Abstract
The present study was conducted to understand changes of seminal fluid sex steroids and milt quality of Russian sturgeon during sequential stripping. Milt samples were collected three times with 24 intervals. According to results, the values of sperm motility duration, sperm production and seminal fluid steroids including 17a-Hydroxyprogesterone (OHP), testosterone (T) and estradiol-17β (E2) decreased significantly during sequential stripping. The levels of OHP showed a close positive correlation with percentage and duration of sperm motility. Similar correlation was found between T and E2 with sperm concentration. The P concentrations did not have significant correlation with sperm quality parameters. Our results revealed that the ability of Russian sturgeon in production of milt with desirable quality decreased with repetition of milt collection. Changes of seminal fluid steroids may be an image of their serum changes during spermiation period. As well as, seminal fluid sex steroids may affect milt quality after spermiation.
1. Introduction
Sturgeon populations in nature have decreased dramatically due to the habitat destruction and over-fishing during recent decade (IUCN 2010). Russian sturgeon is of the important sturgeon fish of Caspian Sea that has been considered for biological conservation. Due to the shortage of male brooders, each Russian sturgeon male is stripped more than one time during spawning season. Many studies showed that sequential stripping affect milt quality (Gjerde Citation1984; Piironen Citation1985; Sequet et al. Citation1992). It was well recognized that sex steroids have an important role in hormonal control of the reproduction in fish (Kime Citation1993; Barannikova et al. Citation2002). Steroids may be involved in the control of the ionic composition of the seminal fluid and of the volume of milt (Baynes & Scott Citation1985; Marshall & Bryson Citation1988; Marshall et al. Citation1989). In comparison with other component of seminal fluid (Lahnsteiner et al. Citation1996, Citation1998; Ingermann et al. Citation2002; Perez et al. Citation2003; Hajirezaee et al. Citation2010a; Hajirezaee et al. Citation2010b), information about changes of seminal fluid sex steroids during sequential stripping is rare especially about sturgeons. Seminal fluid has a unique composition of organic and inorganic component minerals (potassium, sodium, magnesium, calcium, chloride), pH, osmolality, proteins, glucose and triglycerides – all of which seem to play a role in sperm quality (Morisawa et al. Citation1983; Stoss Citation1983; Lahnsteiner et al. Citation1993a, Citation1993b; Ciereszko et al. Citation2000; Lahnsteiner et al. Citation2004). The present study was conducted to understand changes of seminal fluid sex steroids and milt quality of Russian sturgeon during sequential stripping.
2. Materials and methods
Ten spermiated Russian sturgeon males (TL= 134.3 ± 8.1 cm, weight = 24.2 ± 2.1 kg) were considered for experiment in Shahid Beheshti Artificial Sturgeon Propagation and Rearing Center (SBAPRC), Iran, Rasht. The milt samples were collected three times with 24 intervals using special syringe. Sperm density was measured by haemocytometer counting chamber according to Alavi et al. (Citation2006). In this regard, milt was diluted 1000 times in a 0.7% of NaCl solution. Then, a droplet of the diluted milt was placed on a haemocytometer slide (depth 0.1 mm) with a coverslip. After 10 min (a time to allow sperm sedimentation), the spermatozoa were counted in 16 individual cells of haemocytometer under a light microscopy. At the end, the number of spermatozoa was calculated as follows:
To induce the initiation of sperm motility, a 50 µl drop of freshwater placed on a glass slide and then a drop of 1µl fresh sperm was diluted using a microsampler. Then, sperm motility was estimated by a semi-quantitative method (Rurangwa et al. Citation2004). In this regard, the motility was recorded by a video camera coupled with the optical lens of microscope. At the end, the video recordings were reviewed and the motility was presented as the percentage and duration of motility after the onset of motility until 100% of cells were immotile. Only forward-moving sperm were judged motile, those simply vibrating or turning on their axes was considered immotile (Aas et al. Citation1991).
2.1. Steroid assays
Some milt samples were centrifuged (Heraeus, Sepatech, Labofuge 200, Germany, 5000 rpm for 10 min) to separate the seminal fluid. The seminal fluid samples were stored at 20°C until hormonal assay. For hormonal assay, the assay kits of T, P, OHP and E2 were obtained from IMMUNOTECH, France. The kit properties and methods of assay have been mentioned in detail by Hajirezaee et al. (Citation2010a).
2.2. Statistical analysis
The SPSS software was used for data analysis. One-way analysis of variance was employed to analyze between means. When significant F-ratios were calculated by analysis of variance (ANOVA), the Tukey test was applied to identify which groups were different. The relationships between the steroid concentrations of the seminal fluid and sperm motility characteristics (duration and percentage of motility) and sperm concentration were tested using the bivariate correlation coefficients of Pearson. Then, the linear and non-linear regression models were investigated using regression fits. The total duration of sperm motility and the percentage of motile spermatozoa were used as dependent and the sex steroids of the seminal fluid as independent variables.
3. Results
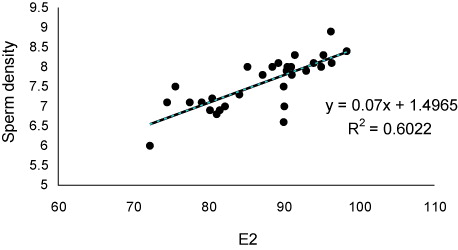
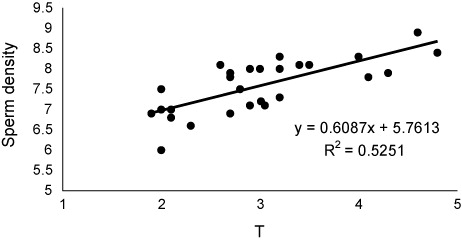
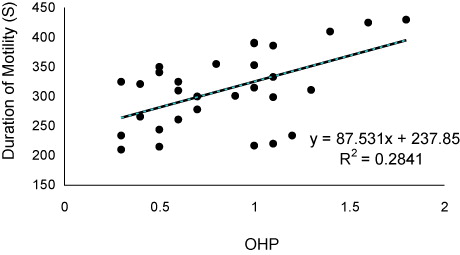
The values of sex steroids in seminal fluid have been presented in . The T and P had the highest and lowest concentrations in seminal fluid. The values of sperm motility duration, sperm production and seminal fluid steroids including 17a-Hydroxyprogesterone (OHP), testosterone (T) and estradiol-17β (E2) decreased significantly during sequential stripping (). Significant positive relationship was found between OHP levels and duration () of motility (P < 0.05), although such relationships were not observed for P (P > 0.05). As well as, significant positive correlations (P < 0.05) were observed between E2 () and T () with sperm density. There was no relationship between P levels and sperm density (P > 0.05).
Table 1. The mean concentration of sex steroids in the seminal fluid of Russian sturgeon.
Table 2. The concentration of sex steroids in the seminal fluid of Russian sturgeon during sequential stripping.
4. Discussion
In this study, the values of sperm motility duration and sperm production decreased with increasing of milt collection frequency. These decreases in sperm density may be due to the hydration of testis or reduction in spermiation rate. Morisawa et al. (Citation1979) have reported that hypotonicity of freshwater environment establishes the hydration of testis, possibly causing the dilution of milt and leading to a higher milt volume and subsequently lower sperm density and spermatocrit and also the activation of spermatozoa before ejaculation.
The secretion of seminal fluid is of important events during final maturation of fish spermatozoa. The seminal fluid contains special composition including ions, lipids, carbohydrates and proteins which support the viability of spermatozoa after spermiation (Hajirezaee et al. Citation2011). Sex steroids are organic components, which have key role in hormonal control of the reproduction in fish (Kime Citation1993; Barannikova et al. Citation2002; Viayeh et al. Citation2006). In our study, variations were observed in concentrations of sex steroids Russian sturgeon seminal fluid. The main source of seminal fluid steroids may be blood, although few studies have demonstrated the urine contamination as additional origin (Scott et al. Citation1991). These authors with investigation on five species including Pleuronectes platessa, dab Limanda limanda, flounder Platichthys flesus, gold fish Carassius auratus and Rainbow trout Oncorhynchus mykiss suggested that urine contamination may be one of the source of steroids in seminal fluid. Such contamination could decreases the milt quality such as decline in sperm motility and also sophisticates the precise analysis of steroids and probably other materials in the seminal fluid (Linhart & Kvasnicka Citation1992; Billard et al. Citation1995; Linhart et al. Citation1995; Perchec et al. Citation1995; Billard Citation1998; Dreanno et al. Citation1998; Perchec et al. Citation1998; Linhart et al. Citation1999). In the present study, as the motility indices of the Russian sturgeon spermatozoa were in an acceptable range, therefore, the urine contamination of sampled milt is improbable and the measured steroid values are reliable. Also, decreases of seminal fluid steroids during sequential striping may return to reduction of their concentrations in the blood serum. In Russian sturgeon, the good relationship was found between T and E2 concentrations in seminal fluid and sperm production. In addition, a close relationship was found between OHP levels and percentage and duration of sperm motility. These data indicate that the seminal fluid levels of T, E2 and OHP could be considered as milt quality biomarkers. During final maturation of fish spermatozoa, the spermatozoa acquire motility ability in spermatic duct under control of sex steroids (Kime Citation1993; reviewed by Miura & Miura Citation2003). The 17a,20b-dihydroxy-4-pregnen-3-one (DHP) was considered as key steroid inducing final maturation in many teleosts (Miura et al. Citation1991; Miura et al. Citation1992). DHP indirectly mediate the increasing of seminal plasma pH, which in turn increases the sperm content of cAMP. In our study, the close relationships between OHP and sperm motility probably suggest a similar role for this steroid in the Russian sturgeon, especially that DHP up-regulated by OHP during steroidogenesis pathway. In the males of teleosts, T has been identified to be the main steroids involved in spermatogenesis and initiation of spermiation (Nagahama Citation1983; Yaron Citation1995). As well as, some studies have discussed about the role of E2 in the spermatogenesis, although the E2 is considered mostly as a vitellogenic hormone. In Russian sturgeon, the close positive correlation of seminal fluid T and E2 with sperm density may be in relation to important their roles on sperm production. In conclusion, our results showed that milt quality of Russian sturgeon decreased with increasing of stripping frequency. This may be due to the changes of serum and seminal fluid steroids.
References
- Aas GH, Refstie T, Gjerde B. 1991. Evaluation of milt quality of Atlantic salmon. Aquaculture.95:125–132. 10.1016/0044-8486(91)90079-M
- Alavi SMH, Cosson J, Kazemi R. 2006. Semen characteristics in Acipenser persicus in relation to sequential stripping. J Appl Ichthyol. 22:400–405. 10.1111/j.1439-0426.2007.00994.x
- Barannikova IA, Dyubin VP, Bayunova LV, Semenkova TB. 2002. Steroids in the control of reproductive function in fish. Neurosci Behav Physiol. 32:141–148. 10.1023/A:1013923308125
- Baynes SM, Scott AP. 1985. Seasonal variations in parameters of milt production and in plasma concentration of sex steroids of male rainbow trout (Salmo gairdneri). Gen Comp Endocrinol. 57:150–160. 10.1016/0016-6480(85)90211-4
- Billard R. 1998. Initiation of carp spermatozoa motility and early ATP reduction after milt contamination by urine. Aquaculture. 160:317–328. 10.1016/S0044-8486(97)00301-3
- Billard R, Cosson G, Perchec G, Linhart O. 1995. Biology of sperm and artificial reproduction in carp. Aquaculture. 129:95–112. 10.1016/0044-8486(94)00231-C
- Ciereszko, A, Glogowski J, Dabrowski K. 2000. Cryopreservation of aquatic species. In: Tiersch TR, Mazik PM, editors. Biochemical characteristics of seminal plasma and spermatozoa of freshwater fishes. Baton Rouge, LA: World Aquaculture Society; p. 20–48.
- Dreanno C, Suquet M, Desbruyeres E, Cosson J, Delliou H, Billard R. 1998. Effect of urine on semen quality in turbot (Psetta maxima). Aquaculture. 169:247–262. 10.1016/S0044-8486(98)00262-2
- Gjerde, B. 1984. Variation in semen production of farmed Atlantic salmon and rainbow trout. Aquaculture. 40:109–114. 10.1016/0044-8486(84)90349-1
- Hajirezaee S, Amiri BM, Mirvaghefi AR. 2010a. Changes in sperm production sperm motility and composition of seminal fluid in Caspian brown trout, Salmo trutta caspius over the course of a spawning season. J Appl Aquaculture. 22:157–170. 10.1080/10454431003736482
- Hajirezaee S, Amiri BM, Mirvaghefi AR, Sheikh Ahmadi A. 2010b. Evaluation of semen quality of endangered Caspian brown trout (Salmo trutta caspius) in different times of spermiation during a spawning season. Czech J Anim Sci. 50:445–455.
- Hajirezaee S, Rafiee GhR, Hushangi R. 2011. Comparative analysis of milt quality and steroid levels in blood and seminal fluid of Persian sturgeon males, Acipense persicus during final maturation induced by hormonal treatment. Biologia. 66:160–169. 10.2478/s11756-010-0140-5
- Ingermann RL, Bencic DC, Gloud JG. 2002. Lowseminal plasma buffering capacity corresponds to high pH sensitivity of sperm motility in salmonids. Fish Physiol Biochem. 24:299–307. 10.1023/A:1015037422720
- Kime DE 1993. “Classical” and “non-classical” reproductive steroids in fish. Rev Fish Biol Fisher. 3:160–180. 10.1007/BF00045230
- Lahnsteiner F, Berger B, Weismann T, Patzner RA. 1996. Motility of spermatozoa of Alburnus alburnus (Cyprinidae) and its relationship to seminal plasma composition and sperm metabolism. Fish Physiol Biochem. 15:167–179. 10.1007/BF01875596
- Lahnsteiner F, Berger B, Weismann T, Patzner RA. 1998. Determination of semen quality of the rainbow trout by sperm motility, seminal plasma parameters and spermatozoa metabolism. Aquaculture. 163: 163–181. 10.1016/S0044-8486(98)00243-9
- Lahnsteiner, F, Mansour, N, Berger B. 2004. Seminal plasma proteins prolong the viability of rainbow trout (Oncorynchus mykiss) spermatozoa. Theriogenology. 62:801–808. 10.1016/j.theriogenology.2003.12.001
- Lahnsteiner, F, Patzner, RA, Weismann T. 1993a. The testicular main duct and the spermatic duct in some cyprinid fishes. II. Composition of seminal fluid. J Fish Biol. 44:459–467.
- Lahnsteiner, F, Patzner, RA, Weismann T. 1993b. Energy resources of spermatozoa of the rainbow trout (Oncorhynchus mykiss) (Pices, teleostei). Repord Nutr Dev. 33:349–360. 10.1051/rnd:19930404
- Linhart O, Kvasnicka P. 1992. Artificial insemination of tench (Tinca tinca L.). Aquacul Fish Manage. 23:125–130.
- Linhart O, Mims SD, Shelton WL. 1995. Motility of spermatozoa from shovelnose sturgeon and paddle fish. J Fish Biol. 47:902–909. 10.1111/j.1095-8649.1995.tb06011.x
- Linhart O, Walford J, Sivaloganathan B, Lam TJ. 1999. Effects of osmolality and ions on the motility of stripped and testicular sperm of freshwater and seawater-acclimated tilapia, Oreochromis mossambicus. J Fish Biol. 55:1344–1358.
- Marshall WS, Bryson SE. 1988. Evidence for Cl dependent K+ secretion by the blood-testis bamer of brook trout. Can J Zool. 66:1603–1609. 10.1139/z88-234
- Marshall WS, Bryson SE, Idler DR. 1989. Gonadotropin stimulation of K+ secretion and Na+ absorption by brook trout (Salvelinus fontinalis) sperm duct epithelium. Gen Comp Endocrinol. 75:118–128. 10.1016/0016-6480(89)90016-6
- Miura T, Miura CI. 2003. Molecular control mechanisms of fish spermatogenesis. Fish Physiol Biochem. 28:181–186. 10.1023/B:FISH.0000030522.71779.47
- Miura T, Ninzeki M, Hirai H, Yamauchi K. 1991. Induction of final maturation by injection of chum salmon pituitary homogenate in the male Japanese huchen (Hucho perryi). Bull Fac Fish Hokkaido Univ. 42:16–25.
- Miura T, Yamauchi K, Takahashi H, Nagahama Y. 1992. The role of hormones in the acquisition of sperm motility in salmonid fish. J Exp Zool. 261:359–363. 10.1002/jez.1402610316
- Morisawa M, Hirano T, Suzuki K. 1979. Changes in blood and seminal plasma composition of the mature salmon (Oncorhynchus keta) during adaptation to freshwater. Comp Biochem Physiol. 64:325–329. 10.1016/0300-9629(79)90451-1
- Morisawa M, Suzuki K, Shimizu H, Morisawa S Yasuda K. 1983. Effects of osmolality and potassium on spermatozoan motility of fresh water salmonid fishes. J Exp Biol. 107:105–113.
- Nagahama Y. 1983. The functional morphology of teleosts gonads. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish physiology. New York, NY: Academic Press; p. 223–275.
- Perchec G, Cosson J, Andre F, Billard R. 1995. Degradation of the quality of carp sperm by urine contamination during stripping. Aquaculture. 129:135–136. 10.1016/0044-8486(95)91958-X
- Perchec G, Paxion C, Cosson J, Jeulin C, Fierville F, Billard R. 1998. Initiation of carp spermatozoa motility and early ATP reduction after milt contamination by urine. Aquaculture. 160:317–328. 10.1016/S0044-8486(97)00301-3
- Perez L, Asturiano JF, Martinez S, Tomas A, Olivares L, Moce E, Lavara R, Vicente JS, Jover M. 2003. Ionic composition and physiochemical parameters of the European eel (Anguilla anguilla) seminal plasma. Fish Physiol Biochem. 28:221–222. 10.1023/B:FISH.0000030536.30570.9d
- Piironen J. 1985. Variation in the properties of milt from the finfish landlocked salmon (Salmo salar M. sehago Girard) during a spawning season. Aquaculture. 48:337–350. 10.1016/0044-8486(85)90136-X
- Rurangwa E, Kime DE, Ollevier F, Nash JP. 2004. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture. 234:1–28. 10.1016/j.aquaculture.2003.12.006
- Scott AP, Canario AVM, Sherwood NM, Warby CM. 1991. Levels of steroids, including cortisol and 17a, 20P-dihydroxy-4-pregnen-3-one, in plasma, seminal fluid, and urine of Pacific herring (Clupea haren guspallasi) and North Sea plaice (Pleuronectes platessa L.). Can J Zool. 69:111–116. 10.1139/z91-016
- Sequet, M, Omnes MH, Normant Y, Fauvel DK. 1992. Assessment of sperm concentration and motility in Turbot, Scophthalmus maximus. Aquaculture. 101:177–185. 10.1016/0044-8486(92)90241-C
- Stoss, J. 1983. Fish physiology. In: Hoar WS, Randall DJ, Donaldson E, editors. Fish gamete preservation and spermatozoon physiology. Vol 9. New York, NY: Academic Press; p. 305–350.
- Viayeh RM, Webb MAH, Hallajian A, Kazemi R, Yali MP. 2006. Biochemical and morphometric parameters as indicators of sex and gonadal stages inmaturing Persian sturgeon, Acipenser persicus. J Appl Ichthyol. 22:364–368. 10.1111/j.1439-0426.2007.00986.x
- Yaron Z. 1995. Endocrine control of gametogenesis and spawning induction in the carp. Aquaculture. 129:49–73. 10.1016/0044-8486(94)00229-H