The significantly higher CT response obtained in the melatonin-treated group at 18:00 h showed that the treated layer hens had higher metabolic or production processes at this hour than the early hours of the day. The findings agree with the results of Weaver and Lockley (Citation2009), who found that melatonin regulates circadian rhythmicity of various physiological parameters in vertebrates. The results of the present study also show that CT values in the hens were maintained within the normal physiological range of 40.0–42.0°C, established for chickens. The diurnal range of 1.8°C, with a mean value of 1.09 ± 0.12, demonstrated that the fluctuation in CT of the laying hens was within the established normal range of 40.0–42.0°C (Zaytsev et al. Citation1971; Ayo et al. Citation2007). The AT range during the hot-dry season, which was 25.0–36.5°C, fell outside the established thermoneutral zone of 12–24°C for poultry species in the temperate region (Selyansky Citation1975; Plyaschenko & Sidorov Citation1987) and 18–28.5°C in the tropics (Oluyemi and Roberts Citation2000). This finding shows that melatonin maintained the fluctuation in CT values of the treated layer hens within the normal range, in spite of the high AT (25.0–36.5°C), prevailing inside the poultry house during the hot-dry season.
The result shows that melatonin modulated the CT fluctuations by maintaining the values within the normal physiological range. Although the proximate mechanism underlying the action of melatonin was not investigated in the present study, melatonin is a potent antioxidant (Reiter et al. Citation2003) and anti-stress (Pierpaoli & Maestroni Citation1987) agent, which decreased malondialdehyde (MDA) concentration (Umosen et al. Citation2012) and increased the heterophil/lymphocyte ratio (Minka et al. Citation2012; Minka & Ayo Citation2013). The result of the present study further demonstrates that melatonin may be of value in the amelioration of adverse effects of heat stress in layer hens during the thermally stressful hot-dry season. Thus, its supplementation may be of value in the management of layer hens reared during the hot-dry season.
The results of the present study on EOF demonstrate that melatonin significantly decreased the fragility of the erythrocytes of layer hens. This finding is in consonance with that of Allegra et al. (Citation2002), and that of Haldar and Kharwar (Citation2012), who reported that melatonin restored to normal the EOF in humans and possesses antioxidant capacity, respectively. The decrease in EOF in melatonin-treated layer hens compared to the controls was, apparently, due to higher antioxidant capacity in melatonin-treated layer hens. The ability to scavenge oxygen or free radicals in melatonin-treated birds may decrease oxidative stress, reducing lipoperoxidation of erythrocyte membranes. It is worth noting that the lower EOF, a marker of oxidative stress (Olaifa et al. Citation2012), in melatonin-treated birds is a protective haematologic mechanism. This is because melatonin, while on one hand enhancing haematopoiesis and/or erythropoiesis (Othman et al. Citation2004), decreased the osmotic fragility of the newly formed erythrocytes on the other hand. This finding suggests that melatonin administration may be of value in consolidating improving the integrity of nucleated erythrocytes of domestic fowls, lizards and toads, whose EOFs have been reported to decrease significantly with time of storage or age of the erythrocytes (Oyewale Citation1994).
5. Conclusion
In conclusion, administration of melatonin decreased wide diurnal fluctuations in body temperature and EOF in layer hens during the thermally stressful hot-dry season, and may enhance their productivity. It is recommended that melatonin be administered to layer hens during the hot-dry season in order to reduce the risk of adverse effects of heat stress.
Acknowledgement
The authors thank Mr. D. D. Dagai for technical assistance.
References
- Adenkola AY, Agbendeh J, Okpe J. 2011. Comparative assessment of erythrocyte osmotic fragility of apparently healthy goats and cattle during the hot-dry and harmattan season in Makurdi, Nigeria. J Anim Plant Sci. 11:1474–1480.
- Adenkola AY, Ayo JO. 2009. Effect of road transportation on erythrocyte osmotic fragility of pigs administered ascorbic acid during the harmattan season in Zaria, Nigeria. J Cell Anim Biol. 3:4–8.
- Ajakaiye JJ, Ayo JO, Ojo SA. 2010. Effects of heat stress on some blood parameters and egg production of Shika brown layer chickens transported by road. Biol Res. 43:183–189. 10.4067/S0716-97602010000200006
- Allegra M, Gentile C, Tesoriere L, Livrea MA. 2002. Protective effect of melatonin against cytotoxic actions of malondialdehyde: an in vitro study on human erythrocytes. J Pineal Res. 32:187–193. 10.1034/j.1600-079x.2002.1o852.x
- Asala OO, Ayo JO, Rekwot PI, Minka NS, Omoniwa DO, Adenkola AY. 2011. Effect of ascorbic acid administration on erythrocyte osmotic fragility of pigs transported by road during the hot dry season. Vet Res Commun. 35:245–254. 10.1007/s11259-011-9469-7
- Ayo JO, Minka NS, Fayomi A. 2005. Effects of ascorbic acid on rectal temperature of pullets transported by road during the hot-dry season. Trop J Anim Sci. 8:43–48.
- Ayo JO, Owoyele OO, Dzenda T. 2007. Effects of ascorbic acid on diurnal variations in rectal temperature of Bovine Nera pullets during the harmattan season. Int J Poult Sci. 6:612–616. 10.3923/ijps.2007.612.616
- Ayo JO, Sinkalu VO. 2007. Effects of ascorbic acid on diurnal variations in rectal temperature of Shaver Brown pullets during the hot-dry season. Int J Poult Sci. 6:642–646. 10.3923/ijps.2007.642.646
- Bentley GE, Perfito N, Calisi RM. 2013. Season- and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm Behav. 63:829–835. 10.1016/j.yhbeh.2012.11.015
- Bianca W. 1976. The significance of meteorology in animal production. Int J Biometeorol. 20:139–156. 10.1007/BF01553047
- Brzezinska-Slebodzinska E. 2003. Species differences in the susceptibility of erythrocytes exposed to free radicals in vitro. Vet Res Commun. 27:211–217. 10.1023/A:1023344607691
- Cable NT, Dust B, Gregson WA. 2007. The impact of altered climatic conditions and altitude on circadian physiology. Physiol Behav. 90:267–273. 10.1016/j.physbeh.2006.09.002
- Deacon S, Arendt J. 1995. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 688:77–85. 10.1016/0006-8993%2895%2996872-I
- EL-Sheikh SEM, Salama AA. 2010. Effect of vitamins C and E as water additives on productive performance and egg quality of heat-stressed local laying hens in Siwa town. Egypt Poult Sci. 30:679–697.
- Franco-Jimenez DJ, Scheideler SE, Kittok RJ, Brown-Brandl TM, Robeson LR, Taira H, Beck MM. 2007. Differential effects of heat stress in three strains of laying hens. J Appl Poult Res. 16:628–634. 10.3382/japr.2005-00088
- Haldar C, Kharwar RK. 2012. Daily variation in antioxidant enzymes and lipid peroxidation in lungs of a tropical bird Perdicula asiatica: role of melatonin and nuclear receptor ROR α. Comp Biochem Physiol, Part A. 162:296–302. 10.1016/j.cbpa.2012.01.021
- Kadim IT, Al-Marzooqi W, Mahgoub O, Al-Jabri A, Al-Waheebi SK. 2008. Effect of acetic acid supplementation on egg quality characteristics of commercial laying hens during hot season. Int J Poult Sci. 7:1015–1021.
- Khan SH, Sardar R. 2005. Effect of vitamin C supplementation on the performance of Desi, Fayoumi and commercial White Leghorn chicken exposed to heat stress. Pak Vet J. 25:163–166.
- Lin H, Decuypere E, Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol, Part A. 144:11–17. 10.1016/j.cbpa.2006.01.032
- Marais M, Maloney SK, Gray DA. 2011. Ambient temperature modulates the magnitude of LPS-induced fevers in Pekin ducks. J Therm Biol. 36:121–127. 10.1016/j.jtherbio.2010.12.003
- Minka NS, Adeiza AA, Hassan FB, Ayo JO, 2012. Effects of melatonin and transportation on rectal temperature, heterophil/lymphocyte ratio and behaviour of Japanese male quails (Coturnix japonica). NY Sci J. 5:52–59.
- Minka NS, Ayo JO. 2010a. Behavioural and rectal temperature responses of Black Harco pullets administered vitamins C and E and transported by road during the hot-dry season. J Vet Behav Clin Appl Res. 5:134–144. 10.1016/j.jveb.2009.12.022
- Minka NS, Ayo JO. 2010b. Physiological responses of erythrocytes of goats to transportation and modulatory role of ascorbic acid. J Vet Med Sci. 72:875–881. 10.1292/jvms.09-0531
- Minka NS, Ayo JO. 2013. Ameliorating effect of melatonin on colonic temperature and erythrocyte osmotic fragility of Japanese quails (Coturnix coturnix Japonica) transported by road. Archiv für Geflügelkunde (Eur Poult Sci). 77:137–143.
- Moller AP. 2010. Body temperature and fever in a free-living bird. Comp Biochem Physiol, Part B. 156:68–74. 10.1016/j.cbpb.2010.02.006
- Nardone A, Ronchi B, Lacetera N, Bernabucci U. 2006. Climatic effects on productive traits in livestock. Vet Res Commun. 30:75–81. 10.1007/s11259-006-0016-x
- Nigerian Veterinary Formulary. 2007. Handbook of essential veterinary drugs, biologics and pesticide chemicals – a publication of the veterinary council of Nigeria. In: Aliu YO, editor. Nigerian Veterinary Formulary. 1st ed. Zaria: Tamaza Publishing Company Limited; p. 159.
- Nisbet DJ, Edrington TS, McReynolds JL, Callaway TR, Byrd JA. 2008. Influence of exogenous melatonin administration on Salmonella enteritidis colonisation in molted layers. Poult Sci. 87:1083–1088. 10.3382/ps.2008-00016
- Oladele SB, Ayo JO, Ogundipe SO, Esievo KAN. 2003. Seasonal and species variations in erythrocyte osmotic fragility of indigenous poultry species in Zaria, Northern Guinea Savannah zone of Nigeria. Bull Anim Health Prod Afr. 51:204–214.
- Olaifa F, Ayo JO, Ambali SF, Rekwot PI. 2012. Effect of packaging on changes in erythrocyte osmotic fragility and malondialdehyde concentration in donkeys administered with ascorbic acid. Onderstepoort J Vet Res. 79:413–418. 10.4102/ojvr.v79i1.413
- Oluyemi JA, Roberts FA. 2000. Poultry production in urban wet climates. Revised edition. Ibadan: Spectrum Books Limited; p. 244.
- Osei P, Robins KR, Shirley HV. 1989. Effects of exogenous melatonin on growth and energy metabolism of chickens. Nutr Res. 9:69–81. 10.1016/S0271-5317%2889%2980105-8
- Othman AI, Al Sharawy S, El-Missiry EA. 2004. Role of melatonin in ameliorating lead induced haematotoxicity. Pharmacol Res. 50:301–307. 10.1016/j.phrs.2004.01.013
- Oyewale JO. 1991. Osmotic fragility of erythrocytes of guinea-fowls at 21 and 156 weeks of age. Veterinarski Arhiv. 61:49–56.
- Oyewale JO. 1994. Changes in osmotic fragility of nucleated erythrocytes resulting from blood storage. J Vet Med. 41:475–479. 10.1111/j.1439-0442.1994.tb00114.x
- Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. 2006. Melatonin: nature's most versatile biological signal. FEBS J. 273:2813–2838. 10.1111/j.1742-4658.2006.05322.x
- Pierpaoli W, Maestroni GJ. 1987. Melatonin: a principal neuroimmunoregulatory and anti-stress hormone: its anti-ageing effects. Immunol Lett. 16:355–361. 10.1016/0165-2478%2887%2990169-6
- Pis T. 2010. The link between metabolic rate and body temperature in galliform birds in thermoneutral and heat exposure conditions: the classical and phylogenetically corrected approach. J Therm Biol. 35:309–316. 10.1016/j.jtherbio.2010.06.010
- Plyaschenko SI, Sidorov VT. 1987. Stresses in farm animals. Moscow: Agropromizdat; p. 192. Russian.
- Purswell JL, DozierIII WA, Olanrewaju HA, Davies JD, Xin H, Gates RS. 2012. Effect of temperature-humidity index on live performance in broiler chickens grown from 49 to 63 days of age. ASABE Paper No. ILES 12-0265, at the 9th International Livestock Environment Symposium; Valencia, Spain, July 8–12, 2012.
- Ramnath V, Rekha PS, Sujatha KS. 2008. Amelioration of heat stress-induced disturbances of antioxidant defence system in chickens by Brahma Rasayana. Evid-Based Complement Alternat Med. 5:77–84. 10.1093/ecam/nel116
- Reiter RJ. 2000. Melatonin: lowering the high price of free radicals. News Physiol Sci. 15:246–250.
- Reiter RJ, Dun-xian T, Juan CM, Rosa MS, Josefa L, Zbigniew C. 2003. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochimica Polonica. 50:1129–1146.
- Sahin K, Kucuk O. 2003. Zinc supplementation alleviates heat stress in laying Japanese quails. J Nutr. 133:2008–2011.
- Sahin K, Ozbey O, Onderci M, Cikim G, Aysondu MH. 2002. Chromium supplementation can alleviate negative effects of heat stress on egg production, egg quality and some serum metabolites of laying Japanese quail. J Nutr. 132:1265–1268.
- Sahin N, Onderci M, Sahin K, Gursu MF, Smith MO. 2004. Ascorbic acid and melatonin reduce heat-induced performance inhibition and oxidative stress in Japanese quails. Brit Poult Sci. 45:166–122. 10.1080/00071660410001668941
- Sandhu MA, Mirza FQ, Afzal F, Mukhtar N. 2012. Effect of heat stress on cellular and humoral immunity and its cure with α-tocopherol in meat type birds. Livest Sci. 148:181–188. 10.1016/j.livsci.2012.06.005
- Selyansky VM. 1975. Microclimate in poultry houses. Moscow: Kolos Publishing House; p. 304. Russian.
- Sinkalu VO, Ayo JO, Adelaiye AB, Hambolu JO. 2008. Effects of vitamin E on diurnal variation in rectal temperature of Black Harco pullets during the hot-dry season. J Therm Biol. 33:32–36. 10.1016/j.jtherbio.2007.09.006
- Sinkalu VO, Ayo JO, Adelaiye AB, Hambolu JO. 2010. Effects of heat stress on leucocytes and haematocrit values of broiler chickens administered with melatonin during the hot-dry season. Proc Ann Congress Niger Vet Med Assoc. 47:61–62.
- Surai PF, Sparks NHC, Speake BK. 2006. The role of antioxidants in reproduction and fertility of poultry. European Poultry Conference; Verona, Italy; p. 354.
- Tao X, Xin H. (2003). Acute synergistic effects of air temperature, humidity, and velocity on homeostasis of market-size broiler. Trans Am Soc Agric Eng. 46:491–497.
- Umosen AJ, Ambali SF, Ayo JO, Mohammed B, Uchendu C. 2012. Alleviating effects of melatonin on oxidative changes in the testes and pituitary glands evoked by subacute chlorpyrifos administration in Wistar rats. Asian Pac J Trop Biomed. 2:645–650. 10.1016/S2221-1691%2812%2960113-0
- Weaver DR, Lockley SW. 2009. Melatonin regulation of circadian rhythmicity in vertebrates. Encyclopaedia of Neuro science. Oxford: Academic Press; p. 721–732.
- Yardibi H, Turkay G. 2006. The effects of vitamin E on the antioxidant system, egg production, and egg quality in heat stressed laying hens. Turk J Vet Anim Sci. 32:319–325.
- Zaytsev VI, Sinev AV, Ionov PS, Vasilyev AV, Sharabrin IG. 1971. Clinical diagnosis of internal diseases of farm animals. Moscow: Kolos Publishing House; p. 336. Russian.