Abstract
In this study, we investigated the polymorphisms of Caprine osteopontin (OPN) gene and their association with placental traits and prolificacy in goats. The placental and reproductive traits of 171 litters on three goat breeds with different prolificacy (Dazu Black, n = 94; Lezhi Black, n = 27; Hexi Cashmere, n = 50) were evaluated. The polymorphisms in the 5′ promoter and exon 7 of OPN gene were detected by PCR-SSCP methods. Their association was further achieved by using least squares means. It showed that the litter size, litter and placenta weight for Dazu Black and Lezhi Black goats were higher significantly than those of Hexi Cashmere goats (P < 0.01). However, the placental efficiency was not significantly different (P > 0.05). Hexi Cashmere does have more cotyledons in placental than that of Dazu Black and Lezhi Black does (P < 0.01). There were only two genotypes (nominated AA and AB) detected whether by the 5′ promoter primer pairs or by exon 7 of OPN gene. They were associated with litter number, with a trend of AA > AB. At the exon 7 locus, the Dazu Black does with genotype AA had 0.88 more litter numbers than those with genotype AB (P < 0.05). It suggested that OPN gene could be a potential candidate gene on goat prolificacy.
1. Introduction
Osteopontin (OPN), also known as secreted phosphoprotein 1 (SPP1), is a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of extracellular matrix proteins/cytokines. OPN was first described in bone matrix, and also detected in many organs, tissues, such as kidneys, mammary gland, oviduct, uterus and placenta (Zhu et al. Citation2012). This protein plays important role as a mediator of cell–cell and cell-extracellular matrix (ECM) communication (Johnson et al. Citation2003a). Particularly, OPN is persistently expressed on the maternal–foetal interface, and it suggests that OPN is also important in maintaining uterine-embryonic microenvironment (Johnson et al. Citation2003b). In particular, the role of OPN has been studied extensively in reproduction studies in human, mice, primates, pigs and sheep. These studies have revealed that OPN has the potential to profoundly impact pregnancy, embryo implantation and placental development (Garlow et al. Citation2002; Li et al. Citation2013). All these above characteristics make OPN a strong candidate gene for reproductive traits in animals. However, detailed analyses of OPN in the goat breeds with different fecundity have not been reported.
Goat fecundity is one of the most economically important traits in production and breeding, which regulated synergistically by genetic and environmental factors. Because of the restrictions of low heritability and negative maternal effects, genetic improvement on goat fecundity is not obvious. Type and size at birth are critical in determining life expectancy and are dependent primarily on the placental supply of nutrients (Fowden et al. Citation2009), so the placental development and efficiency could affect reproductive rate. It has been reported that placental efficiency (PE, defined as the ratio of total birth weight to placental weight) has a higher heritability and significant positive correlation with the litter size on swine (Mesa et al. Citation2005; Mesa et al. Citation2012) and sheep (Dwyer et al. Citation2005). Thus, PE, rather than litter size is a potential selection trait in evaluation goat fecundity.
The Dazu Black goat is one kind of prolific local goat breeds in Chongqing, Southwest China. The mean litter size for Dazu Black goats as reported is 2.72 (Zhao et al. Citation2012b). Lezhi Black is a moderate prolific goat breed, and Hexi Cashmere, a single-birth breed characterized by adapting to cold, hypoxic ecological conditions in the Gansu Province, is a low-prolific goat breed (Zi et al. Citation2013). Here, we investigated the polymorphisms of caprine OPN gene and the associations of OPN gene with the placental and reproductive traits in the above three goat breeds.
2. Materials and methods
2.1. Animals and sample collection
Dazu Black goats were supplied by the Dazu Black Goat Breeding Farm in Dazu County and by the Research Farm of College of Animal Science and Technology, Southwest University in Beibei District, Chongqing. Lezhi Black goats were supplied by the Lezhi Black Goat Breeding Farm in Lezhi and Fengdu Counties. All the goats were housed and fed a daily ration of 300–500 g concentrate. In addition, all experimental goats were allowed to an unrestricted access to straw, mineral salt lick and water. Hexi Cashmere goats were grazed on the pasture in Gansu Province. All does were naturally bred by bucks from the same breed.
Blood samples were collected from 181 does (Dazu Black, n = 60; Lezhi Black, n = 70; Hexi Cashmere, n = 51) for investigating polymorphism of OPN gene. Venous blood (5–6 mL) was collected using EDTA as an anticoagulant. DNA extraction was performed within 24 h of blood collection, according to Sambrook and Russell (Citation2001). After checking the quality and quantity, DNA was diluted to a final concentration of 20 ng/µL in water and stored at 4°C for immediate use while for long term kept at –20°C.
2.2. Data collection
In this study, we investigated the placental and reproductive performance traits of a total of 171 litters on three goat breeds (Dazu Black, n = 94; Lezhi Black, n = 27; Hexi Cashmere, n = 50).After birth, the kids were sexed and weighed using suspended scales with a range of 0–20 kg, to record litter weight (LW) in grams. Placentas were collected immediately after delivery and weighed fresh in scales, as placenta weight (PW). Individual placental cotyledons were sub-divided by the size and diameter (cm) (small: <1 cm; medium: 1–5 cm; large: >5cm). The cotyledon number (CN) of every type, from each delivered placenta, was counted and recorded. Placental efficiency (PE) was defined as the ratio of birth litter weight LW (g) to placental weight (PW) (g) (Ocak & Onder Citation2011) and cotyledon density (CD) was defined as cotyledon number (CN) per gram placental weight (PW) (Dwyer et al. Citation2005).Cotyledon efficiency (CE) was defined as the ratio of birth litter weight LW (g) to cotyledon number (CN). The samples collected from three goat breeds were in .
Table 1. The litter size distribution of three goat breeds.
2.3. PCR amplification and SSCP assay
The specific PCR primers were designed based on the GenBank sequence (NCBI Accession No. AY540129and AJ431207) for OPN gene (). PCR was performed in a 25 µL total volume reaction mixture containing 2 µL genomic DNA, 1µL each of primers and 0.5 µL Taq DNA polymerase (Promega). The conditions of PCR were as follows: initial denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 45 s, annealing at 49°C/53°C for 45 s and extension at 72°C for 60 s and final extension at 72°C for 10 min in thermal cycler T-gradient (Bio-Rad).
Table 2. Primer sequence, product size, amplified region and annealing temperature used in analyses of the goat OPN gene.
The PCR products (3–4 µL) were mixed with 10 µL denaturing solution (95% formamide, 25 mM EDTA, 0.025% xylene–cyanole and 0.025% bromophenol blue), heated at 98°C for 10 min, and then chilled on ice for 5 min. All denatured DNA samples were then subjected to 10–12% PAGE (polyacrylamide gel electrophoresis) in 1× TBE buffer and at a constant temperature (4°C) under voltage of 250 V for 60 min, 100 V for 15 h. The gel (29:1 acrylamide: bis) was stained with 0.1% silver nitrate for 10 min. After the polymorphisms were detected, each of the DNA bands on the SSCP gel was extracted and the PCR products of the different electrophoresis patterns were sent for sequencing in both directions (repeated three times) in an ABI 377 DNA analyzer (Applied Biosystems) and the sequences were determined with DNAstar software (version 7.1) and blast in NCBI (National Center for Biotechnology Information).
2.4. Statistical analysis
Population genetic parameters such as gene heterozygosity (He), effective number of alleles (Ne) and polymorphism information content (PIC) were calculated by Nei's methods (Nei & Li Citation1979). The statistical software program of SPSS 17.0 was used for data analyses. Differences between the means of placental and reproductive traits were tested for significance by the Duncan's new multiple range tests.
The following fixed effects model was employed for analysis of litter size in Dazu Black goat and least squares means were used for multiple comparisons in litter size among the different genotypes:
where yijklm is the phenotypic value of litter size; µ is the population mean; Si is the fixed effect of the ith sire (i = 1,2, 3, 4, 5); KSj is the fixed effect of the jth lambing season (j = 1, 2, 3, 4); Pk is the fixed effect of the k the parity (k = 1, 2, 3); Gl the fixed effect of the lth genotype and eijklm is the random residual effect of each observation. The association of the OPN gene with placental and reproductive traits was achieved using Proc GLM of SAS. Correlations and differences with P < 0.05 were considered as significant.
3. Results
3.1. Placental and reproductive performance traits of goats
The placental and reproductive performance traits of three goat breeds were given in . There were significant differences in mean litter size, litter weight and placenta weight (P < 0.01). The litter size, litter weight and placenta weight for Dazu Black and Lezhi Black goats were higher significantly than those of Hexi Cashmere goats (P < 0.01). In addition, the average Dazu Black kid weight was lighter than that of Lezhi Black (P < 0.05).
Table 3. The comparison of kid, litter and placenta weights, and placental components among three goat breeds.
There was no significant difference found of PE among the three goat breeds studied. The numbers of placental cotyledons were compared among three goat breed. And we found a significant increase in cotyledon number in Hexi Cashmere does compared to Dazu Black and Lezhi Black (P < 0.01). Most changes were found in different size cotyledons. Hexi Cashmere placentas had a higher number of small (P < 0.01) and medium sized (P < 0.05) cotyledons compared to Dazu Black and Lezhi Black, but fewer large cotyledons (P < 0.01) ().
We also compared the placental and reproductive performance traits of three goat breeds with the same litter size and the results were given in . Under the condition that the litter size was single the litter weight and placenta weight of Dazu Black were larger than those of Hexi Cashmere (P < 0.05).There was no significant difference of PE and the total number of placental cotyledons among the three goat breeds (P > 0.05). Hexi Cashmere placentas had a higher number of small size cotyledons compared to Dazu Black and Lezhi Black (P < 0.01), but fewer medium cotyledons (P < 0.01) and large cotyledons ().
Table 4. The comparison of kid, litter and placenta weights, and placental components among three goat breeds with the litter size is single.
3.2. PCR-SSCP analysis of the caprine OPN gene
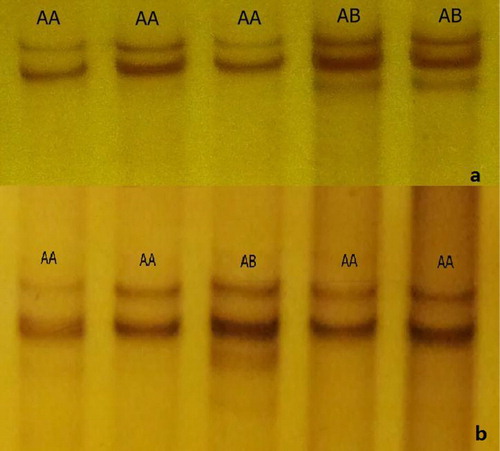
There were two genotypes (nominated AA and AB) detected by two primer pairs (the 5’ promoter and exon 7 of OPN gene) of three goat breeds (). Genotypic frequencies and genetic polymorphism parameters of OPN gene in three goat breeds were shown in . Meanwhile, the frequencies of the 2 loci at the promoter and the exon7 of OPN of Dazu Black goat and Hexi Cashmere goat gene were analyzed by using x2-test, and it showed that the 2 loci were with Hardy-Weinberg equilibrium (P > 0.05). In 5′ promoter and exon 7 of OPN gene in Lezhi Black goat, frequencies of AA genotype were 0.200 and 0.243; frequencies of AB genotype were 0.800 and 0.757, respectively.
Table 5. Genotypic frequencies and genetic polymorphism parameters at different locus of OPN gene in three goat breeds.
3.3. DNA sequence analysis
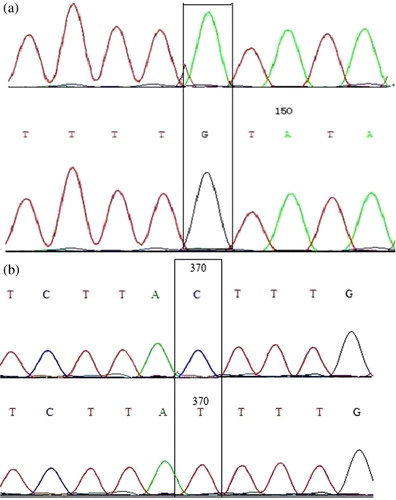
Regarding the primer of 5′ promoter, there were three mutations at A112G, A113C and G172A of this fragment in all of the two genotypes (AA and AB) (). But all the mutations did not lead to the change of the amino acid. Concerning the primer of Exon 7, C to T transversion at the 370 locus ().
3.4. Associations of polymorphisms of OPN with placental efficiency and litter size in Dazu Black goat
The association of variations of the promoter and the exon 7 of OPN gene with placental efficiency and litter size were analyzed and given in . The results showed that the genotype of OPN gene locus was associated with litter number, with a trend of AA > AB. At the promoter locus, the Dazu Black does with genotype AA had 0.33 more litter numbers than those with genotype AB, which no significant difference was found (P > 0.05).When considering about the placental efficiency, there was no significant difference with different genotype (P > 0.05).At the exon 7 locus, the Dazu Black does with genotype AA had 0.88 more litter numbers than those with genotype AB (P < 0.05), which indicates that in exon 7 of goat OPN gene, allele A is the dominant gene and has significant effects on litter size.
Table 6. Least squares means and standard error of PE and reproduction traits at different locus of OPN gene in Dazu Black goat.
4. Discussion
Litter size trait is one of the most economically important traits in goat production and breeding. But litter size trait which shows low heritability is hard to be improved by traditional breeding technologies. However, placental growth and development of its functional ability are important because they are the means by which the foetus receives metabolic substrates for growth. So selection on placental size and efficiency may provide a valuable tool for optimizing litter size in commercial livestock, such as swine and sheep(Wilson et al. Citation1999; Mesa et al. Citation2012).
In our study, there is no significant difference on placental efficiency among three goat breeds with different prolificacy. It is similar to the previous studies (Ocak & Onder Citation2011).Three goat breeds, including Saanen, German Fawn and Damascus goats were investigated and the PE were 6.10 ± 0.78, 6.19 ± 0.41 and 6.23 ± 0.55, respectively. In our study, the PE of Dazu Black, Lezhi Black and Hexi Cashmere goats were 9.75 ± 3.59,10.21 ± 3.70 and 9.20 ± 2.67, respectively. They were higher than those of the western goat breeds. However, the litter weight and placenta weight of western goat breeds were higher than those of Chinese goat breeds studied in this paper. The PE were 19.16 and 16.39 of Blackface and Suffolk sheep (Dwyer et al. Citation2005), and was only 6.35 ± 1.48 of Large White Pig (Chen et al. Citation2009). PE was also very significantly different between first parity and multiparous female animals (Dwyer et al. Citation2005; CitationZhao et al. 2012a).So the placental size and efficiency were species- and breed-dependence traits and were affected by many factors.
And we found significant different in cotyledon number and size among the three goat breeds. Konyali et al. (Citation2007) studied the relationships between placental characteristics and litter weight in Turkish Saanen goats and demonstrated that there were positive correlations between litter weight and cotyledon number. Butthere were negative correlations between litter weight and cotyledon number compared among goat breeds in our study. The effect of litter size on number and size of cotyledons was due to increased recruitment of caruncles during placental development in multiple pregnancies. Twin, triplet or multiple pregnancies were associated with an increase in cotyledon size compared to singleton pregnancies. Total cotyledon number, however, declined with each increase in litter size. In the previous study, it demonstrated an increase in cotyledon number in the twin pregnancies over singletons in the same ewes (Dwyer et al. Citation2005). In our study, however, each twin or triplet is associated with fewer cotyledons than singles, and triplets fewer than twins, which suggested an decrease in cotyledon number in the multiple pregnancies over singletons. Higher PE may have been achieved by the compensatory increase in large cotyledons in the multiple pregnancies. The main reason for the increase in both weight and placental efficiency appeared to be the increase in cotyledon size and weight with prolific goats.So we provided a new term here, cotyledon efficiency (CE), which defined as the ratio of birth litter weight to cotyledon number. And we found the CE of Hexi Cashmere was significantly smaller compared to Dazu Black and Lezhi Black (P < 0.05).
There were three mutations in the 5′ promoter, and one mutation in the exon 7 revealed in goat OPN by PCR-SSCP methods in three Chinese domestic goat breeds. According to the classification of PIC (low polymorphism if PIC value < 0.25, moderate polymorphism if 0.25 < PIC value < 0.50, and high polymorphism if PIC > 0.50), Lezhi Black in the 5′ promoter and exon 7 of OPN gene had moderate genetic diversity. And the 2 loci were in Hardy–Weinberg disequilibrium (P < 0.05), which showed that the genotypic frequencies were affected by selection, mutation or migration. The frequencies of AA and AB genotypes of OPN are higher than that of BB (P > 0.05). Few studies have been taken on the association of OPN gene with placental efficiency and prolificacy in goats (Chen et al. Citation2009). OPN appears to play a key role in conceptus implantation and maintenance of pregnancy in mice, sheep and pigs(Garlow et al. Citation2002; Johnson et al. Citation2002; Johnson et al. Citation2003b). The reproductive traits are complex quantitative traits involving multiple genes, loci and interactions, so it is important to analyze the combined effect of multiple genes or loci on reproductive traits. In the study, association between multiple locus and litter size was analyzed. Individuals with genotype AA had higher litter size than those with genotype AB. The biochemical and physiological functions, together with the results obtained in our study, indicate that OPN gene could be as a molecular breeding marker in goats.
The following conclusions are drawn: There is no significant difference on PE among three goat does. Based on its important role in implantation and placentation, OPN gene was considered as a potential candidate gene for the prolificacy of Dazu Black goats. However, its genetic mechanism of reproduction in goat breeds should be further investigated.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 31172195); the Fundamental Research Funds for the Central Universities [grant number XDJK2014A010]; the 2013 Innovation Team Building Program in Chongqing universities [grant number KJTD201334]; and Natural Science Foundation Project of CQ CSTC [No. CSTC, grant number 2011BB1015].
Acknowledgements
The authors thank Dr Jian Wang for technical assistance during the project, and Dr Xiaochuan Chen for helpful discussions about the placental analysis.
References
- Chen L-H, Wang L-X, Ji Y-G, Zhang L-C, Yan H. 2009. Association of polymorphism for porcine BF gene with reproductive traits and placental efficiency in large white. Yichuan. 31:615–619.
- Dwyer CM, Calvert SK, Farish M, Donbavand J, Pickup HE. 2005. Breed, litter and parity effects on placental weight and placentome number, and consequences for the neonatal behaviour of the lamb. Theriogenology. 63:1092–1110. 10.1016/j.theriogenology.2004.06.003
- Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. 2009. Placental efficiency and adaptation: endocrine regulation. J Physiol (London). 587:3459–3472. 10.1113/jphysiol.2009.173013
- Garlow JE, Ka H, Johnson GA, Burghardt RC, Jaeger LA, Bazer FW. 2002. Analysis of osteopontin at the maternal-placental interface in pigs. Biol Reprod. 66:718–725. 10.1095/biolreprod66.3.718
- Johnson GA, Burghardt RC, Bazer FW, Spencer TE. 2003a. Osteopontin: roles in implantation and placentation. Biol Reprod. 69:1458–1471. 10.1095/biolreprod.103.020651
- Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Gray CA, Pfarrer C. 2003b. Osteopontin is synthesized by uterine glands and a 45-kDa cleavage fragment is localized at the uterine-placental interface throughout ovine pregnancy. Biol Reprod. 69:92–98. 10.1095/biolreprod.102.013573
- Johnson GA, Joyce MM, Burghardt RC. 2002. Osteopontin/early T-cell activation factor-1 is expressed by fetal placental immune cells after day 20 of pregnancy in sheep but not pigs. Biol Reprod. 66:272–273. 10.1095/biolreprod66.2.272
- Konyali A, Tolu C, Das G, Savas T. 2007. Factors affecting placental traits and relationships of placental traits with neonatal behaviour in goat. Anim Reprod Sci. 97:394–401. 10.1016/j.anireprosci.2006.09.008
- Li MC, Fang Q, He ZM, Gao Y, Zhou Y. 2013. Placental expression of osteopontin(OPN) in monochorionic twins with discordant growth. Placenta. 34:288–290. 10.1016/j.placenta.2012.12.014
- Mesa H, Cammack KM, Safranski TJ, Green JA, Lamberson WR. 2012. Selection for placental efficiency in swine: conceptus development. J Anim Sci. 90:4217–4222. 10.2527/jas.2011-5001
- Mesa H, Safranski TJ, Fischer KA, Cammack KM, Lamberson WR. 2005. Selection for placental efficiency in swine: genetic parameters and trends. J Anim Sci. 83:983–991.
- Nei M, Li WH. 1979. Mathematical-model for studying genetic-variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 76:5269–5273. 10.1073/pnas.76.10.5269
- Ocak S, Onder H. 2011. Placental traits and maternal intrinsic factors affected by parity and breed in goats. Anim Reprod Sci. 128:45–51. 10.1016/j.anireprosci.2011.08.011
- Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual. 3rd ed. 271–275. New York: Cold Spring Harbor Laboratory Press.
- Wilson ME, Biensen NJ, Ford SP. 1999. Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J Anim Sci. 77:1654–1658.
- Zhao Y, Xie C, Mao J. 2012a. Association of osteopontin polymorphisms with placental efficiency and reproductive performance in Lezhi Black goat. Reprod Domestic Anim. 47:501–501. 10.1111/j.1439-0531.2011.01911.x
- Zhao Z, Liu X, Zhang X, Zhao Y, Li Z, Zhang J. 2012b. Introduction about indigenous goat breed resources in chongqing municipality and genetic relationship research about these goat breeds with other goat breeds in adjacent provinces. J Anim Vet Advances. 11:488–493. 10.3923/javaa.2012.488.493
- Zhu F, Shen F, Fan Y, Xie Y, Xia Y, Kong Y. 2012. Osteopontin increases the expression of beta 1, 4-Galactosyltransferase-I and promotes adhesion in human RL95-2 cells. Glycoconj J. 29:347–356. 10.1007/s10719-012-9426-x
- Zi X-D, Huang L, Wang Y, Lu J-Y. 2013. Comparative messenger RNA expression of FSHbeta, LHbeta, FSHR, LHR, and ERbeta in high and low prolific goat breeds. Anim Biotechnol. 24:307–311. 10.1080/10495398.2013.790824