Abstract
In this work, we evaluated the effect of the probiotic Enterococcus gallinarum L-1 on the cellular immune system of four different fish species of great interest in aquaculture such as gilthead sea bream (Sparus aurata), European sea bass (Dicentrarchus labrax), meagre (Argyrosomus regius) and red porgy (Pagrus pagrus). Phagocytic activity, respiratory burst and peroxidase content of leucocytes were observed 30 minutes after incubation with the probiotic E. gallinarum strain L-1, alive or inactivated with heat shock or ultraviolet (UV) light at different concentrations of 107, 108 and 109 cfu mL−1 (final concentration 106, 107 and 108 cfu mL−1). E. gallinarum produced dose-dependent increments in respiratory burst in red porgy, sea bream and sea bass leucocytes. About 106 and 107 cfu mL−1 of live and inactivated bacteria with no stimulation of the respiratory burst activity of sea bream and red porgy head kidney leucocytes was shown. The highest values of peroxidase content were observed in red porgy cells with stimulation indexes higher than 1 in each treatment. Statistical analysis revealed that differences were only significant in sea bream where UV light-inactivated bacteria denote statistically significant differences (P < 0.05) with respect to other treatments. Highest values of phagocytic activity were obtained in sea bream leucocytes incubated with live bacteria (26% ± 1.88), where significant differences (P < 0.05) with other species were detected. Our results suggest that the in vitro assays may be useful in optimising their effective dose and viability for the immunomodulatory effects of probiotic bacteria, although in vivo studies are necessary to confirm the immunomodulatory effect of this strain.
1. Introduction
In the last few years, the use of probiotics and immunostimulants has had a remarkable increase in aquaculture both due to the intensification of this area and due to the general tendency of reducing the use of antibiotics (Thyssen & Ollevier Citation2001). The environmental impact caused by the presence of chemotherapeutic agents in the marine environment and the appearance of bacterial resistance are the main reasons why probiotics and immunostimulants are considered as excellent candidates to control fish diseases (Gatesoupe Citation1999; Panigrahi et al. Citation2004; Balcázar et al. Citation2006; Díaz-Rosales et al. Citation2009; García de la Banda et al. Citation2010; Sharifuzzaman & Austin, Citation2010). According to the currently adopted definition by Food and Agricultural Organization/World Health Organization, probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host (FAO Citation2001). There are some limitations caused by the administration of live bacteria into fish pens or cages for their potential interaction with the marine environment. The use of inactivated bacteria clearly solves such safety-related issues since they can no longer interact with other aquatic organisms. Probiotics have, indeed, been redefined as microbial complements, not necessarily alive, that have beneficial effects on host health (Díaz-Rosales et al. Citation2006). Numerous microbes have been identified as probiotics for aquaculture practices, many of which differ markedly in their mode of action such as competition of adhesion sites, production of antibiotic substances, improvement in feed efficiency and competition for energy sources (Díaz-Rosales et al. Citation2009).
Among the numerous beneficial and awaited effects of probiotics, modulation of immune system may be one of the commonly purposed and more important consequences. Most of the earlier studies in fish dealt with growth-promoting and disease-protective ability of probiotics (Nayak Citation2010). However, in recent years, much attention has been paid towards the immunomodulating effects of probiotics in aquaculture (Irianto & Austin Citation2003; Nikoskelainen et al. Citation2003; Panigrahi et al. Citation2005; Salinas et al. Citation2005; Díaz-Rosales et al. Citation2009; García de la Banda et al. Citation2010; Sharifuzzaman et al. Citation2011). A clear evidence of the benefit effect of probiotics do exist, particularly on the immune system after administration of one or several strains along different periods of time (Balcázar et al. Citation2007; Villamil et al. Citation2002; Salinas et al. Citation2008; Son et al. Citation2009; Chiu et al. Citation2010). However, in vitro studies that evaluated the immunomodulator effects of bacteria on the immune cells are particularly scarce (Salinas et al. Citation2006; Román et al. Citation2012). Taking into account that in most of the studies, the results obtained in vitro show correspondence to those obtained in vivo (Salinas et al. Citation2005; Díaz-Rosales et al. Citation2006), the in vitro assays are being developed to reliably identify the most interesting bacterial strains (Morelli Citation2000).
The present work is the first study including the effect of the probiotic Enterococcus gallinarum strain L-1 on the innate immune parameters of different relevant fish species for marine aquaculture such as gilthead seabream (Sparus aurata), European sea bass (Dicentrarchus labrax), meagre (Argyrosomus regius) and red porgy (Pagrus pagrus).
The in vitro effects of viable or heat-killed and UV-light killed bacteria were studied, such as phagocytic activity, peroxidase content and respiratory burst activities of head kidney (HK) leucocytes. Thus we do not only analyze the effect of the same strain on different fish species but we can also better adjust the concentration to feed animals in the future.
2. Materials and methods
2.1. Bacteria
E. gallinarum strain L-1 isolated from gilthead sea bream intestine, according to the in vitro and in vivo characteristics tested in our laboratory made them suitable as potential fish probiotic (Sorroza et al. Citation2013). E. gallinarum strain L-1 was evaluated in vitro through various mechanisms of selection, such as production of antagonistic effects against pathogens (Austin et al. Citation1992), production of antibacterial substance (Nikoskelainen et al. Citation2001; Kim & Austin Citation2008), fish bile and pH resistance (Nikoskelainen et al. Citation2001) adhesion and growth in intestinal mucus (Olsson et al. Citation1992; Gómez & Balcázar Citation2008; Van der Marel et al. Citation2008), and the in vivo study of the protective effect against Vibrio anguillarum European sea bass (D. labrax) (Sorroza et al. Citation2013).
The strain was correctly identified as Enterococcus spp by Polymerase Chain Reaction (PCR) through a sequencing of the 16S rRNA (Sorroza et al. Citation2013). E. gallinarum L-1 was grown at 22°C in tubes containing 10 mL of brain heart infusion broth (BHIB; Pronadisa) supplemented with 1.5% NaCl, with continuous shaking for 24 h. Bacterial suspensions were washed with sterile phosphate-buffered saline (PBS) and the final concentration adjusted to 109 cfu mL−1 by espectrophotometry (Biophotometer, Eppendorf, USA). E. gallinarum strain L-1 was used both live and inactivated for the three different assays. All bacterial cultures were heat-inactivated for 2 h at 60°C and UV-light inactivated for 2.5 h. Then all bacterial cultures were plated on Brain Heart Infusion Agar (BHIA) to check the bacteria inactivation and finally stored at −80°C with until required.
2.2. Fish
Four different healthy species of marine fish were used: gilthead sea bream ((S. aurata), sea bass (D. labrax), meagre, (A. regius) and red porgy (P. pagrus) with an average body weight of 200 g. Sea bass, sea bream and meagre were kindly provided by a local fish farm Canexmar (Canary Island), while red porgy were gently provided by Instituto Canario de Ciencias Marinas (Telde, Spain). Prior to the study, some fish were checked in our laboratory for the absence of pathogen bacteria that could interfere with the study.
2.3. Isolation of HKleucocytes and tested with the bacteria
Previously, fish were slaughtered in a local fish farm and in the Instituto Canario de Ciencias Marinas (ICCM) with an overdose of 2-phenoxyethanol (Panreac) and then they were transferred to the Instituto Universitario de Sanidad Animal y Seguridad alimentaria (IUSA). HK leucocytes were isolated from each fish analysed (n = 30) under sterile conditions following the technique described by Secombes (Citation1990) with some modifications. Briefly, HK leucocytes were excised, cut into small fragments and transferred to 8 mL of Leibovitz L-15 medium (L-15 medium, Gibco, Gaithersburg, MD, USA) with 0.35% sodium chloride, 500 U mL−1 penicillin-streptomycin (Sigma), 0.005 mg mL−1 gentamicyn (Sigma; Penicillin/Streptomycin/Gentamycin [P/S/G]), 10 U mL−1 heparin (Sigma) and 2% foetal bovine serum (FBS, Sigma). Cell suspensions were obtained by forcing the fragments of the organ through a 100-µm nylon mesh. After centrifugation (400× g for 10 min), cells suspension was layered on a Ficoll gradient (Lymphoprep) suspension and centrifuged at 1100× g for 30 min at 4°C. The interface layer was harvested and diluted in 1 mL of supplemented L-15 medium and again centrifuged at 450× g for 10 min at 4°C to remove residual Ficoll and finally resuspended in L-15 medium supplemented with P/S/G. Viable cells were stained with trypan blue and counted in a Neubawer chamber. For the study of the respiratory burst and peroxidase content, aliquots of 100 µL containing 106 cells mL−1 in L-15 medium supplemented with P/S/G were added to 96-well microtitre plates (Nunc, Roskilde, Denmark) and aliquots of 500 µL containing 107 cells mL−1 in L-15 medium supplemented with P/S/G were seeded onto 20-mm diameter glass coverslips in 6-well plates (Nunc, Roskilde, Denmark). After 3 h of incubation at 22°C, the non-adherent cells were removed, and the medium was substituted by L-15, and P/S/G was supplemented with 2% FBS. HK leucocytes were incubated overnight at 22°C.
E. gallinarum live and inactivated were adjusted in PBS to 107, 108 and 109 cfu mL−1, and 100 µL of each suspension was added to the samples of 100 µL of HK leucocytes after 30 min of incubation, (final concentration 106, 107 and 108 cfu mL−1) and peroxidase content and respiratory burst activities were measured. To study phagocytic activity a MOI 1:1 technique was realized after one-hour incubation with live and inactivated bacteria.
2.4. Study of the immune parameters
2.4.1. Peroxidase content
The total peroxidase content present inside HK leucocytes was measured according to Quade and Roth (Citation1997). Live and inactivated bacteria, adjusted in PBS to 107, 108 and 109 cfu mL−1 and 100 µL of each suspension, was added to the samples of 100 µL of HK leucocytes and incubated for 30 min. After the incubation, leucocytes were lysed with 75 µL of 0.02% cetyltrimethylammonium bromide (CTAB, Sigma). Afterwards, 50 µL of 10 mM 3,3′,5,5′-tetramethylbenzidine hydrochloride (TMB, Sigma) and 25 µL of 5 mM H2O2 were added producing a colour-change reaction. This reaction was stopped after 2 min by adding 50 µL of 2 M sulphuric acid (H2SO4), and the optical density was read at 450 nm in a multiscan spectrophotometer (MultiskanFc, Thermo, Chicago). Controls consisted of leucocytes incubated with 100 µL of PBS without bacterial cells.
2.4.2. Respiratory burst activity
The generation of intracellular superoxide radicals by phagocytes was determined by the reduction of nitro blue tetrazolium (NBT, Sigma) according to the technique described by Secombes (Citation1990) and Boesen et al. (Citation2001). All experiments were carried out in triplicate. Respiratory burst activity of isolated phagocytes from the assay was expressed as a stimulation index, which was calculated as the ratio between the absorbance obtained with phagocytes from fish incubated with bacterial cells and the absorbance of the controls phagocytes incubated with phorbol myristate acetate (PMA).
2.4.3. Phagocytic activity
The phagocytic activity was measured as described by Puangkaew et al. (Citation2004). Then phagocyte monolayer was incubated with 10 µL of 109 cfu mL−1 (MOI 1:1; bacteria/macrophage cell ratio) of live and inactivated probiotic for 1 hour at 22°C. After washing with PBS, the cells were stained with Diff-Quick solution (Panreac). One hundred macrophages per slide were counted, and the phagocytic capacity was determined as the percentage of macrophages with phagocytic ability.
2.5. Statistical analysis
The results are expressed as the stimulation index (mean ± SE). Thus, values higher than 1 are mean activation, while lower values reflect inhibition. All statistical analyses were carried out using the statistical software SPSS program version 17 (SPSS, Inc., Chicago, IL, USA). Data were analysed by two-way analysis of variance and Tukey's comparison of means when necessary in the case of peroxidase content and respiratory burst activity data, and one-way for the results obtained for the phagocytic activity. Differences were considered statistically significant when P < 0.05.
3. Results and discussion
The peroxidase content of HK leucocytes of the sea bass, sea bream, meagre and red porgy incubated with live and inactivated bacteria with three different concentrations showed variations among species studied (). Live and inactivated bacteria were stimulated in vitro in sea bream (), sea bass () and red porgy leucocytes (). Statistical analysis revealed that the differences were only significant (P < 0.05) in sea bream luecocytes where UV-light inactivated bacteria denote statistically significant differences (P < 0.05) with respect to other treatments.
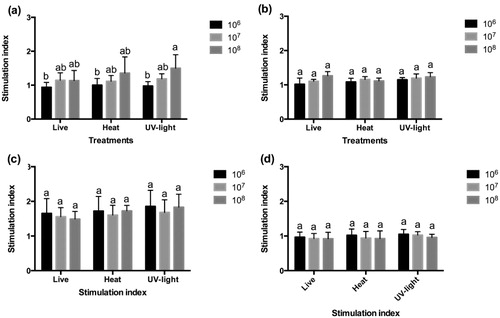
After incubation with 106 and 107 cfu mL−1 of live and inactivated bacteria (), no stimulation of the respiratory burst activity of sea bream () and red porgy HK leucocytes was shown (). However, when 108 cfu mL−1 was added, stimulation indexes higher than 1 were observed in these two species and the UV-light inactivated bacteria denote statistically significant differences (P < 0.05) with respect to other treatments.
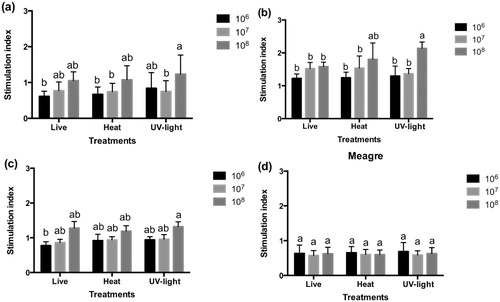
Maximum slope of respiratory burst activity was higher after the addition of 108 cfu mL−1 UV-light inactivated bacteria in sea bass HK leucocytes, showing stimulation indexes higher than 1 in all treatments (). Statistically significant differences (P < 0.05) were found in 108 cfu mL−1 UV-light inactivated bacteria with the other doses.
Considering that the correlation between in vivo and in vitro results have been reported in previous studies (Villamil et al. Citation2002; Salinas et al. Citation2006), in this work, we studied for the first time the immunomodulator effect of E. gallinarum L-1 on HK leucocytes of four different fish species of economical relevance. Thus we could compare how leucocytes of different fish species responded to the same probiotic and if viability of this strain influenced the immune modulation.
In general, it was observed that both alive and inactivated probiotic produced response in leucocytes of the analysed fish species and that this response was dose-dependent. Our results matched to some extend with those reported by Salinas et al. (Citation2006) in HK sea bream leucocytes and Román et al. (Citation2012) in HK sea bream and sea bass leucocytes both incubated for a short time with different probiotics strains. Salinas et al. (Citation2006) studied the respiratory burst, peroxide content and cytotoxic activity of sea bream leucocytes incubated with probiotics and found a dose-dependent effect in each of these parameters. In our study, a dose-dependent effect in the respiratory burst of leucocytes incubated with E. gallinarum was observed, as described by Salinas et al. (Citation2006) in sea bream and Román et al. (Citation2012) in sea bass with the only exception for meagre leucocytes where no stimulation was induced. The dose of 108 cfu mL−1 and the UV-light inactivated bacteria induced the highest response in sea bream, sea bass and red porgy, and our results are similar to those obtained by Román et al. (Citation2012). While in sea bream and red porgy no difference between alive and inactivated strain was found, in sea bass, the UV-light inactivated bacteria seemed to produce the highest stimulation. Regarding the peroxide content, red porgy leucocytes showed the highest stimulation levels with no differences among treatments and doses. In this study, the respiratory burst and the peroxidase content of HK leucocytes of sea bream and red porgy were similar. Both species belong to Sparidae family of fish, then it may have an evidence of the relationship between fish species in response to the same probiotic strain.
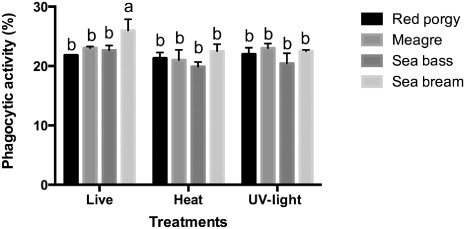
shows the phagocytic activity of leucocytes incubated with live and killed bacteria inactivated by both UV-light and heat shock. Results do not show any differences neither among fish species nor among treatments. Highest values were obtained in sea bream leucocytes incubated with live bacteria (26% ± 1.88), where significant differences with other species were detected. In all species, an internal vacuole could be seen in the cell cytoplasm with or without digested bacteria inside. Phagocytic activity appears at the early stage of fish's innate immune response and plays an important role against bacteria entrance in the organism. It has been reported that probiotics stimulate in vivo phagocytic activity of grouper (Sun et al. Citation2010) after being fed for 60 days with the probiotic bacteria Bacillus spp. Díaz-Rosales et al. (Citation2006) observed significant differences in the phagocytic activity among groups of sea bream fed four weeks with heat shock inactivated probiotic strains. On the contrary, Lactococcus lactis does not produce any effect on phagocytic activity of turbot (Villamil et al. Citation2002). Salinas et al. (Citation2006) studied the phagocytic activity of four probiotic strains heat-inactivated and observed by electron microscopy the internalisation of bacteria inside leucocytes, on the contrary in this work, we used the same method than Román et al. (Citation2012). In our study, only the sea bream leucocytes incubated with live bacteria showed significant difference (P < 0.05) with other groups, in contrast to the results obtained by Román et al. (Citation2012) where the best response was in HK leucocytes of sea bream incubated with UV-light inactivated bacteria. We can consider the probiotic E. gallinarum L-1 to have a stimulant effect on leucocytes that remained within basal levels when comparing with data reported on sea bream (Montero et al. Citation2008) or sea bass (Torrecillas et al. Citation2007), and no data are available for other fish species studied. Modulator effect of probiotic E. gallinarum L-1 was not the same in every fish species: gilthead sea bream, sea bass, meagre and red porgy, studied in this work. However, it seems to show a similar response when attending to fish of the same family. And the reason for this difference can be related to the intestinal microbiota, the fish environment as well as some particular requirements of diet.
4. Conclusion
In conclusion, our results showed that probiotic E. gallinarum strain L-1 is capable of stimulating leucocytes of sea bream, sea bass and red porgy, but no stimulation was found in meagre. This stimulation is dose-dependent in most of the cases, and the dose of 108 cfu mL−1 performed the highest values and no significant differences between treatments were found. Our data confirm that in vitro studies are essential not only to calculate better the effective dose of a probiotic but also to analyse how the viability of the bacteria influence the phagocytic activity, respiratory burst and peroxidase content in order to perform, in the future, the in vivo studies.
Acknowledgements
Lorena Román was recipient of Ph.D. grant by Cabildo Insular de Gran Canaria. The authors wish to thank CANEXMAR SL and Instituto Canario de Ciencias marinas (ICCM) for providing the fish for this research.
Additional information
Funding
References
- Austin B, Baudet E, Stobie M. 1992. Inhibition of bacterial fish pathogens by Tetraselmis suecica. J Fish Dis. 15:55–61. 10.1111/j.1365-2761.1992.tb00636.x
- Balcázar JL de Blas I, Ruiz-Zarzuel I, Cunningham D, Vendrell D, Muzquiz JL. 2006. The role of probiotics in aquaculture. Vet Microbiol. 114:173–186. 10.1016/j.vetmic.2006.01.009
- Balcázar JL, de Blas I, Ruiz-Zarzuela I, Vendrell D, Calvo AC, Márquez I, Gironés O, Muzqui JL. 2007. Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout (Salmo trutta). Brit J Nutr. 97:522–527. 10.1017/S0007114507432986
- Boesen HT, Larsen MH, Larsen LJ, Ellis AE. 2001. In vitro interactions between rainbow trout (Oncorhynchus mykiss) macrophages and Vibrio anguillarum serogroup O2a. Fish Shellfish Immunol. 11:415–431. 10.1006/fsim.2000.0328
- Chiu CH, Cheng CH, Gua WR, Guu YK, Cheng W. 2010. Dietary administration of the probiotics, Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coloides. Fish Shellfish Immunol. 29:1053–1059. 10.1016/j.fsi.2010.08.019
- Corr SC, Hill C, Gahan CGM. 2009. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res. 56:1–15.
- Das S, Ward LR, Burke C. 2008. Prospects of using marine Actinobacteria as probiotics in aquaculture. Appl Microbiol Biot. 81: 419–429. 10.1007/s00253-008-1731-8
- Díaz-Rosales P, Arijo S, Chabrillón M, Alarcón FJ, Tapia-Paniagua ST, Martínez- Manzanares E, Balebona MC, Moriñigo MA. 2009. Effects of two closely related probiotics on respiratory burst activity of Senegalese sole (Solea senegalensis, Kaup) phagocytes, and protection against Photobacterium damselae subsp. piscicida. Aquaculture. 293:16–21. 10.1016/j.aquaculture.2009.03.050
- Díaz-Rosales P, Salinas I, Rodríguez A, Cuesta A, Chabrillón M, Balebona MC, Moriñigo MA, Esteban MA, Meseguer J. 2006. Gilthead seabream (Sparus aurata L.) innate immune response after dietary administration of heat-inactivated potential probiotics. Fish Shellfish Immunol. 20:482–492. 10.1016/j.fsi.2005.06.007
- Food and Agriculture Organization of the United Nations (FAO). 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. In the Joint FAO/WHO expert consultation report on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria (October 2001).
- García de la Banda I, Lobo C, León-Rubio JM, Tapia-Paniagua S, Balebon MC, Moriñigo MA, Moreno-Ventas X, Lucas LM, Linares F, Arce F, Arijo S. 2010. Influence of two closely related probiotics on juvenile Senegalese sole (Solea senegalensis, Kaup 1858) performance and protection against Photobacterium damselae subsp. piscicida. Aquaculture. 306:281–288. 10.1016/j.aquaculture.2010.05.008
- Gatesoupe FJ. 1999. The use of probiotics in aquaculture. Aquaculture. 180:147–165. 10.1016/S0044-8486(99)00187-8
- Gómez GD, Balcázar JL. 2008. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol. 52:145–154.
- Irianto A, Austin B. 2003. Use of dead probiotic cells to control furunculosis in rainbow trout (Oncorhynchus mykiss (Walbaum)). J Fish Dis. 26:59–62. 10.1046/j.1365-2761.2003.00414.x
- Kim DH, Austin B. 2008. Characterization of probiotic carnobacteria isolated from rainbow trout (Oncorhynchus mykiss) intestine. J Appl Microbiol. 47:141–147. 10.1111/j.1472-765X.2008.02401.x
- Montero D, Grasso V, Izquierdo MS, Ganga R, Real F, Tort L,Caballero MJ,Acosta F. 2008. Total substitution of fish oil by vegetable oils in gilthead sea bream (Sparus aurata) diets: effects on hepatic Mx expression and some immune parameters. Fish Shellfish Immunol. 24:147–155. 10.1016/j.fsi.2007.08.002
- Morelli L. 2000. In vitro selection of probiotic Lactobacilli: a critical appraisal. Curr Issues Intest Microbiol. 1:59–67.
- Nayak SK. 2010. Probiotics and immunity: a fish perspective. Fish Shellfish Immunol. 29:2–14. 10.1016/j.fsi.2010.02.017
- Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius EM. 2003. Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol. 15:443–452. 10.1016/S1050-4648(03)00023-8
- Nikoskelainen S, Salminen S, Bylund G, Ouwehand AC. 2001. Characterization of the properties of human and dairy-derived probiotics for prevention of infectious diseases in fish. Appl Environ Microbiol. 58:551–556.
- Olsson JC, Westerdahl A, Conway P, Kjelleberg S. 1992. Intestinal colonization of potential turbot (Scophthalmus maximus) and dab (Limanda limanda) associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 67:2430–2435.
- Panigrahi A, Kiron V, Kobayashi T, Puankaew J, Satoh S, Sugita H. 2004. Immune response in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunop. 102:379–388. 10.1016/j.vetimm.2004.08.006
- Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H. 2005. The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout, Oncorhynchus mykiss. Aquaculture 243:241–254. 10.1016/j.aquaculture.2004.09.032
- Puangkaew J, Kiron V, Somamoto T, Okamoto N, Satoh S, Takeuchi T, Watanabe T. 2004. Nonspecific immune response of rainbow trout (Oncorhynchus mykiss, Walbaum) in relation to different status of vitamin E and highly unsaturated fatty acids. Fish Shellfish Immunol. 16:25–39. 10.1016/S1050-4648(03)00028-7
- Quade MJ, Roth JA. 1997. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vete Immunol Immunop. 58:239–248. 10.1016/S0165-2427(97)00048-2
- Román L, Real F, Sorroza L, Padilla D, Acosta B, Grasso V, Bravo J, Acosta F. 2012. The in vitro effect of probiotic Vagococcus fluvialis on the innate immune parameters of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol. 33:1071–1075. 10.1016/j.fsi.2012.06.028
- Salinas I, Abelli L, Bertoni F, Picchieti S, Toque A, Furones D, Cuesta A, Meseguer J, Esteban MA. 2008. Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effect in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 25:114–123. 10.1016/j.fsi.2008.03.011
- Salinas I, Cuesta A, Esteban MA, Meseguer J. 2005. Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on Gilthead seabream cellular innate immune responses. Fish Shellfish Immunol. 19:67–77. 10.1016/j.fsi.2004.11.007
- Salinas I, Díaz-Rosales P, Cuesta A, Meseguer J, Chabrillón M, Moriñigo MA, Esteban, MÁ. 2006. Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet Immunol Immunop. 111:279–286. 10.1016/j.vetimm.2006.01.020
- Secombes CJ. 1990. Isolation of salmonid macrophages and analysis of their killing activity. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB, editors. Techniques in Fish Immunology. Fair Haven, NJ: SOS; p. 137–154.
- Sharifuzzaman SM, Abbass A, Tinsley JW, Austin B. 2011. Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout (Oncorhynchus mykiss, Walbaum) against Vibrio anguillarum. Fish Shellfish Immunol. 30:347–353. 10.1016/j.fsi.2010.11.005
- Sharifuzzaman SM, Austin B, 2010. Develpoment of protection in rainbow trout (Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum following use of the probiotic Kocuria SM1. Fish Shellfish Immunol. 29:212–216. 10.1016/j.fsi.2010.03.008
- Son VM, Changa CC, Wu MC, Guu YK, Chiu CH, Cheng W. 2009. Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol. 26:691–698. 10.1016/j.fsi.2009.02.018
- Sorroza L, Real F, Acosta F, Acosta B, Déniz S, Román L, El Aamri F, Padilla D. 2013. A probiotic potential of Enterococcus gallinarum against Vibrio anguillarum infection. Fish Pathol. 48:9–12. 10.3147/jsfp.48.9
- Sun YZ, Yang HL, Ma RL, Lin WY. 2010. Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol. 29:803–809. 10.1016/j.fsi.2010.07.018
- Thyssen A, Ollevier F, 2001. In vitro antimicrobial susceptibility of Photobacterium damselae subsp. piscicida to 15 different antimicrobial agents. Aquaculture 200:259–269. 10.1016/S0044-8486(01)00517-8
- Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Sweetman J, Tort L, Izquierdo MS. 2007. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannanoligosaccharides. Fish Shellfish Immunol. 23:969–981. 10.1016/j.fsi.2007.03.007
- Van der Marel M, Schroers V, Neuhaus H, Steinhagen D. 2008. Chemotaxis towards adhesion to and growth in carp gut mucus of two Aeromonas hydrophila strains with different pathogenicity for common carp, Cyprinus carpio L. J Fish Dis. 31:321–330. 10.1111/j.1365-2761.2008.00902.x
- Villamil L, Tafalla C, Figueras A, Novoa B. 2002. Evaluation of immunomodulatory effects of lactic acid bacteria in turbot (Scophtalmus maximus). Clin Diagn Lab Immun. 9:1318–1323.