Abstract
Quantitative analysis of the kidney was undertaken in three species of bird: domestic fowl (Gallus domesticus), house sparrow (Passer domesticus) and domestic pigeon (Columba livia var. domestica). The volume and surface area of the renal components were measured. The fine structure of the proximal convoluted tubules showed intercellular spaces that were occupied by interdigitating folds at the basolateral membrane level. The distal tubules showed microvilli in the apical basement membrane into the tubular lumen. Microscopic study revealed that the domestic fowl had a greater volume of cortical tissue in the kidney compared with the other two species; this bird also had a greater medullary tissue volume than the other species. The volume and surface area of the renal corpuscles and proximal and distal convoluted tubules in the cortex were highest in the domestic fowl and lowest in the house sparrow. The volume and surface area of the proximal tubules, thin and thick limbs of the loop of Henle, and collecting ducts were lowest in the house sparrow, while the domestic pigeon had slightly higher values for these parameters. Blood capillaries in the domestic fowl had the greatest volume and surface area in the cortex and medulla. This study suggests that the volume and surface area in structural elements of the renal nephron in these birds play an important role in the concentration of urine.
1. Introduction
The kidneys of birds consist of lobules, each comprising the cortex and the medullary cones. Unlike in the mammalian kidney, the medullary cones are not divided into inner and outer regions (Poulson Citation1965; Dantzler & Braun Citation1980; Boykin & Braun Citation1993). Nephrons with and without loops of Henle are the main structures in the avian kidney. This population of nephrons is separated from the medullary cones and then converges to form unique single ducts. The anatomical organization of the medullary cones does not allow production to move from one medullary cone to another; hence, all the medullary cones operate as separate units (Boykin & Braun Citation1993). Research has shown that there are three types of nephron in the kidneys of some birds. The cortical nephrons are similar to those in reptiles, which include a small glomerulus and are limited to the cortex. The medullary nephrons are similar to the mammalian type, comprising a large glomerulus which is located partly within the medulla. The intermediate nephrons have a structure that is in between the reptilian and mammalian types (Johnson & Mugaas Citation1970).
The roles of the avian kidney, similarly to other vertebrate kidneys, are filtration, excretion or secretion, and absorption. Kidneys also play an important role in conserving water and reabsorbing needed substances (Roberts & Schmidt-Nielsen Citation1966). There is a direct relationship between the number of medullary cones per unit of renal cortex and effective water conservation (Johnson & Mugaas Citation1970). The current study was conducted to obtain a quantitative description of the nephron elements and renal components in three species of birds by stereological models and electron microscopy. A further aim of this research was to provide important information that will help to evaluate the composition of the kidneys in these birds.
2. Material and methods
2.1. Animals and sampling
Six healthy domestic fowl (Gallus domesticus), three house sparrows (Passer domesticus) and two domestic pigeons (Columba livia var. domestica) (male and female), representing three species and three orders (Columbiformes, Galliformes and Passeriformes), were collected in Shahrekord district, Iran. The birds were kept in cages, then immediately taken to the laboratory for tissue processing. The birds' body weights and renal dimensions were measured to obtain the biometric proportions of the kidneys.
The birds were anaesthetized with ketamine (5 mg kg−1) and xylazine (2.5 mg kg−1). A midline abdominotomy was performed to reveal the viscera and then the kidneys were flushed with phosphate buffer (pH 7.4). A half-strength formalin fixative (2% solution) was applied to decrease tissue autolysis before sampling. Then, the kidneys were carefully separated from the synsacrum bone.
Left and right kidneys were selected for light microscopy. Before carrying out the procedures, biometric values including of the length and width of each kidney were measured with callipers () and the quantitative volumes of the samples were evaluated based on the method described by Scherle (Citation1970). To assess tissue shrinkage, biometric values were again calculated for the length and width of the kidneys. Values of tissue shrinkage were determined to range from almost 10% to 25%, as found in a previous investigation (Casotti et al. Citation1998). For the preparation of the tissue blocks, the samples were embedded in paraffin wax, the organ was sliced at regular intervals (20 equal intervals) and the first cut was positioned randomly within the first interval (Boyce et al. Citation2010). The tissue sections were stained with haematoxylin and eosin.
Table 1. Biometric characteristics of the left and right kidneys: weight and length (mean ± SD).
For electron microscopic studies, the left kidneys of two bird species (domestic pigeon and domestic fowl) were processed for transmission electron microscopy. Samples from 1 to 3 mm thickness were taken, immediately prefixed in glutaraldehyde–paraformaldehyde (pH 7.4) and maintained for 24 h. They were then rinsed three times with cacodylate sodium buffer 0.15 M (pH 7.4) at 10 min intervals and postfixed in a phosphate-buffered solution of 1% osmium tetroxide at 37°C for 2 h. Samples were washed again in distilled water and dehydrated by passage through a series of increasing concentrations of ethanol (35%, 50%, 75%, 95% and 100%). Tissue blocks were prepared in small plastic capsules in pure resin, made transparent with polypropylene. Semithin sections were stained with toluidine blue, then ultrathin sections were prepared and observed under the transmission electron microscope.
The volume densities of kidney elements, including cortex, medulla, major blood vessels, and also nephron components, comprising the renal corpuscle, proximal tubule, loops of Henle, distal tubule and collecting ducts, were determined based on the Cavalieri principle by point counting at the light microscopic level (Fabricius et al. Citation2007). Their absolute volume was obtained by measuring the kidney volume, which was estimated by the water displacement method (Scherle Citation1970). The surface area of tubule structures in the kidneys was determined by the equation [(VV /d) × 4] × source volume in serial paraffin sections (Gundersen et al. Citation1988), where d is the mean luminal diameter of the measured constituents and VV is the volume of the measured constituents using Cavalieri's fundamental law, and the source volume with respect to the cortex or medulla, where these measured components are seen in the cortex and/or medulla. Values of volume and measured surface areas make it possible to determine whether the size of renal constituents is the result of a greater total length of smaller tubules or the existence of bigger tubules. Quantitative measurements of the kidney components covered only the tubular structures; the background scaffold and components of the extracellular matrix were not considered in the present study.
2.2. Statistical analyses
Statistical analyses of volume and surface area data were carried out using the SPSS statistical software package version 13.0 for Windows. Data are expressed as mean ± standard deviation (SD) and statistical variations were tested by one-way analysis of variance. The software program Statistica was used to assess variations in body weight. The relative values of variations, including the kidney volume in relation to body weight, were corrected between all the species, the calculated value was converted to a function of the logarithm and measurements were regressed versus a suitably sized covariate. Samples were selected in such a way that there was no relationship between members in the samples, as measured dependent variables were applied depending on different independent variables. For instance, birds' body weight was considered as an independent variable against the dependent variable of kidney volume. On the other hand, kidney volume was considered independent with regard to the volume of the cortex, medulla and principal blood vessels. The volumes of constituents were similarly applied as independent variables when analysing the volumes and surface areas of the renal corpuscle, proximal tubules, distal tubules, cortical collecting duct and cortical capillaries. These calculations were used for the volumes and surface areas of the limbs of the loop of Henle, collecting ducts and medullary capillaries as the dependent variable in comparison with the volume of the medulla, which was considered as the independent variable. Linear regression was used to estimate the coefficients of the linear equation involving different independent variables that best predicted the value of the dependent variable. A stepwise method was used, with the criteria of a probability of F = 0.05 for stepwise entry and F = 0.10 for stepwise removal included as constants in the equation.
3. Results
This study aimed to obtain quantitative microscopic findings on the volume dimensions of the principal constituents of the renal structure in avian species. The relative values of renal components, such as different nephron tubules, corpuscles and blood vessels, were estimated by stereological methods. The study also examined whether changes in the measurable properties of the kidney were reflected in characteristics of order and body weight.
3.1. External anatomy
With regard to macroscopic features, considerable variation was seen in the shape and extension of the lobes among the birds tested in this study. However, each kidney is comprised of three compartments: anterior, posterior and middle. In the pigeon, the lobes of the anterior and posterior kidney were similar in size, but the middle one was somewhat less extensive; a similar anatomy was found in the domestic fowl. There were no clear differences between the middle and posterior lobes in the house sparrow, but the anterior lobe was larger.
3.2. Volumetric findings
The dimensions of the left and right kidneys, body weight and kidney volume are summarized in and . The data are presented as mean ± SD. In all species, the dimensions of the right kidney were larger than those of the left kidney. The mean body weight for the individual species ranged from 32.5 ± 8.4 g in the house sparrow to 1800 ± 124.5 g in the domestic fowl. The mean stabilized kidney volume ranged from 128.3 ± 16.8 mm3 in the house sparrow to 12,500 ± 123.1 mm3 in the domestic fowl. Typically, smaller birds had larger kidneys relative to their body weight compared with larger birds, which had smaller kidneys relative to body weight.
Table 2. Body weight and kidney volume according to bird species (mean ± SD).
The volume ratios of the renal cortex and medulla partitions are presented in . The volume surrounded by cortex and medulla was different among species, with the cortex ranging from 98.5 ± 11.94 mm3 in volume in the house sparrow to 9750 ± 45.9 mm3 in the domestic fowl, and the medulla from 8.6 ± 0.55 mm3 in the house sparrow to 2200 ± 9.54 mm3 in the domestic fowl. The cortex volume ratio was lowest in domestic fowl, at 73.84%, and highest in house sparrow, at 84.33%. The lowest value of the medulla volume ratio was seen in the house sparrow (7.36%) and the highest mean estimation proportion was obtained for the domestic fowl (16.66%). The domestic pigeon had intermediate values between these two groups. The values of volume proportions in the blood vessels ranged from 9.7 ± 3.2 mm3 in the house sparrow to 1254 ± 320 mm3 in the domestic pigeon. The highest ratios for the blood vessels were found in the domestic pigeon (10.55%) and the lowest in the house sparrow (8.34%).
Table 3. Volume of the kidney and its constituent parts (mean ± SD).
The largest kidney components in the cortex were the proximal tubules in the domestic fowl (1320 ± 112 mm3) and the smallest were the collecting ducts in the cortex of the house sparrow (3.8 ± 1.2 mm3) (). In the medulla, the greatest renal constituents were the thick limb of the loop of Henle in the domestic fowl (189.4 ± 8.5 mm3) and the lowest values were found for the thin limb of the loop of Henle in the house sparrow (0.8 ± 0.3 mm3). These data volumes were changed to percentages among species to improve the presentation of the charts in and .
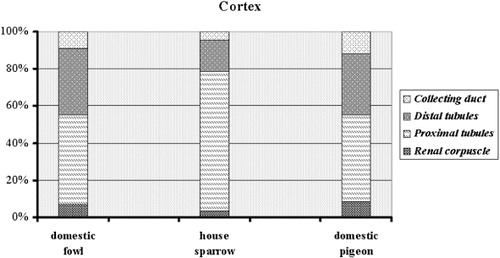
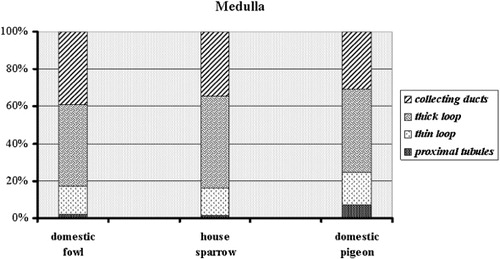
Table 4. Volume density of the tubular components of the nephron (mean ± SD).
3.3. Surface area assessment
Surface area measurements were taken of kidney components in the renal cortex and medulla (). Surface area-based comparison of the cortical partition in all species showed that the renal corpuscle had the smallest surface area in the house sparrow (1.3 ± 0.55 cm2) and the greatest values were found for the proximal tubules in the domestic fowl (425.2 ± 23.5 cm2). In the medulla, the collecting ducts of the house sparrow had the smallest surface area (0.2 ± 0.15 cm2) and the proximal tubules of the domestic fowl had the largest surface area (2.84 ± 2.4 cm2) ( and ).
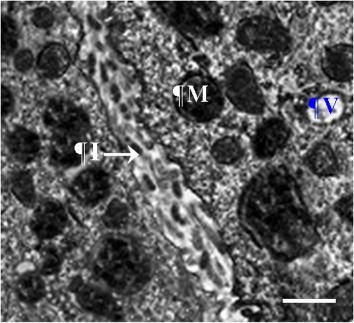
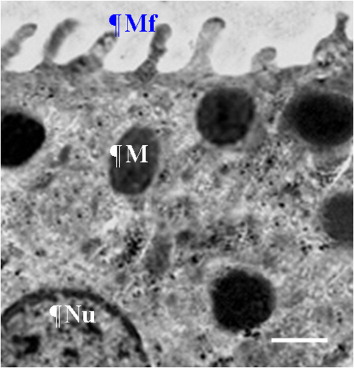
Table 5. Surface area of the tubular components of the nephron (mean ± SD).
3.4. Light microscopy
A similar renal parenchyma was seen in the three bird species. In various regions, populations of different nephron elements as well as renal corpuscles were located at variable depths, so that there was almost no line of demarcation between the cortical and medullary regions. In all birds, the peripheral area comprised the Bowman's capsule with glomerulus, proximal convoluted tubules, distal convoluted tubules and collecting tubules. Index of the glomeruli size was polygonal in shape and large number of these had a small size, however were mix in both house sparrow and domestic pigeon. In the house sparrow, the majority of the cortex was occupied by proximal convoluted tubules, whereas the distribution of the cortical convoluted components was almost equal in the other two species. The renal medulla of all three species was made up of a number of small units called the medullary cones. These medullary cones surrounded bundles of tubules (the collecting tubules) and the thick and thin limbs of the loop of Henle.
4. Discussion
Tissue shrinkage occurred during tissue processing and paraffin embedding but was not considered because the structural constituents had been shrunk in similar relative ratios. The entire kidney volume was used as the criterion for assessing the absolute volume of each component, for which virtual values were estimated by calculating the absolute volume before processing. Point counting was used to identify the principal constituents and to select transverse sections for analysis from the total sample, as previously mentioned by Gundersen et al. (Citation1988).
The relationship of relative growth between body weight and kidney volume was obtained for each of the three species. The internal renal morphology data showed that there were differences between the three orders, Galliformes, Passeriformes and Columbiformes. However, it is not unexpected that variations in the structural constituents of the avian kidney would result in different concentrations of urine. In all species, the proportions of kidney cortex to medulla will have originated from a well-arranged organization. In the cortex, distal convoluted tubules were distributed between the proximal convoluted tubules as well as the renal corpuscles. Collecting ducts were separated by thick and thin loops of Henle throughout the medullary cones. Nishimura et al. (Citation1989) showed that the thin limb of the loop of Henle is operative in the countercurrent multiplication system. They suggested a specific hypothesis for the operation of the avian concentrating mechanism, as a countercurrent multiplier system. They proposed that active transepithelial transport of sodium chloride from the thick epithelium of the ascending limb is the source of the single effect of the avian countercurrent mechanism (Nishimura et al. Citation1989). The current study presents further information regarding the role of secretory units in urine production in some bird species. Changeable gross parameters, including the percentage and volume of nephron components of the kidney, appear to be correlated with bird type and habitat, as some studies have demonstrated that birds from arid zones have a greater volume of renal medulla than birds in moist habitats (Warui Citation1989; Casotti & Richardson Citation1992, Citation1993; Casotti et al. Citation1998).
This study found prominent differences in the size of the renal corpuscles among the three species. In accordance with bird physique, the corpuscles were largest in domestic fowl, smallest in house sparrow and of intermediate size in domestic pigeon. Since large volumes of fluid are filtered in the renal corpuscles, this is reflected in their size and filtration capacity. It is therefore to be expected that greater amounts of filtered fluid are produced by larger corpuscles.
Sabolić et al. (Citation1992) showed that the proximal tubules of the kidney have an important function in near-isosmolar reabsorption from fluid filtered by the glomeruli. The fine structure of proximal tubules revealed a great capacity for reabsorbing both water and ions due to the existence of numerous mitochondria and large interdigitations of the basal lamina (Casotti & Richardson Citation1993). In this study, an individual connection was found between the renal corpuscle and the proximal convoluted tubules. Domestic fowl had greater volume and surface area of proximal convoluted tubules than the other two species. Thus, it may be concluded that birds with more tubules could reabsorb more water than other birds. The fine structure of the proximal tubules revealed an intercellular space, as well as spaces between the basolateral interdigitations. Therefore, the greater capacity for the reabsorption of water and elements may be the result of cellular interdigitation of the proximal tubules and abundant mitochondria (Rhodin Citation1963; Roberts & Schmidt-Nielsen Citation1966). In mammals, the countercurrent system is stabilized by the thin descending limb of the loop of Henle (Kokko Citation1970), and this part of the loop of Henle includes different types of cell (Bachmann & Kriz Citation1982). In contrast to mammals, the thin descending limb of the loop of Henle in bird species includes only one kind of cell, as found in Gambel's and Japanese quail (Braun & Reimer Citation1988; Casotti & Richardson Citation1993), in the initial portions of the thin limb of Henle. The findings of the current study showed that the volume and surface area of the thin descending loop of Henle were lower in the house sparrow than in the other two birds. Therefore, the sparrow has the shortest thin loop of Henle, as could be expected. The same values of volume and surface area of the thick loop of Henle were found in all species. Nishimura et al. (Citation1989) proposed that concentrated urine is produced by the prominent structure and morphological pattern of the epithelium in the thick loop of Henle, together with the structure of the thin loop of Henle.
Despite species variations, the distal convoluted tubules were decreased more than the proximal ones; however, domestic fowl showed the maximum changes by decrease of the volume and surface area of the distal segments. Beside, filtration capacity of the distal tubules at level unit is low, therefore the functional zones would shift more toward the proximal convoluted tubules than the distal. The fine structure of the distal tubules showed fewer mitochondria than in the proximal tubules, similar to findings in other birds (Nicholson Citation1982; Casotti & Richardson Citation1993). The cortical collecting ducts form the terminal part of the distal tubules, with individual traits and types from cells that are similar to those in other birds (Nicholson Citation1982; Casotti and Richardson Citation1993). However, mucus secreted by the principal cells prevents sedimentation of uric acid in the tubular lumen (Guzsal Citation1970; Peek et al. Citation1977). Regulation of sodium balance takes place in the cortical collecting ducts, in which sodium reabsorption is controlled by corticosteroid hormones and processed through mineralocorticoid and/or glucocorticoid receptors (Gaeggeler et al. Citation1993; Bens et al. Citation1999). In the medulla of the fowl, pigeon and sparrow, the nephrons terminate in collecting ducts that are similar to those of other birds described in previous studies (Nicholson Citation1982; Casotti & Richardson Citation1993).
The terminal inner medullary collecting ducts facilitate the transport of urea through the lumen, and undertake the reabsorption of water and some sodium. There were no significant differences in the medullary collecting ducts of domestic fowl, house sparrow and domestic pigeon, although the measured values were slightly greater in the domestic fowl. This may indicate that the collecting ducts in these birds are longer, thus increasing the amount of water that can be reabsorbed in this portion of the nephron. The surface area of the capillaries in the cortex and medulla was higher in the domestic fowl than in the other two species and indicates a larger surface area of the collecting ducts to transport fluid from the ducts towards the medullary matrix. In summary, morphological data in this study were based on an accurate analysis of renal components for the assessment of urine concentration in the studied species. Hence, it seems that there is higher capability in producing concentrated urine in these species than other species.
5. Conclusion
In conclusion, the results of this study suggest that the volume and surface area of the structural elements of the renal nephron in birds play a role in the concentration of urine.
Acknowledgements
This work was performed in the histology laboratory and was financially supported by the University of Shahrekord, Iran.
References
- Bachmann S, Kriz W. 1982. Histotopography and ultrastructure of the thin limbs of the loop of Henle in the hamster. Cell Tissue Res. 225:111–127. 10.1007/BF00216222
- Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. 1999. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 10:923–934.
- Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJG. 2010. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicol Pathol. 38:1011–1025. 10.1177/0192623310385140
- Boykin SLB, Braun EJ. 1993. Entry of nephrons into the collecting duct network of the avian kidney: a comparison of chickens and desert quail. J Morphol. 216:259–269.
- Braun E, Reimer P. 1988. Structure of avian loop of Henle as related to countercurrent multiplier system. Am J Physiol. 255:F500–F512.
- Casotti G, Beuchat C, Braun E. 1998. Morphology of the kidney in a nectarivorous bird, the Anna's hummingbird Calypte anna. J Zool. 244:175–184. 10.1111/j.1469-7998.1998.tb00023.x
- Casotti G, Richardson K. 1992. A stereological analysis of kidney structure of honeyeater birds (Meliphagidae) inhabiting either arid or wet environments. J Anat. 180:281.
- Casotti G, Richardson KC. 1993. A qualitative analysis of the kidney structure of meliphagid honeyeaters from wet and arid environments. J Anat. 182:239–247.
- Dantzler WH, Braun EJ. 1980. Comparative nephron function in reptiles, birds, and mammals. Am J Physiol. 239:R197–R213.
- Fabricius K, Pakkenberg H, Pakkenberg B. 2007. No changes in neocortical cell volumes or glial cell numbers in chronic alcoholic subjects compared to control subjects. Alcohol Alcoholism. 42:400–406. 10.1093/alcalc/agm007
- Gaeggeler HP, Duperrex H, Hautier S, Rossier BC. 1993. Corticosterone induces 11 beta-HSD and mineralocorticoid specificity in an amphibian urinary bladder cell line. Am J Physiol. 264:C317–C322.
- Gundersen H, Bendtsen T, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard J, Pakkenberg B, Sørensen F, Vesterby A, West MJ. 1988. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 96:379–394. 10.1111/j.1699-0463.1988.tb05320.x
- Guzsal E. 1970. Histochemical study of the kidneys of domestic fowls. Acta Veterinaria Hungarica. 20:295–299.
- Johnson OW, Mugaas JN 1970. Some histological features of avian kidneys. Am J Anat. 127:423–435. 10.1002/aja.1001270407
- Kokko JP. 1970. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest. 49:1838–1846. 10.1172/JCI106401
- Nicholson J. 1982. The microanatomy of the distal tubules, collecting tubules and collecting ducts of the starling kidney. J Anat. 134:11.
- Nishimura H, Koseki C, Imai M, Braun EJ. 1989. Sodium chloride and water transport in the thin descending limb of Henle of the quail. Am J Physiol. 257:F994–F1002.
- Peek WD, Shivers RR, Mcmillan DB. 1977. Freeze-fracture analysis of junctional complexes in the nephron of the garter snake, Thamnophis sirtalis. Cell Tissue Res. 179:441–451. 10.1007/BF00219847
- Poulson TL. 1965. Countercurrent multipliers in avia kidneys. Science. 148:389–391. 10.1126/science.148.3668.389
- Rhodin JAG. 1963. Structure of the kidney. In: Strauss MB, Welt LG, editors. Diseases of the kidney. Boston (MA): Little Brown; p. 1–29.
- Roberts JS, Schmidt-Nielsen B. 1966. Renal ultrastructure and excretion of salt and water by three terrestrial lizards. Am J Physiol. 211:476–486.
- Sabolić I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D. 1992. Localization of the CHIP28 water channel in rat kidney. Am J Physiol. 263:C1225–C1233.
- Scherle W. 1970. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 26:57–60.
- Warui CN. 1989. Light microscopic morphometry of the kidneys of fourteen avian species. J Anat. 162:19–31.
