Abstract
Histomorphological and histochemical studies on the different layers of skin of Bakerwali goat was conducted in neonatal, young and adult age groups. Tissue samples were collected form neck, thorax and loin regions. The gross skin thickness increased with advancement of age. Histologically, the total thickness of skin was maximum on the neck dorsal and minimum on thorax ventral region in all age groups. The epidermis consisted of four layers and stratum lucidum was absent in all regions of all age groups. Beneath the epidermis, the dermis layer was observed in which the hair follicles, sweat glands, sebaceous glands, arrector pilli muscles and blood vessels were present. The dermis layer consisted of dense connective tissue and was itself divisible into superficial papillary layer and deep reticular layer. The two layers of dermis were merged into one another. Mast cells were found scattered throughout the dermis. Keratin covered the whole skin externally. The thickness of epidermis and dermis increased as the age advanced. The epidermis showed moderate Periodic acid-Schiff's reaction for carbohydrates. Dermis showed a moderate reaction for bound lipids. The keratin layer showed a strong reaction for bound lipids in all regions of all age groups.
1. Introduction
Bakerwali goat is one among the twenty well recognized breeds of India distributed in northern regions of Jammu and Kashmir State. The Bakerwali goat is reared in same state by Bakerwal and Gaddie tribes so are named after the name of tribe i.e. Bakerwal or Gaddie goat. Bakerwal goat is the main source of milk to these tribes and an important source of meat to the people of Jammu and Kashmir. This goat ranks among the heaviest mature meat producing goats in the country.
Skin is one of the largest and the most important organic system of the body and acts as a barrier between the external and internal environment of the body (Montage Citation1960). It is responsible for protection, thermoregulation, external sensory awareness, immunological defence, wound healing, perception and excretion. The skin is an effective barrier which prevents desiccation of electrolytes and macromolecules from the body (Dyce et al. Citation2002 and Bhattacharya et al. Citation2003a). The skin is the multilayered organ and its layers get modify depending upon the species, habitat and body region of the animal. Enough number of blood vessels, nerves, muscles and glands are present in different layers of skin. Like various other animals, the skin of goats is used as a basic raw material in leather industries so; basic histomorphology and histochemistry of different layers of skin is an important aspect to know. Since there is no methodical record on this aspect of Bakerwali goat, so the work on histomorphology and histochemistry was undertaken in this species of goat.
2. Material and methods
2.1. Animals and sampling
The skin samples (1ʺ × 1ʺ) of Bakerwali goats were collected from different slaughter houses. The skin samples were collected from three different age groups which include goats below 1 year age (neonatal), 2–3 years (young) and above 3 years (adult). Skin samples were collected from eight different regions of body which include neck dorsal, neck lateral, neck ventral, thorax dorsal, thorax lateral, thorax ventral, loin dorsal and loin lateral regions. The samples so collected were fixed in 10% neutral buffered formalin for about 48 hours. The samples were then processed through ascending grades of alcohol-benzene schedule as per standard methods and emblocked in paraffin wax of 58°C–60°C melting points. Thin sections (5µ) both horizontal and vertical were cut with the help of rotatary microtome and stained with H & E (Luna Citation1968), Von Gieson and Verhoeff's (Mallory Citation1942), Gomori's method (Luna Citation1968), Sudan black B (Luna Citation1968), Periodic acid-Schiff's (PAS) reaction (Bancroft & Stevens Citation1996), Combined Alcian blue- PAS technique (Bancroft & Stevens Citation1996), Ayoub-Shklar method (Luna Citation1968) and Masson's trichrome (Mallory Citation1942). Various measurements were taken from the section with the help of ocular micrometre which was standardized by stage micrometre.
2.2. Statistical analyses
The micrometric data was analyzed by using Univariate ANOVA at 5% level. Multiple comparison test was used to compare the difference between the groups and within the groups. For analysis, values of (P < 0.05) were considered significant. All analysis was done by using SPSS-17. Statistical calculations (mean ± standard error) were recorded according to the standard statistical procedures recommended by Snedecor and Cochran (Citation1994).
3. Results and discussion
Histologically the skin of Bakerwali goat consisted of two layers, the epidermis layer followed by dermis layer (). The total skin thickness included thickness of epidermis and dermis as a whole. The total skin thickness values were found to be minimum in neonatal age group and the values showed a gradual increase with advancement of age as shown . The total skin thickness was found to be maximum in dorsal regions and minimum in ventral regions in all age groups of Bakerwali goat. These findings are in accordance with (Saxena et al. 1994) in cattle as on . The total skin thickness was found to be maximum on the neck dorsal region and which is in total agreement with (Mir et al. Citation2011) in Red Madras sheep as on .
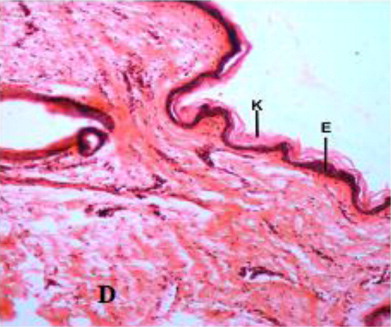
Table 1. Total thickness of skin (µm) at different post-natal age groups and in different regions of Bakerwali goat (Mean ± SE).
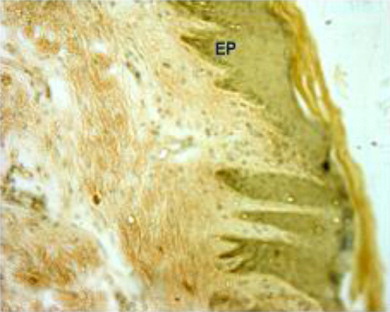
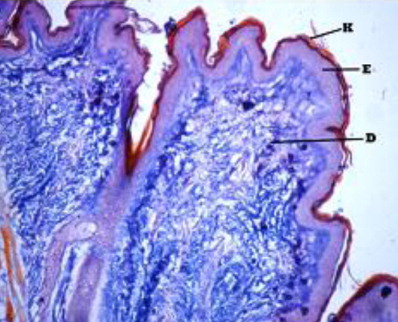
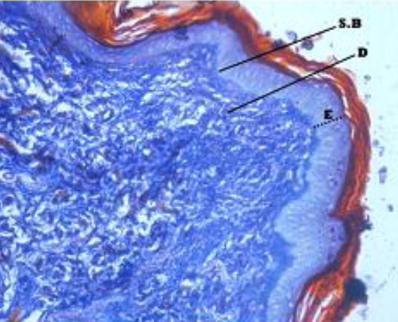
The epidermis formed outermost layer of the skin which was itself covered by a layer of keratin externally. The skin epidermis of the Bakerwali goat was composed of keratinized stratified epithelium which consisted of four layers viz., stratum basale, stratum spinosum, stratum granulosum and stratum corneum (, , ). Stratum lucidium was found to be absent in all regions and age groups in the present study. These findings are in accordance with (Talukdar et al. Citation1972) in horses, (Baba et al.Citation1998) in palpebral epidermis of sheep and (Bhattacharya et al. Citation2003b) in buffalo. On the contrary, the presence of stratum lucidum in llama was reported by (Atlee et al. Citation1997). Further, (Yagci et al. Citation2006) reported that stratun granulosum and stratum lucidium was absent in the skin of rabbit. (Lloyd & Garthwaite Citation1982) reported that the epidermis of skin was composed of 3–6 cell layers in canine, (Gayakee et al. Citation2008) reported that skin epidermis of wild pig consisted of 15–20 layers and (Bacha & Bacha Citation2000) found 3–4 layers of cells in epidermis of domestic pig. In the present study eight to ten layers were found in the epidermis of skin of Bakerwali goat.
Stratum basale
The stratum basale layer of epidermis was found to have single layer of low columnar cells lied on basement membrane. These finding is common as reported by (Atlee et al. Citation1997) in llama, (Mandage et al. Citation2003) in Deccani sheep, (Kapadnis & Bhosle Citation2004) in Osamanabadi goats, (Martin et al. (Citation2007) and (Hole et al. Citation2008) in Kandhari cows.
Stratum spinosum
The stratum spinosum layer was observed to have two to three layers of polyhedral cells in Bakerwali goat in all age regions and in all age groups, which is in agreement with (Atlee et al. Citation1997) in llama. However, (Gayakee et al. Citation2008) found 10–12 layers of cells in the stratum spinosum of wild pig skin.
Stratum granulosum
In the present study, the stratum granulosum consisted of single layer of elongated cells with compressed nuclei. This observation is in agreement with the findings reported by (Mandage et al. Citation2003) in Deccani sheep and (Kapadnis & Bhosle Citation2004) in Osamabadi goats.
Stratum corneum
The stratum corneum was the outermost layer of epidermis which consisted of four to five cell layers in the present study which is in accordance with the finding reported by (Kapadnis & Bhosle Citation2004) in goats. However, (Lloyd et al. Citation1979) reported that the stratum corneum layer of epidermis skin in cattle consisted of about 30 cell layers. The cells of this layer were observed as flat, non-nucleated and keratinized as reported by (Bhattacharya et al. Citation2003b) in non-descriptive buffalo. The cells in this outermost layer were discontinuous and were sloughed off as scales at few places. These findings are in agreement with the (Banks Citation1993) in domestic animals.
Stratum lucidium
The stratum lucidium was found to be absent in all regions of all age groups under study. This finding is in agreement with the finding reported by (Sar & Calhoun Citation1966) in American goats, (Gayen et al. Citation1989) in Black Bengal goats, (Mandage et al. Citation2003) in Decani sheep and by (Kapadnis & Bhosle Citation2004) in Osmanabadi goats.
The epidermis was found to be projected into the underlying dermis at few places to form the epidermal pegs in all regions of all age groups of Bakerwali goat (). The thickness of epidermis was maximum in dorsal regions of neck, thorax and loin than their respective lateral and ventral regions. These findings are in agreement with (Atlee et al. Citation1997) in llama. The epidermal pegs were interdigitated with corresponding dermal papillary layer, seen at the epidermo-dermal junction. The typical arrangement of these epidermal pegs was observed more prominent in thorax ventral region in adult age group (). The thickness of epidermis at epidermal pegs was apparently more than that of other regions of epidermis. The thickness of epidermis increased with advancement of age and was recorded thickest in neck dorsal followed by thorax dorsal in all age groups as on . The thickenss of epidermis was minimum in loin lateral regions of neonatal age group, thorax ventral regions in young and adult age groups as on . The epidermis showed moderate reaction for carbohydrates with PAS indicating the presence of neutral mucoplysaccharides. The basement of the epidermis was found to show moderate to intense positive reaction for neutral mucoplysaccharides. These findings are in agreement with reports of (Baba et al. Citation1990) in palpebral skin of goat.
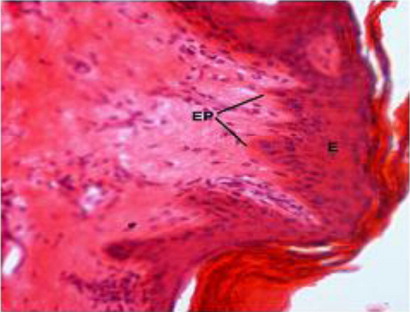
Table 2. Thickness of epidermis (µm) at different post-natal age groups and in different regions of Bakerwali goat (Mean ± SE).
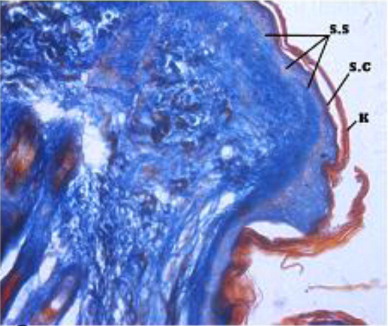
Beneath the epidermis, the dermis layer of skin was found. The dermis consisted primarily of dense irregular connective tissue with a network of collagen and fine reticular fibres, nerve components, hair follicles, sweat and sebaceous glands, blood vessels and arrector pili muscle in all the regions of all the three age groups under study (). The dermis was divisible into superficial papillary layer and deep reticular layer with no clear line of demarcation (). This finding is in accordance with observation reported by (Dellmann & Eurell Citation1998) in domestic animals. In contrary to finding reported by (Talukdar et al.Citation1972) in horses observed well demarcation between these two layers. The papillary layer had more cellular population. Whereas, the reticular layer was found more fibrous with less cellular population in all the three age groups. These observations are in accordance with the findings reported by (Dellmann & Eurell Citation1998) in domestic animals and (Kapadnis et al. Citation2005) in goats. The papillary layer was observed beneath the basement membrane of the epidermis and also formed protrusions into the epidermal pegs. This is in agreement with the finding reported by (Martin et al. Citation2007) in dermis of ferret skin.
Dermal thickness showed a decreasing pattern from dorsal regions towards the lateral and ventral regions of neck, thorax and loin regions in all the age groups of Bakerwali goat, which was in accordance with finding reported by (Atlee et al.Citation1997) in llama. The thickness of papillary dermis was lesser than reticular dermis in all body regions of all age groups of Bakerwali goat. The thickness of reticular dermis layer showed an increasing trend with advancement of age same as reported by (Monte et al. Citation2005) in skin of goat as on , & .
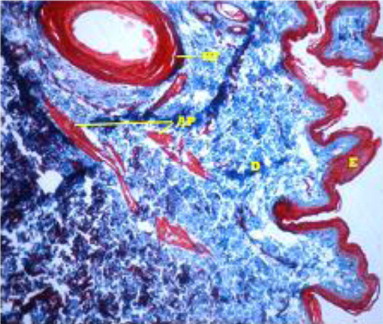
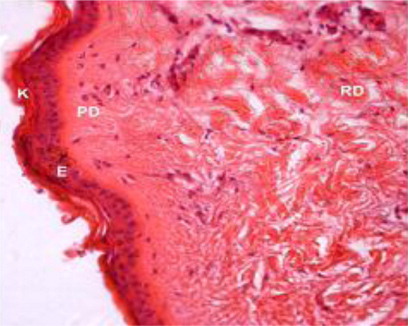
Table 3. Thickness of dermis (µm) at different post-natal age groups and in different regions of Bakerwali goat (Mean ± SE).
Table 4. Thickness of papillary dermis (µm) at different post-natal age groups and in different regions of Bakerwali goat (Mean ± SE).
Table 5. Thickness of reticular dermis (µm) at different post-natal age groups and in different regions of Bakerwali goat (Mean ± SE).
Fine collagen fibres were loosely arranged and distributed irregularly in the papillary layer whereas fibres were coarse and densely arranged in the reticular layer. Collagen fibres surround the hair follicles in deeper layer of dermis but not in superficial layer. These findings coincide with the findings reported by (Talukdar et al.Citation1972) in horses and (Kapadnis et al. Citation2005) in goats. Elastic fibres were not observed in any regions of the age groups under study except in the walls of blood vessels, in disparate with the finding reported by (Talukdar et al.Citation1972) in horses, (Bhayani et al. Citation2005) in sheep.
Dermis showed a moderate reaction for bound lipids with Sudan black B stain. Elastic fibres were not observed in any regions of the age groups except in the walls of blood vessels. Mast cells were found scattered throughout the dermis.
4. Conclusion
The total skin thickness increased as age advanced and dorsal regions showed more thickness as compared to other regions under study. The stratun lucidum was absent in epidermis in all regions of all age groups. Elastic fibres were not observed in any regions of the age groups under study except in the walls of blood vessels.
Acknowledgements
To the Sher-e-Kashmir University of Agriculture science and technology of Jammu (India).
References
- Atlee BA, Stannard AA, Fowler ME, Willemse T, Ihrke PJ, Olivry T. 1997. The histology of normal llama skin. Vet Dermatology. 8:165–176. 10.1046/j.1365-3164.1997.d01-13.x
- Baba MA, Prasad G, Prasad R, Prasad J. 1998. Histology and histochemistry of the palpebral epidermis and dermis of Chotanagpuri sheep. Indian Vete J. 75:934–936.
- Baba MA, Sinha RD, Prasad R, Prasad J. 1990. Histological and histochemical studies on the palpebral epidermis and dermis of goat. Indian J Anim Sci. 60:811–813.
- Bacha WJ, Bacha LM. 2000. Color atlas of veterinary histology. 2nd ed. Lippincott Williams and Wilkins: Blackwell Publishing; p. 85–118.
- Bancroft JD, Stevens A. 1996. Theory and practice of histological techniques. 4th ed. New York (NY): Churchill Livingstone.
- Banks WJ. 1993. Applied veterinary histology. 3rd ed. London: Williams and Wilkins; p. 298–325.
- Bhattacharya M, Sheikh IU, Rajkhowa J. 2003a. Epidermal thickness in the skin of yalk (poephagus poephagus). Indian J Vet Anatomy. 15:73–76.
- Bhattacharya MK, Banerjee R, Singh KS, Guha RK, Ghosh RK. 2003b. Histological and bio-metrical study on epidermis of non-descriptive buffalo (Bubalus bubalis). Indian J Anim Health. 42:151–155.
- Bhayani DM, Vyas KN, Vyas YL, Pandya SP. 2005. Post-natal study on the distribution of mucopolysaccharides, mast cells, lipids and elastic fibers in the skin of sheep. Indian J Vet Anatomy. 17:6–10.
- Dellmann HD, Eurell JA. 1998. Text book of veterinary histology. 5th ed. London: Williams and Wilkins; p. 303–332.
- Dyce KM, Sack WO, Wensing CJG. 2002. Textbook of veterinary anatomy. 3rd ed. Philadelphia: Saunders; p. 347–365.
- Gayakee DE, Ladukar ON, Zade BA, Mainde UP, Dalvi RS. 2008. Histoarchitecture of skin of wild pig (Sus scrofa). Indian J Field Vet. 3:30–31.
- Gayen S, Sinha RD. 1989. Comparative histological studies on the skin of Indian buffalo and black Bengal goats. Indian J Anim Sci. 59:920–924.
- Hole MB, Bhosle NS, Kapadnis PJ. 2008. Histological study of skin epidermis in red kandhari cows. Indian J Anim Res. 42:69–70.
- Kapadnis PJ, Bhosle NS. 2004. Microscopic anatomy of the integument of osmanabadi goat. Indian Vet J. 81:912–914.
- Kapadnis PJ, Bhosle, NS, Mamade CS. 2005. Study in connective tissue fibre in neck skin of goat. Indian J Anim Res. 39:60–62.
- Lloyd DH, Dick WD, Jenkinson DM. 1979. Structure of the epidermis in ayrshire bullocks. Res Vet Sci. 26:172–179.
- Lloyd DH, Garthwaite G. 1982. Epidermal structure and surface topography of canine skin. Res Vet Sci. 33:99–104.
- Luna IG. 1968. Manual of histological staining methods of the armed forces institute of pathology. 3rd ed. New York (NY): McGraw Hill Book Company.
- Mallory FB. 1942. Pathological techniques. Philadelphia: W. B. Saunders Co.
- Mandage ST, Bhosle NS, Kapadnis PJ, Mamade CS. 2003. Microscopic anatomy of the integument of the deccani sheep. Indian J Vet Anatomy. 15:68–69.
- Martin AL, Irizarry-Rovira AR, Bevier DE, Glickman LG, Glickman NW, Hullinger RL. 2007. Histology of ferret skin: preweaning to adulthood. Vet Dermatology. 18:401–411. 10.1111/j.1365-3164.2007.00627.x
- Mir SA, Sathyamoorthy OR, Ramesh G, Balachandran MC. 2011. Micrometrical studies on the skin of Madras red sheep in different age groups. Tamilnadu J Vet Anim Sci. 7:23–28.
- Montage W. 1960. The structure and function of skin. 2nd ed. New York (NY): Academic press.
- Monte MA, Costa BL, Grotta RG, Bragagnoli M, Jacinto G, Medeiros MAC. 2005. Histological evaluation of goat's hair at different ages. Braz J Vet Res Anim Sci. 42:12–18.
- Sar M, Calhoun ML. 1966. Microscopic anatomy of the integument of the common American goat. Am J Vet Res. 27:444–456.
- Saxena SK, Malik MR, Parekh HKB. 1994. Histological character of skin in crossbred cattle. Indian J Vet Anatomy. 6:8–11.
- Snedecor CW, Cochran WG. 1994. Statistical methods. 9th ed. Ames (IA): Iowa state University press.
- Talukdar AH, Calhoun ML, Stinson AW. 1972. Microscopic anatomy of the skin of the horse. Am J Vet Res. 33:2365–2390.
- Yagci A, Zik B, Uguz C, Altunbas K. 2006. Histology and morphometry of white New Zealand rabbit skin. Indian Vet J. 83:876–880.