Abstract
To assess the performance of kids under different disbudding pre-medication regimes, 24 kids of 14–15 days were randomly designated as D0 (control, no medication), DL (local anaesthetics, 2% lignocaine, 1 ml/bud as cornual nerve block), DM (non-steroidal analgesic, meloxicam with 0.25 mg/kg body weight by I/M route) and DL + M (both lignocaine and meloxicam) with six in each group. First fortnight after hot-iron disbudding, ADG was higher (P < 0.05) in DM than control. FCR was significantly (P < 0.05) better in DM and DL over control. PER was non-significantly higher in DM, DL and DL + M over control by 24.86%, 22.70% and 17.84%, respectively. Hb and PCV was higher (P < 0.01) in DM followed by DL, DL + M and D0. Neutrophil, lymphocyte, eosinophil and basophil counts were higher in DL than other treatment groups. Neutrophil-to-lymphocyte ratio was higher (P < 0.01) in DM than other treatment groups. Effect of disbudding pre-medication on serum biochemistry like glucose, total protein, albumin and globulin was significantly affected (P < 0.01) by pre-disbudding treatments among different groups. During entire study period, serum cortisol was lower (P < 0.01) in DM, T3 was higher in D0 and T4 was higher in DL than other groups; however, TSH was lower (P < 0.01) in DM than other three groups. It was concluded that non-steroidal analgesic–medicated kids had better performance and disbudding distress persists up to 15 days irrespective of different pre-disbudding medication regime.
1. Introduction
Indian goat production system, with highest population of 157 million in the world (FAOSTAT Citation2012), is observing fast transformation from extensive to stall-feeding system for a variety of reasons (Argüello Citation2011). Probably some of them are shrinkage of natural grazing land caused by changed cropping pattern, urbanisation, industrialisation, etc. Goat production under stall-fed conditions requires economisation of housing space. Therefore, production of hornless goats will be the need of hour, as horned animal pose a threat to pen mates, are more likely to destroy facilities than animals without horns and are more dangerous for the persons who handle them (Al-Sobayil Citation2007). Additionally, horned animals need more floor and feeding space. Horns are sometimes also responsible for the different painful conditions like horn gore, fight-end bruises, maggoted wound in brocken horns, horn cancer etc., which severly affect animal welfare by imparting pain-related stresses, affecting critical body functions, or sometimes even inflicts death. So, disbudding is one of the important and much required management techniques in kids (Alvarez et al. Citation2009) to avoid above problems and to accommodate about 10–15% more animals in unit area.
Disbudding causes growth distress, especially, during early part of life when they have higher growth potential; however, reports for goats are scanty (Stafford & Mellor Citation2011). During and after disbudding, animals are exposed to various stressful conditions that affect their welfare (Alvarez et al. Citation2009) and cortisol level abruptly increases in response to pain, which remained high for 1.5–2.5 hours (Alvarez & Galindo Citation2008) to 4–5 hours (Sylvester et al. Citation2004). In response to animals' trauma, pain or inflammation, animal's performance may also get affected. Disbudding by pre-medication of local anaesthetics and non-steroidal anaelgesics is indicated as improved techniques by many workers (Al-Sobayil Citation2007). However, local anaesthetics loses its effectiveness within 2–4 hours and cortisol increased thereafter and becomes similar to animals disbudded or dehorned without anaesthesia (Stafford & Mellor Citation2011). Due to this, use of non-steroidal anti-inflammatory drugs (NSAIDs) has been recommended. NSAID when combined with local anaesthesia, significantly, reduces cortisol responses (Heinrich et al. Citation2010) in calves.
It has been reported that circulating corticosteroids after disbudding may affect hypothalmus-pituitary-adrenal axis (Stafford et al. Citation2003). Therfore, we hypothesised that disbudding may affect the metabolic profile of kids by upsetting the release of thyroid hormones like T3, T4 and TSH. Pretentious metabolic profiles may also affect growth, feed intake, serum biochemicals and haemogram of animals through various interlinked processes. Due to irritation of local anaesthetics at injected site, the eosinophile profile may also get affected. Furthermore, none of the study has shown prolonged effect of disbudding on performance of kids. According to our knowledge, information regarding kid performance, feed and fodder intake, haematology, serum biochemistry and hormonal profile due to disbudding pre-medication in kids up to pre-weaning period, especially under Indian condition, is scanty. Hence, present study was undertaken to investigate the kid welfare through growth performances, hormonal, serum biochemical and haematological profiles by the use of single injected dose of local anaesthetics and non-steroidal analgesics in Beetal kids up to weaning period of three months under stall-fed conditions.
2. Materials and methods
2.1. Location and experimental design
This study was conducted, from May to August, 2010, at University Goat Research Farm. It is located at 30o54′N latitude, 75o48′E longitude and 247 m above mean sea level. The said study was undertaken after permission of the Institutional Animal Ethics Committee. Twenty-four farm-born Beetal kids of almost equal weight, litter size and dam parity, aged 14–15 days, were randomly divided into four groups with six in each (3 males and 3 females). Kids disbudded without any medication were designated as D0 (Control). Kids medicated with local anaesthetics like 2% lignocaine ((Loxicard®, Neon Laboratories, Mumbai, India) at a dose rate of 1 ml/bud as cornual nerve block, non-steroidal analgesics like meloxicam (Melonex®, Intas Pharmaceutical Ltd., Ahmadabad, India) at 0.25 mg/kg body weight by I/M route and with both lignocaine and meloxicam at above dose and route, were designated as DL, DM and DL + M, respectively.
2.2. Medication and disbudding
To achieve cornual nerve block for goats, lignocaine in DL group was injected (10 minutes before disbudding) at two sites in each horn buds as described by Ommer and Harshan (Citation1995). Similarly, meloxicam was injected intramuscularly in DM group. For combined medication (DL + M), lignocaine was injected first (10 minutes before) followed by meloxicam after 5 minutes. Rest management practices were similar in four treatments. Five minutes after medication, disbudding was performed by hot iron cauterisation method (using manually heated disbudder) between 08:30 and 11:00 hrs over a period of six days with four kids per day (one kid from each treatment). Following disbudding, framycetin skin cream (Soframycin skin cream®, Sanofi India Ltd., Goa, India) and Topicure Herbal Spray® (Natural Remedies Pvt. Ltd., Bangalore, India) as fly repellent were applied on each buds till complete healing of the wound.
2.3. Housing and general management
Kids, after disbudding till completion of study, were kept individually in an enclosure of wire meshed iron panels with an effective area of 1.44 m2. Adjustable feeders of 12 × 8 × 5.5 inches and 18 × 12 × 6 inches for concentrate and roughages, respectively, were fixed in each enclosure. Kids during first month was fed with milk at 10% body weight or 100 ml/kg (Argüello et al. Citation2004), followed by at 6.6% and 5.0% during second and third month, respectively, by baby feeder as described by Castro-Alonso et al. (Citation2008). Milk was offered three times a day at equal intervals, that is, at 6:00, 14:00 and 22:00 hours. The creep feed and green fodder were introduced from day 15 onwards to all kids at ad libitum basis. The creep feed was offered in the morning (10 am) and green fodder in the evening (3.00 pm).
2.4. Observations recorded
2.4.1. Daily milk, feed and fodder intake
Daily milk, feed and fodder intake was recorded individually by offering weighed amount of milk, creep feed and green fodder to animal and taking records of daily residue on next day. The creep feed comprised of maize 40, soybean meal 33, de-oiled rice bran 24, salt 1 and mineral mixture 2 parts by weight and mixed with 25 g vitamin premix per 100 kg feed. The feed, fodder and residue samples, collected on fortnightly basis under different treatments, were subjected to proximate analysis as per methodology of AOAC (Citation1997).
2.4.2. Body weight
The fortnightly body weight of kids was recorded in the morning hours before feeding and watering on a 200-kg capacity digital platform weight bridge having 50g least count. Recorded body weight was used for calculation of different growth indices like average daily gain (ADG), feed conversion ratio (FCR) and protein efficiency ratio (PER).
2.4.3. Haematology, serum biochemistry and hormonal profiles
Blood was collected 10 minutes before medication and then during first, third, sixth, twelfth and twenty-fourth hours after disbudding on day zero (first day) and once daily from second to fifth day, followed by at fortnightly intervals up to 3 months. The blood sample of individual animal (2 ml) was collected in K3 EDTA-coated vials (Accuvet®, Quantum Biologicals Pvt. Ltd., India) from jugular vein under aseptic conditions as described by Jain (Citation1986). Haemoglobin (Hb) and packed cell volume (PCV) were estimated by Sahli's acid haematin and haematocrit centrifugation method, respectively. Total leukocytic count (TLC) and differential leukocytic count (DLC) were determined by the methods of Benjamin (Citation1985) and Jain (Citation1986), respectively.
For serum biochemical profiles, blood samples were collected in acid-free vials without any anticoagulant. Serum was separated and transferred to a dry clean eppendorfs tube in triplicate. The samples were stored at 20°C temperature till further evaluation. For serum glucose estimation, blood was collected in sodium fluoride vials (Quantum Biologicals Pvt. Ltd., India). VITROS DT 60/DT 60 II Chemistry System (Ortho-Clinical Diagnostics, Johnson & Johnson, USA) was used for analysis of different serum biochemical profiles like glucose, total protein (TP) and albumin. Serum globulin level was calculated through difference between total protein and albumin. The serum hormones, i.e., cortisol, T3, T4 and TSH were estimated by the use of LumaxTM Model 4101 chemiluminescence Immunoassay (CLIA) Strip Reader (Monobind, Inc., USA) using Acculite CLIA microwells (Monobind, Inc., USA) as per the standard protocol.
2.4.4. Statistical analysis
The recorded data were analysed by SPSS 16. The recorded data were subjected to one-way ANOVA (Snedecor & Cochran Citation1989) to test the difference between various treatments. The significant means between different treatments were compared by Tukey's b test.
3. Results
3.1. Milk, feed and fodder intake
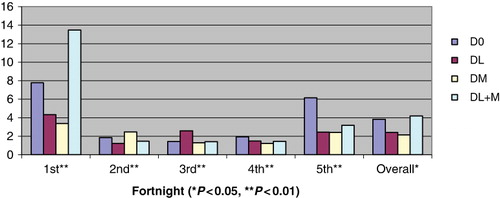
During first fortnight, milk intake was significantly higher (P < 0.01) in DM than other three groups (). However, during second and third fortnights, it was significantly lower (P < 0.01) in both DL and DL + M groups than control or DM. During fourth fortnight, intake was significantly higher (P < 0.01) in DL + M. Total milk intake during study period of 10 weeks was similar in all four treatments. Daily creep feed, green fodder and dry matter intake (DMI) was significantly higher (P < 0.01) in control than the other three groups (). The proximate composition (%) of creep feed in terms of DM, OM, CP, EE, NDF, ADF, cellulose, lignin and total ash was 87.69, 79.03, 24.85, 2.60, 25.67, 7.19, 6.43, 2.46 and 8.66, respectively. Similarly, composition of offered green fodder was 24.63, 14.35, 7.89, 1.88, 69.04, 44.68, 29.21, 7.05 and 10.28 for DM, OM, CP, EE, NDF, ADF, cellulose, lignin and total ash, respectively.
Table 1. Daily milk intake (ml/day) by the kids under different treatments during the experiment.
Table 2. Dry matter (DM) consumption (g/day) by the kids from milk, concentrate and roughages during the experimental period.
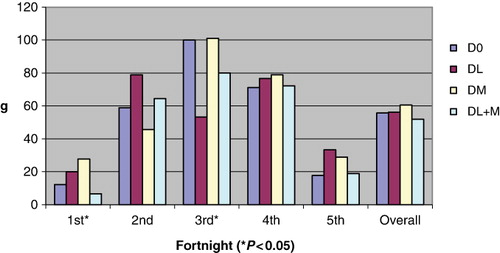
3.2. Body weight and average daily gain (ADG)
The kids of DM gained significantly higher (P < 0.05) weight than DL + M group (). Later on there was no differences in the body weight gain up to fifth fortnight. During first fortnight, the ADG was significantly (P < 0.05) higher in DM, lower in control and DL + M group (). During third fortnight, the ADG was higher (P < 0.05) both in control and DM over DL + M or DL group. The average ADG during study period of 10 weeks was similar in all groups.
Table 3. Average body weight of kids at fortnightly intervals in different treatments.
3.3. Feed conversion ratio (FCR) and protein efficiency ratio (PER)
FCR over a period of 10 weeks was significantly (P < 0.05) better in DM and DL over DL + M or control (). PER () also followed the same pattern as FCR where it was higher (P < 0.01) in DM during second, fourth and fifth fortnight followed by DL and DL + M over control. Average PER was non-significantly higher in DM, DL and DL + M over control by 24.86, 22.70 and 17.84%, respectively.
3.4. Haematological profile
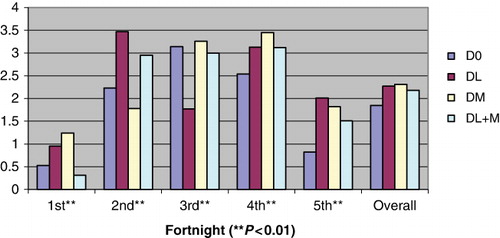
Haemoglobin (Hb) level was significantly higher (P < 0.01) in DM followed by DL, DL + M and control (). Same pattern was observed for PCV under different treatments. However, these values were within normal range. All other blood profiles like TLC and absolute DLC, though differed significantly (P < 0.01) between groups, were within normal range (Jain Citation1986). Absolute neutrophils, lymphocytes, eosinophils and basophils were significantly higher in DL than other treatment groups. Neutrophil-to-lymphocyte (N/L) ratio was significantly (P < 0.01) higher in DM than other treatment groups.
Table 4. Haematological profile of kids under different treatment groups.
3.5. Serum biochemistry
Glucose concentration was significantly higher (P < 0.01) in DL than other three groups (). Total protein level was higher (P < 0.01) in DL + M, followed by DM and lower in control and DL. The concentration of albumin was higher (P < 0.01) in DM than other three groups. Serum globulin concentration was significantly higher in mixed (DL + M) group as compared to control (D0) during almost entire study period.
Table 5. Serum biochemical profile of kids under different treatment groups.
3.6. Hormonal attributes
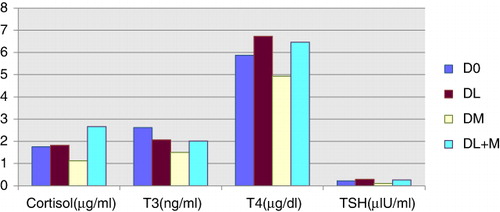
During entire study period, cortisol level was significantly (P < 0.01) lower in DM and higher in DL + M followed by DL and D0 group in descending order (). T3 level was higher in D0, followed by DL, DL + M and DM in decreasing order. Serum T4 level was higher in DL, followed by DL + M, D0 and DM in descending order. Concentration of TSH was significantly lower (P < 0.01) in DM than other three groups.
4. Discussion
Reasons for selecting kids of same litter size and dam parity was to avoid any variation on disbudding stress due to transfer of immunoglobulin (IgG) through colostrum after birth due to suckling. Argüello et al. (Citation2006) concluded that the chemical and physical characteristics of colostrum in terms of IgG and nutrients were slightly affected by litter size of kids and lactation number of dam. Higher milk intake by kids of DM than other three groups during first fortnight may be due to lesser degree of pain and stress just after disbudding. Mixed response of milk intake from second to fourth fortnight may either be due to disbudding or environmental stresses. Higher (P < 0.01) milk intake during fourth fortnight in DL + M may be due to normalisation of stressful conditions. These results also indicate that the effect of disbudding stress irrespective of pre-medication persists only up to 15 days. Slighly better intake in DM may be due to action of NSAID that prevents inflammation by inhibiting cyclooxygenase (COX-1 and COX-2) enzyme, which produce prostaglandins (Heinrich et al. Citation2010) associated with pain and inflammation that resulted from tissue injury. Meloxicam, one of the latest NSAID, with comparatively prolonged half-life (Heinrich et al. Citation2010) preferentially inhibits COX-2 isoform and caused less interference with normal homeostatic process and have fewer deleterious effects on gastrointestinal system. Therefore, meloxicam seems to be a better and potent pre-disbudding medication for kids than local anaesthetics or combination of both. Probably slightly higher degree of pain was felt by kids of DL and DL + M than DM after disbudding, which may be due to non-inhibitive nature of lignocaine for release of prostaglandins and pain boosters after disbudding. Similar observations had been recorded by Alvarez and Gutiérrez (Citation2010) for non-steroidal analgesics (NSAIDs) or local anaesthetics used separately. Slightly higher degree of pain in mixed medicated group (DL + M) may be due to twice handling and five-time needle pricking to the kids, which might had released more cortisol () along with nullification of the action of pain boosters. Complete absence of meloxicam-induced action in control group might be the reason for higher degree of pain in kids of D0, and lesser milk intake during first fortnight.
Significantly higher (P < 0.01) creep intake in kids of D0 may be due to lower milk intake and more consumption of concentrate feed to satisfy their appetite. Lower creep intake by DM group may be due to higher milk intake. Lesser creep intake of both lignocaine and mixed groups can be correlated with prolonged stress, both of disbudding and environment. Significantly higher (P < 0.01) green fodder intake in control may be explained on the same pattern as for creep feed intake. Due to same reason, total DMI was higher (P < 0.01) in control followed by DL and DM and lower in DL + M, which differs significantly (P < 0.01) from rest groups (). Duffield et al. (Citation2010) observed that ketoprofen (a NSAID)-treated calves tend to consume more calf starter following dehorning than control group. Pattern of feed intake was suggestive of increased levels of comfort in the ketoprofen-treated calves, since appetite suppression is known to occur due to inflammatory response during dehorning sickness and pain. Significantly higher (P < 0.05) weight gain by the kids of DM group than control clearly revealed the significance of disbudding pre-medication. These findings also signify that effect of disbudding stress remained up to 15 days, as after first fortnight there was no difference in the body weight gain. During entire study period, animals of DM group showed slightly higher growth rate with a fortnight increment of 14.13% followed by 13.83% in DL, 13.65% in D0 and 13.04% in DL + M. These findings indicated that non-steroidal analgesics mitigate pain for longer duration after hot-iron disbudding, which might result in higher weight gain. Faulkner and Weary (Citation2000) observed that the calves treated with ketoprofen gained more weight during the 24 hours after dehorning than control calves. However, no difference for weight gain was observed by Milligan et al. (Citation2004). Significantly (P < 0.05) higher ADG during first fortnight in DM than other groups might be due to disbudding distress of higher degree in control and DL + M group followed by DL group over a period of 15 days. The ADG of this experiment was lower than the average ADG of this farm over same period (Chandrahas Citation2011), which indicates that disbudding itself is a stressful condition as observed by Faulkner and Weary (Citation2000). Higher (P < 0.05) ADG during third fortnight in DM and control over DL + M or DL group may be indicative of persistent stressful situation in lignocaine or mixed group up to that period. It may again be due to deep burn and prolonged healing in kids of both lignocaine groups due to absence of any resistance by animal during disbudding. Significantly (P < 0.01) better FCR and PER in DM during entire study period again confirmed lesser stress in meloxicam-treated groups over others.
Findings of significantly higher (P < 0.01) Hb in DM followed by DL, DL + M and control may be correlated with the cortisol level, which was lower in DM than control and DL + M (). Identical pattern of the PCV probably due to similar reason mentioned for Hb. Continuous stress after disbudding in lignocaine (DL) and control (D0) groups might be the probable reason for the increased total leukocyte count (TLC) than other two treatment groups. Weiss and Walcheck (Citation2008) reported that cortisol depresses the neutrophil function, and this may be the reason for marked neutropenia in DL + M group. Lymphocytosis and eosinophilia in lignocaine (DL) group may be due to the effect of continuous stress and irritation by lignocaine injection. Taylor (Citation2000) observed increased lymphocyte and monocyte with a mild eosinopenia under conditions like excitement and stress in ruminants. These findings were in agreement with Doherty et al. (Citation2007) who observed that the percentage of circulating neutrophils was greatest (P < 0.001) at 12 hours with saline- and 2% lignocaine -treated animals; however higher neutrophil:lymphocyte ratio in lignocaine-treated group may be due to species difference.
Findings of serum biochemistry can be correlated with rise or fall in different hormones especially cortisol. Increasing cortisol might have reduced glucose uptake and utilisation followed by marked gluconeogenesis, from non-carbohydrate sources like protein and albumin, in order to increase blood glucose level. This might had again resulted in simultaneous fall in protein and albumin level (Kaneko Citation2008). Kannan et al. (Citation2000) in his study on transportation stress concluded that as a response to stress, elevation of glucose concentration was preceded by an elevation of cortisol concentration. Fall in serum protein in D0 or DL may again be due to catabolic effect of thyroxine elevation as reported by Eckersall (Citation2008). Due to inflammation, fluids and proteins moved to tissue and might have also contributed in decreased plasma albumin in groups having more stress (Eckersall Citation2008). However, these findings do not support the findings of Doherty et al. (Citation2007), who reported similar total plasma protein in calves dehorned after administrating lignocaine in 5%/2% lignocaine or saline or 5% lignocaine followed by sham dehorning. This may be due to species variation or different environmental conditions.
Present findings, for cortisol, were in agreement with the observations of workers like Doherty et al. (Citation2007), Alvarez et al. (Citation2009) and Alvarez and Gutiérrez (Citation2010). Higher concentration of the serum cortisol in DL + M, before and after disbudding, may be due to fear of thrice handling to animals of this group, i.e., once for hair cutting and twice for medication. It may also be due to local irritation of lignocaine, probable interaction of lignocaine with meloxicam etc. Similarly, Doherty et al. (Citation2007) also observed that regardless of treatment, plasma cortisol concentration was greater (P < 0.001) in all calves 30 minutes post-dehorning compared with the other sampling times. Alvarez et al. (Citation2009) and Alvarez and Gutiérrez (Citation2010) reported that disbudding by thermal cauterisation in goats caused an acute and significant increase in cortisol concentration and the values remained high for 2 hours. The cortisol concentration was higher in the disbudded groups (P < 0.05), even when local anaesthesia was used.
Higher serum T3 or T4 concentration, either in control (D0) or lignocaine (DL) pre-medicated groups (), may be due to effect of cortisol on hypothalamic-pituitary-adrenal (HPA) axis (Dunlop Citation2004) for reduction in basal metabolic rate (BMR) of kids. To maintain BMR, compensatory rise of T3 and T4 hormones might have taken place in all groups except DM during different periods of blood collection. Since T3 and T4 remained unavailable for the further use, TSH continuously increased to raise the serum T3 and T4 levels by stimulating thyroid gland. Stillwell et al. (Citation2008) opined that it is not known about the possibility that NSAID may influence the functioning of HPA during a stress/pain response to burns during chemical disbudding. However, in present study, possibility of NSAID action through HPA exists, which might have affected, cortisol controlled thyroid gland action in meloxicam-treated groups.
5. Conclusion
For improved kids' welfare, meloxicam pre-medication may be more effective than lignocaine or lignocaine and meloxicam combined. This is because cornual nerve block requires frequent pricking of needle, which may add further stress to kids. In combined therapy also kids needed to be handled frequently, which may add more stress and welfare may be affected. Moreover, under Indian conditions, disbudding is mostly performed by goat-keepers, themselves. So they may not be proficient in cornual nerve block and single I/M injection of meloxicam may prove to be more effective to avoid post-disbudding pain and stress in disbudded kids. Although, the modest increase in body weight may not be economically significant but it is biologically important and plays important role in animal welfare. Therefore, it can be concluded that disbudding after pre-medication with non-steroidal analgesics had better effect on the performance in the Beetal kids and impact of disbudding distress persists maximum up to 15 days post-disbudding.
Funding
The financial assistance received from Guru Angad Dev Veterinary and Animal Sciences University, World Bank supported National Agricultural Innovative Project and M/s American Soybean Association is thankfully acknowledged.
Additional information
Funding
References
- Al-Sobayil FA. 2007. A new simple device for dehorning in small ruminants. Small Ruminant Res. 67:232–234. 10.1016/j.smallrumres.2005.10.010
- Alvarez L, Galindo F. 2008. Daily cortisol profile in lactating and non-lactating dairy goats (Capra hircus). Res J Anim Sci. 2:72–77.
- Alvarez L, Gutiérrez J. 2010. A first description of the physiological and behavioural responses to disbudding in goat kids. Anim Welfare. 19:55–59.
- Alvarez L, Nava RA, Ramirez A, Ramirez E, Gutiérrez J. 2009. Physiological and behavioural alterations in disbudded goat kids with and without local anaesthesia. Appl Anim Behav Sci. 117:190–196. 10.1016/j.applanim.2009.01.001
- AOAC. 1997. Official methods of analysis. 16th ed. Maryland: Association of Official Analytical chemists.
- Argüello A. 2011. Trends in goat research, a review. J Appl Anim Res. 39:429–434. 10.1080/09712119.2011.637362
- Argüello A, Castro N, Alvarez S, Capote J. 2006. Effect of the number and litter size on chemical composition and physical characteristics of goat colostrums. Small Ruminant Res. 64:53–59. 10.1016/j.smallrumres.2005.03.016
- Argüello A, Castro N, Capote J, Tyler JW, Holloway NM. 2004. Effect of colostrum administration practices on serum IgG in goat kids. Livest Prod Sci. 90:235–239. 10.1016/j.livprodsci.2004.06.006
- Benjamin MM. 1985. Outline of veterinary clinical pathology. 3rd ed. New Delhi: Kalyani.
- Castro-Alonso A, Castro N, Capote J, Morales-delaNuez A, Moreno-Indias I, Sanchez-Macias D, Herraez P, Argüello A., 2008. Apoptosis regulates passive immune transfer in newborn kids. J Dairy Sci. 91:2086–2088. 10.3168/jds.2007-0814
- Chandrahas. 2011. Studies on some managemental interventions for sustainability of Beetal Goats under stall-fed conditions [Ph.D. thesis]. Ludhiana: Guru Angad Dev Veterinary and Animal Sciences University.
- Doherty TJ, Kattesh HG, Adcock RJ, Welborn MG, Saxton AM, Morrow JL, Dailey JW. 2007. Effects of a concentrated lignocaine solution on the acute phase stress response to dehorning in dairy calves. J Dairy Sci. 90:4232–4239. 10.3168/jds.2007-0080
- Duffield TF, Heinrich A, Millman ST, DeHaan A, James S, Lissemore K. 2010. Reduction in pain response by combined use of local lignocaine anesthesia and systemic ketoprofen in dairy calves dehorned by heat cauterization. Can Vet J. 51:283–288.
- Dunlop RH. 2004. Pathophysiology of homeostatic and toxic disorders. In: Dunlop RH, Malbert CH, editors. Veterinary pathophysiology. 1st ed. Ames, IA: Blackwell; p. 481.
- Eckersall PD. 2008. Proteins, proteomics and the dysproteinemias. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 6th ed. Burlington, MA: Elsevier Academic Press; p. 117–155.
- FAOSTAT. 2012. Food and Agriculture Organization statistical database. http://faostat.fao.org/ Food and Agriculture Organization of United Nations.
- Faulkner PM, Weary DM. 2000. Reducing pain after dehorning in dairy calves. J Dairy Sci. 83:2037–2041. 10.3168/jds.S0022-0302(00)75084-3
- Heinrich A, Duffield TF, Lissermore KD, Millman ST. 2010. The effect of meloxicam on behaviour and pain sensitivity of dairy calves following cautery dehorning with a local anesthetic. J Dairy Sci. 93:2450–2457. 10.3168/jds.2009-2813
- Jain NC. 1986, In: Schalm's veterinary hematology. 4th ed. Philadelphia, PA: Lee and Febiger.
- Kannan G, Terrill TH, Kouakou B, Gazal OS, Gelaye S, Amoah EA, Samaké S. 2000. Transportation of goats: effects on physiological stress responses and live weight loss. J Anim Sci. 78:1450–1457.
- Kaneko JJ. 2008. Carbohydrate metabolism and its diseases. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 6th ed. Burlington, MA: Elsevier Academic Press; p. 55–56.
- Milligan BN, Duffield T, Lissemore K. 2004. The utility of ketoprofen for alleviating pain following dehorning in young dairy calves. Can Vet J. 45:140–143.
- Ommer PA, Harshan KR. 1995. Applied anatomy of the domestic animals. 1st ed. New Delhi: Jaypee Brothers Medical Publishers; p. 27.
- Snedecor GW, Cochran WG. 1989. Statistical methods. 8th ed. New Delhi: Affiliated East-West Press.
- Stafford KJ, Mellor DJ. 2011. Addressing the pain associated with disbudding and dehorning in cattle. Appl Anim Behav Sci. 135:226–231. 10.1016/j.applanim.2011.10.018
- Stafford KJ, Mellor DJ, Todd SE, Ward RN, McMeekan CM. 2003. The effect of different combinations of lignocaine, krtoprofen, xylazine and tolazoline on the acute cortisol response to dehorning in calves. N Z Vet J. 51:219–226. 10.1080/00480169.2003.36370
- Stillwell G, Lima MS, Broom DM. 2008. Comparing plasma cortisol and behaviour of calves dehorned with caustic paste after non-steroidal-anti-inflammatory analgesia. Livest Sci. 119:63–69. 10.1016/j.livsci.2008.02.013
- Sylvester SP, Stafford KJ, Mellor DJ, Bruce RA, Ward RN. 2004. Behavioral responses of calves to amputation dehorning with and without local anaesthesia. Aust Vet J. 82:697–700. 10.1111/j.1751-0813.2004.tb12162.x
- Taylor JA. 2000. Leukocyte responses in ruminants. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's veterinary hematology. Maryland: Lippincott Williams & Wilkins; p. 392–404.
- Weiss DJ, Walcheck B. 2008. Neutrophil function. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 6th ed. Burlington, MA: Elsevier Academic Press; p. 331–350.