Abstract
An eight-week feeding trial was conducted to evaluate the effects of dietary Macsumsuk® supplementation on growth performance, haematological parameters, disease resistance and body composition of juvenile Nile tilapia, Oreochromis niloticus. A total of 375 fish averaging 1.0 ± 0.05 g (mean ± SD) were randomly distributed into 15 tanks in groups of 25, and each tank was then randomly assigned to one of three replicates of five diets containing 0(M0), 0.3(M0.3), 0.6(M0.6), 1.2(M1.2) and 2.4(M2.4)% dietary Macsumsuk®. At the end of eight weeks of feeding trial, average weight gain (WG), specific growth rate (SGR), feed efficiency (FE) and protein efficiency ratio (PER) of fish fed M1.2 diet were significantly higher than those fed M0 diet (P < 0.05). Significantly higher values for haematological parameters, such as red blood cell (RBC), haemoglobin (Hb) and haematocrit (HCT), were obtained in fish fed M0.6 diet compared to the fish offered M2.4 diet. Although an analysis of variance (ANOVA) test suggested that the optimum level of dietary Macsumsuk® in juvenile Nile tilapia, O. niloticus, could be 1.2%, broken-line analysis of WG indicated a level of 0.803% when Macsumsuk® is used as the dietary feed additive.
1. Introduction
In recent years, aquaculture industry has developed rapidly in many countries. The aquaculture production improves by 15% annually and is predicted to continue to grow fast in future (Dadgar et al. Citation2010). The sustainability of aquaculture development is absolutely dependent on the availability of quality and cheap feedstuffs. Feed costs generally account for more than half of operating costs of aquaculture operations (Cho & Slinger Citation1979). Any reduction in feed costs would have a direct positive effect on profitability of aquaculture (Francis et al. Citation2005; Henry & Alexis Citation2009). The feed additives improve the immunity, productivity and economic efficiency of fish via its improvement body weight (BW) of the fish (Carnevali et al. Citation2006), weight gain (WG; Venkat et al. Citation2004), feed conversion ratio and efficiency (Abdel Hamid & Mohamed Citation2008). For all these reasons and the effect of feed additives into the fish ration, the returns and net profit will be improved with minimization of the fish production costs (Abdel Hamid & Mohamed Citation2008; Henry & Alexis Citation2009). Consequently, numerous studies have investigated the efficacy of various additives in aquafeeds that could be used to improve the performance of fish: plant products such as herbs (Yin et al. Citation2009), root (Sharma et al. Citation2010), seed meal (Ahmad & Abdel-Tawwab Citation2011), onion (Cho & Lee Citation2012) and green tea (Hwang et al. Citation2013); macroalgae such as Ulva pertusa (Satoh et al. Citation1987) and Undaria (Park et al. Citation2003); microalgae such as Chlorella ellipsoidea (Kim et al. Citation2002) and Dunaliella (Supamattaya et al. Citation2005); chitosan (Cha et al. Citation2008); and yeast glucan (Baulny et al. Citation1996). However, more studies to develop dietary additives to improve the performance of fish in fish farming are required.
Macsumsuk® is a kind of rare earth mineral that radiates more than 90% of eco-friendly beneficial far infrared rays in a range of 8–10 µm, a bio-absorbable wavelength range. Macsumsuk® contains abundant natural minerals such as silica (SiO2), aluminium (Al), calcium (Ca), magnesium (Mg), ferrous (Fe) and various other beneficial material. Because of this, it is very beneficial to bioactivity. Refined Macsumsuk® through sorting, firing, burning and grinding processes has been routinely used for medical products, medicine and purification of water and air. Macsumsuk® has also been reported to have a strong antibacterial effect, vitalize the cells, help promote the growth and development and boost the immunity against diseases (Macsumsuk GM, CO., LTD Citation2012).
Nile tilapia, Oreochromis niloticus (L.) is one of the most economically important species in aquaculture to culture because of its rapid growth, good survival in high density culture and disease tolerance (El-Sayed Citation2006). However, currently no reports are available regarding the effect of Macsumsuk® as a feed additive on fish growth. Therefore, this study aimed at elucidating the potential effects of Macsumsuk® on growth performance, feed utilization, blood parameters and disease resistance of juvenile Nile tilapia challenged with Edwardsiella tarda.
2. Materials and methods
2.1. Preparation of experimental diet
Dietary additive Macsumsuk® powder () was provided by the Macsumsuk® GM company, Republic of Korea. Five experimental diets were formulated to be isonitrogenous and isoenergetic and to contain 42.0% crude protein and 15.9 kJ available energy/g diet (NRC 2001). Dietary Macsumsuk® was supplemented at 0(M0), 0.3(M0.3), 0.6(M0.6), 1.2(M1.2) and 2.4(M2.4)% in place of cellulose. Fish meal and dehulled soybean meal were used as the protein sources, soybean oil and fish oil as the lipid sources, and wheat flour and corn starch as the carbohydrate sources in the experimental diets. Dietary formulation and proximate composition of the basal diet are shown in .
Table 1. Mineral composition of Macsumsuk.
Table 2. Dietary formulation and proximate composition of the basal diet for juvenile Nile Tilapia, Oreochromis niloticus.a
Procedures for diet preparation and storage were followed as previously described by Bai and Kim (Citation1997). Diets were prepared by mixing the dry ingredients in an electric mixer, followed by the addition of oil and water. This mixture was formed into dough and dry pellets were made by passing the dough through a screw-type pelleting machine and air drying the formed pellets for approximately 48 h. After drying, the pellets were broken up, sieved into the proper diameter size, sealed and stored at −20°C until use for feeding trial.
2.2. Experimental design
The feeding trial was carried out at the Feeds and Foods Nutrition Research Center, Pukyong National University, Busan, South Korea. Fish were transported to the experimental station and acclimated to the experimental conditions for one week before the feeding trial began. At the start of the experiment, 25 Nile tilapia with an initial weight averaging 1.0 ± 0.05 g (mean ± SD) were randomly distributed into each of the 15 tanks. Each tank was then randomly assigned to one of three replicates of five dietary treatments. Fish were fed twice daily (09:00 and 18:00 h) for eight weeks at a fixed rate of 5% of wet BW/day. Total fish weight in each tank was determined every two weeks and the feeding rate was adjusted accordingly. The feeding trial was conducted by using the semi-recirculating system with 15 tanks (40 L) receiving filtered seawater at the rate of 0.8 L/min from the centre tank. Supplemental aeration was provided to maintain the dissolved oxygen at 6.0 ± 0.2 mg L−1, and also water temperature, ammonia nitrogen, nitrite and pH during the experiment were maintained at 26 ± 0.5°C, 0.05 ± 0.01 mg NH4-N/L, 0.13 ± 0.05 mg NO2-N/L and 8.13 ± 0.13, respectively. Siphoning was done every morning in each rearing tank; also, dead fish were removed, weighed and recorded daily.
2.3. Sample collection and analysis
At the end of the feeding trial, fish were weighed and counted to determine WG, feed efficiency (FE), specific growth rate (SGR), protein efficiency ratio (PER) and survival. After the final weighing, blood samples were obtained from the caudal vessels of three individual fish per tank (nine fish per treatment group) by using a heparinized syringe and pooled into treatment groups for blood analysis. Haematocrit (HCT; packed cell volume, PCV) was determined by the micro HCT e method (Brown Citation1980), and haemoglobin (Hb) was measured with the same fish by the cyanmethaemoglobin procedure using Drabkin's solution. Hb standard prepared from human blood (Sigma Chemical Co., St Louis, MO, USA) was used.
Five fish were freeze-dried as whole body and held at −20°C until used for proximate composition analysis. The tested diets and whole-fish body from each treatment were analysed according to the standard methods of AOAC (Citation2000) for moisture, protein, lipid and ash. Samples of diet and fish were dried at 135°C for 2 h to a constant weight to determine their moisture content. Ash content was determined by incineration at 600°C. Crude protein was determined using the Kjeldahl method (N × 6.25) after acid digestion, and crude lipid was determined by soxhlet extraction using Soxtec system 1046 (Tecator Ab, Hoganas, Sweden).
2.4. Bacterial challenge
Challenge test was performed following the method described by Choi et al. (Citation2008). At the end of the feeding trial, fish were redistributed based on their earlier dietary treatments in 40-L tanks as triplicate groups of five fish and allowed to stabilize for 24 h. E. tarda (FPC 799) was grown in heart infusion broth (Nissui, Japan) for 24 h at 37°C. The culture broth was centrifuged at 3000× g for 10 min. Fish from each experimental group were injected intraperitoneally with 0.1 mL of the bacterial suspension per fish at a concentration of 1× 108 colony forming units (cfu) mL−1. Twenty-four hours after challenge, the flow rate was established (0.8 L/min) and mortality of the challenged fish from each tank was observed up to 12 days.
2.5. Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) test using Statistix 3.1 (Analytical Software, St Paul, MN, USA). When a significant treatment effect was observed, a least significant difference test was used to compare means. Treatment effects were considered significant at P < 0.05. The breakpoint for optimum dietary Macsumsuk® was estimated by using the broken-line model of Robbins et al. (Citation1979).
3. Results
3.1. Growth performance and haematological parameters
shows the growth performance and haematological parameters of fish fed the different experimental diets. At the end of eight weeks of feeding trial, WG, SGR, FE and PER of fish fed M1.2 diet were significantly higher than those fed M0 diet (P < 0.05). However, there were no significant differences in WG, SGR, FE and PER among fish fed M0.3, M0.6, M1.2 and M2.4 diets, or among those fed M0, M0.3, M0.6 and M2.4 diets (P > 0.05), respectively. There was no significant difference in survival of fish fed all the experimental diets. RBC, Hb and HCT of fish fed M0.6 diet were significantly higher than those fed M2.4 diet. However, there were no significant differences in RBC, Hb and HCT among fish fed M0, M0.3, M0.6 and M1.2 diets, or among those fed M0, M0.3, M1.2 and M2.4 diets. Broken-line analysis of WG indicated that the optimum inclusion level of dietary Macsumsuk® as an additive in juvenile Nile tilapia, O. niloticus (1.0–7.4 g) could be approximately 0.803% () under the given experimental conditions.
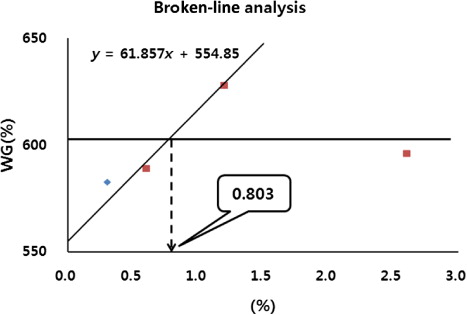
Table 3. Growth performances and haematological parameters of juvenile Nile Tilapia, Oreochromis niloticus, fed five experimental diets.
3.2. Whole-body proximate composition
Whole-body proximate composition of juvenile Nile tilapia fed different levels of dietary Macsumsuk® is shown in . Whole-body crude lipid content was significantly higher in fish fed M0.3 and M0.6 diets than in those fed M2.4 diet (P < 0.05). However, there were no significant differences in whole-body crude lipid content among fish fed M0, M0.3, M0.6 and M1.2 diets, or among those fed M0, M1.2 and M2.4 diets (P > 0.05). Whole-body moisture content was significantly higher in fish fed M0 diet than in those fed M0.6 diet. However, there were no significant differences in whole-body moisture content among fish fed M0, M0.3, M1.2 and M2.4 diets, or among those fed M0.3, M0.6, M1.2 and M2.4 diets. There were no significant differences in whole-body crude protein and ash contents of fish among all treatments.
Table 4. Whole-body proximate composition of juvenile Nile Tilapia, Oreochromis niloticus, fed five experimental diets.
3.3. Challenge test
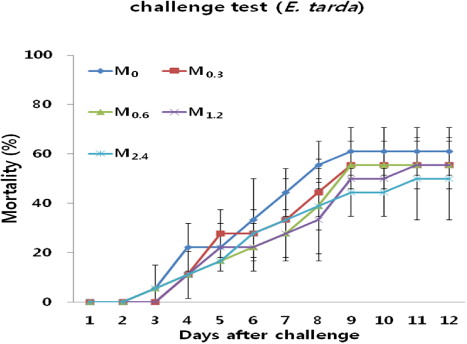
shows a cumulative mortality rate (%) of juvenile Nile tilapia over the duration of 12 days after intraperitoneal injection with E. tarda fed five different diets for eight weeks. Mortality was recorded right from the first day of the injection. After nine days of the injection, more than 40% of fish fed M0, M0.3, M0.6, M1.2 and M2.4 diets had died. However, no significant differences were recorded in mortality rate of fish among all treatments (P > 0.05).
4. Discussion
To the best of our knowledge, no previous work has been done to investigate the effect of Macsumsuk® on growth performance and feed utilization in fish. In the present study, fish fed diet containing 1.2% dietary Macsumsuk® had a significantly higher growth performance and feed utilization efficiency as compared to fish fed the control diet. The present study demonstrates that the dietary supplementation of Macsumsuk® enhanced WG, SGR, FE and protein efficiency ratio of Nile tilapia, as a feed additive. Likewise, Dada (Citation2012) who reported that feeding Superliv® as a feed additive in Nile tilapia can enhance growth and feed utilization. In addition, Meurer et al. (Citation2009) showed that the diet supplemented with brown propolis improved WG of fingerlings Nile tilapia, O. niloticus. These results may be possibly due to the Macsumsuk® as a feed additive can enhance feed ingestion and absorption and nutrient metabolism in Nile tilapia; various types of feed additives enhance the digestibility and utilization efficiency of nutrients, including enzyme secretion; stimulators of in the digestive process by improving absorption, mobilization and transport of nutrients; and feeding stimulants that reduce feed/nutrient waste (Ceulemans et al. Citation2009).
Haematological parameters are used as valuable biological indicators in response to dietary manipulations (Adhikari et al. Citation2004; Shah & Altindag Citation2005; Maheswaran et al. Citation2008). In the present study, haematological parameters of Nile tilapia were taken into account and significant differences were found among different dietary treatments. Higher red blood cell (RBC), HCT and Hb values were found in fish fed Macsumsuk® level of 0.6%. On the contrary, RBC, HCT and Hb decreased above this level, indicating that high inclusion levels of Macsumsuk® above the optimum level negatively affect the haematological parameters of Nile tilapia. In other words, adequate supplementation of Macsumsuk® improves growth, FE and haematological parameters of Nile tilapia; however, excess supplementation could adversely affect the health of fish. This suggests that care should be taken to maintain the optimum supplementation level. To our knowledge, there is no available information on the effects of dietary Macsumsuk® on haematological parameters of fish species. Similarly, the obtained results in HCT concerning the effect of Macsumsuk® are supported by the findings of some researchers such as Bae et al. (Citation2012) who showed that HCT concentration of the juvenile eel, Anguilla japonica, decreased above the optimum level of dietary Propolis.
E. tarda, FPC 799 is a gram-negative bacterium associated with freshwater ecosystems. Known to colonize in a wide variety of amphibians, reptiles and fish, E. tarda can also cause disease in these animals, as exemplified by emphysematous putrefactive disease of catfish (Meyer & Bullock Citation1973). Edwardsiella septicaemia occurs in numerous freshwater and marine fish species economically important to aquaculture such as Nile tilapia, O. niloticus (Meyer & Bullock Citation1973; Austin & Austin Citation1999). The Nile tilapia is quite susceptible to E. tarda infection; consequently, in order to assess the disease tolerance of Nile tilapia in this trial, fish were infected with E. tarda. In the present study, there was no significant difference among different treatments in mortality of Nile tilapia infected with E. tarda. Therefore, Macsumsuk® improved survival in Nile tilapia fed diets supplemented with 0.3–2.4% Macsumsuk® nine days after intraperitoneal injection with the bacteria. It can be anticipated that dietary Macsumsuk® was able to enhance the immune system in Nile tilapia against E. tarda infection.
In conclusion, results indicated the optimum Macsumsuk® supplementation levels of 0.803–1.2% based on the broken-line analysis of WG and ANOVA test for optimum growth and FE and 0.3–2.4% for enhanced disease resistance in juvenile Nile tilapia, O. niloticus. This study may suggest that the dietary Macsumsuk® can enhance growth performances, feed utilization, haematological parameters and induce higher protection of Nile tilapia against E. tarda infection.
Additional information
Funding
References
- Abdel Hamid E, Mohamed KA. 2008. Effect of using probiotic as growth promoters in commercial diets for monosex Nile tilapia (Oreochromis niloticus) fingerlings. 8th International Symposium on Tilapia in Aquaculture; 2008 Oct 12–14; Cairo: International Convention Center (CICC).
- Adhikari S, Sarkar B, Chatterjee A, Mahapatra CT, Ayyappan S. 2004. Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicol Environ Saf. 58:220–226. 10.1016/j.ecoenv.2003.12.003
- Ahmad M, Abdel-Tawwab M. 2011. The use of caraway seed meal as a feed additive in fish diets: growth performance, feed utilization, and whole-body composition of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Aquaculture. 314:110–114. 10.1016/j.aquaculture.2011.01.030
- AOAC (Association of Official Analytical Chemists). 2000. Official methods of analysis. 17th ed. Virginia: AOAC.
- Austin B, Austin DA. 1999. Bacterial fish pathogens: disease in farmed and wild fish. Chichester: Ellis Horwood Limited.
- Bae JY, Park GH, Lee JY, Okorie EO, Bai SC. 2012. Effects of dietary propolis supplementation on growth performance, immune responses, disease resistance and body composition of juvenile eel, Anguilla japonica. Aquacult Int. 20:513–523. 10.1007/s10499-011-9482-4
- Bai SC, Kim KW. 1997. Effects of dietary animal protein sources on growth and body composition in Korean rockfish, Sebastes schelegeli. J Aquacult. 10:77–85.
- Baulny MOD, Quentel C, Fournier V, Lamour F, Gouvello RL. 1996. Effect of long-term oral administration of β-glucan as an immunostimulant or an adjuvant on some non-specific parameters of the immune response of turbot Scophthalmus maximus. Dis Aquat Organ. 26:139–147. 10.3354/dao026139
- Brown BA. 1980. Routine hematology procedures. In: Brown BA, editor. Hematology: principles and procedures. Philadelphia (PA): Lea and Febiger; p. 71–112.
- Carnevali O, Vivo L, Sulpizio R, Gioacchini G, Olivotto I, Silvi S, Cresci A. 2006. Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture. 258:430–438. 10.1016/j.aquaculture.2006.04.025
- Ceulemans S, Coutteau P, Robles R. 2009. Innovative feed additives improve feed utilization in Nile tilapia. Global Aquacult Adv, Nov/Dec. 63–65.
- Cha S, Lee J, Song C, Lee K, Jeon Y. 2008. Effects of chitosan-coated diet on improving water quality and innate immunity in the olive flounder, Paralichthys olivaceus. Aquaculture. 278:110–118. 10.1016/j.aquaculture.2008.01.025
- Cho SH, Lee SM. 2012. Onion powder in the diet of the olive flounder, Paralichthys olivaceus: effects on the growth, body composition, and lysozyme activity. J World Aquacul Soc. 43:30–38. 10.1111/j.1749-7345.2011.00489.x
- Cho CY, Slinger SJ. 1979. Apparent digestibility measurement in feedstuffs for rainbow trout. In: Halver JE, Tiews K, editors. Finfish nutrition and fish feed technology. Berlin: Heenemann GmbH, 2; p. 239–248.
- Choi SH, Park KH, Yoon TJ, Kim JB, Jang YS, Choe CH. 2008. Dietary Korean mistletoe enhances cellular non-specific immune responses and survival of Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 24:67–73. 10.1016/j.fsi.2007.08.007
- Dada AA. 2012. Effects of herbal growth promoter feed additive in fish meal on the performance of Nile tilapia (Oreochromis niloticus (L.)). Egypt Acad J Biol Sci. 4:111–117.
- Dadgar S, Saad CRB, Alimon AR, Kamarudin MS, Nafisi Bahabadi M. 2010. Comparison of Soybean meal and Cottonseed meal variety Pak (CSMP) on growth and feed using in rainbow trout (Oncorhynchus mykiss). Iran J Fish Sci. 9:49–60.
- El-Sayed AFM. 2006. Tilapia culture. Wallingford, Oxfordshire: CABI Publishing, CABI International.
- Francis G, Makkar HPS, Becker K. 2005. Quillaja saponins-a natural growth promoter for fish. Stuttgart, Germany: Department of Animal Nutrition and Aquaculture, Institute for Animal Production in Tropics and Subtropics, University of Hofenheim.
- Henry MA, Alexis MN. 2009. Effects of in vitro lactoferricin and lactoferrin on the head kidney cells of European sea bass (Dicentrarchus labrax, L.). Vet Immunol Immunopathol. 130:236–242. 10.1016/j.vetimm.2009.02.014
- Hwang JH, Lee SW, Rha SJ, Yoon HS, Park ES, Han KH, Kim SJ. 2013. Dietary green tea extracts improves growth performance, body composition, and stress recovery in the juvenile black rockfish, Sebastes schlegeli. Aquacul Int. 21:525–538. 10.1007/s10499-012-9586-5
- Kim KW, Bai SC, Koo JW, Wang X, Kim SK. 2002. Effects of dietary Chlorella ellipsoidea supplementation on growth, blood characteristics, and whole-body composition in juvenile Japanese flounder Paralichthys olivaceus. J World Aquacul Soc. 33:425–431. 10.1111/j.1749-7345.2002.tb00021.x
- Macsumsuk GM, CO., LTD. 2012. Yeongcheon-si. Available from: http://www.macsumsuk.com/.
- Maheswaran R, Devapaul A, Muralidharan S, Velmurugan B, Ignacimuthu S, 2008. Haematological studies of freshwater fish, Clarias batrachus (L.) exposed to mercuric chloride. IJIB. 2:49–54.
- Meurer F, De Costa MM, De Barros DAD, De Oliveira STL, De Paixao PS. 2009. Brown propolis extract in feed as a growth promoter of Nile tilapia (Oreochromis niloticus, Linnaeus 1758) fingerlings. Aquacul Res. 40:603–608. 10.1111/j.1365-2109.2008.02139.x
- Meyer FP, Bullock GL. 1973. Edwardsiella tarda, a new pathogen of channel catfish, Ictalurus punctatus. Appl Microbiol. 25:155–156.
- Park SU, Kwon MG, Lee YH, Shin IS, Min SM. 2003. Effects of supplemental Undaria, obosan and wasabi in the experimental diets on growth, body composition, blood chemistry and non-specific immune response of juvenile flounder, Paralichthys olivaceus. J Aquacul. 16:210–215.
- Robbins KR, Norton HW, Baker DH. 1979. Estimation of nutrient requirements from growth data. J Nutr. 109:1710–1714.
- Satoh K, Nakagawa H, Kasahara S. 1987. Effect of Ulva meal supplementation on disease resistance of red seabream. Nippon Suisan Gakkaishi. 53:1115–1120. 10.2331/suisan.53.1115
- Shah SL, Altindag A. 2005. Alterations in the immunological parameters of tench (Tinca tinca L. 1758) after acute and chronic exposure to lethal and sub lethal treatments with mercury, cadmium and lead. Turk J Vet Anim Sci. 29: 1163–1168.
- Sharma A, Deo A, Riteshkumar S, Tandel C, Thongam I, Das A. 2010. Effects of Withania somnifera (L. Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita (Hamilton) fingerlings. Fish Shellfish Immunol. 29:508–512. 10.1016/j.fsi.2010.05.005
- Supamattaya K, Kiriratnikom S, Boonyaratpalin M, Borowitzka L. 2005. Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). Aquaculture. 248: 207–216. 10.1016/j.aquaculture.2005.04.014
- Venkat HK, Sahu NP, Jain KK. 2004. Effect of feeding lactobacillus-based probiotics on the gut microflora, growth and survival of Macrobacterium resenbergii (de man). Aquacul Res. 35:501–507. 10.1111/j.1365-2109.2004.01045.x
- Yin G, Ardo K, Thompson D, Adams A, Jeney Z, Jeney G. 2009. Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol. 26:140–145. 10.1016/j.fsi.2008.08.015