Abstract
The aim of this research was conducted to investigate the effect of interaction between dietary enzymes [β-mannanase enzyme (M) and β-glucanase enzyme (G)] and metabolizable energy (ME) on small intestine morphology in male broilers. In the present study, 160 male broilers were assigned and analyzed under a 23 factorial arrangement of treatments. The treatments were carried out for 21 days and chickens were subsequently slaughtered and samples of different parts of small intestine were collected for morphological assessment. Results showed that G, M and ME × G × M interaction had significant effect on duodenal villus length. Mannanase, ME × M interaction and ME × G interaction had significant effect on duodenal villus width. Mannanase and ME × G × M interaction had significant effect on duodenum crypts depth. Also, ME × G interaction had significant effect on jejunal villus length. ME level, M, ME × G interaction and ME × G × M interaction had significant effect on jejunal crypts depth. Also, G, M and ME × G interaction had significant effect on ileal villus length. ME × M interaction and ME × G × M interaction had significant effect on ileal villus width. ME level, M, ME × G interaction and ME × G × M interaction had significant effect on ileal crypts depth. In conclusion, it seems that feeding of enzyme-supplemented diet in different energy levels can alter some morphological characteristics of small intestine.
Keywords:
1. Introduction
Improvements in the performance of poultry due to different diets containing some enzymes were first reported more than 50 years ago. Mixed linkage β-(1-3), (1-4)-D-glucan (β-glucan) and β-mannan are the major constituents of barley and soybean endosperm cell walls, respectively (Buliga et al. Citation1986; Wood et al. Citation2003). The β-glucans have been identified as a major cause of low growth rate and nutrient digestibility in the broiler chickens (Ward & Marquardt Citation1987). These anti-nutritive effects of non-starch polysaccharides (NSPS) are attributed to an increase in intestinal digesta viscosity (Choct & Annison Citation1992a). The mucosa of small intestine is arranged into two fundamental structures: villi and crypt. Villi are projections into the lumen covered predominantly with enterocytes, along with occasional mucus-secreting goblet cells. These cells are alive only for a few days; then they will die and drop into the lumen to become part of the ingesta. Crypts of Lieberkuhn are moat-like invaginations of the epithelium around the villus, and are lined with epithelial cells. The intestinal villus and crypt morphological status in chickens has been associated with intestine function and chicken growth (Tarachai & Yamauchi Citation2000). In general, the effect of some factors such as thermal conditions (Uni et al. Citation2001), body weight of the laying hen (Yamauchi & Isshiki Citation1991), feed restriction, dry or wet diets, quantity of dietary protein and energy, exogenous enzymes and NSPs and probiotics (Al-Marzooqi & Leeson Citation2000; Batal & Parsons Citation2002; Wang et al. Citation2005) on intestinal morphology of broiler chicks has been studied.
On the other hand, some processing methods can affect apparent metabolizable energy (AME) content of feed ingredients in poultries. It has been reported that processing of a wheat-based diet may facilitate digestion of energy substrates, therefore enhancing values of AME (Amerah et al. Citation2007a). The beneficial effects of processed feed on digestibility of nutrients may arise from their influence on intestinal morphology (Amerah et al. Citation2007b; Zang et al. Citation2009). However, the aim of the present study was to clarify the ME levels and endogenous enzyme effects and their interaction effect on small intestine morphological status in male broiler chickens fed a barley–soybean-based diet.
2. Materials and methods
2.1. Animals, diets and treatments
One hundred and sixty one-day-old male broiler chicks (Ross 308) were sexed by vent sexing method and randomly housed in floor pens (five birds in each) containing litter composed of wood shaving. Pens were located in one room providing environmental control. The experimental design was a completely randomized one with a 2 × 2 × 2 factorial arrangement of treatments. At one day of age, all the chickens were divided into eight groups (with three replicates of five male broiler chicks each). Starter (1–12 days) and grower (after 12 day) basal diets () were formulated to provide a similar nutrient profile. Hemicell (ChemGen Co, USA) is used as a source of β-mannanase enzymes (EC 3.2.1.78) which include 140 million unit/kg of this enzyme, and Rovabio® Excel (ADISSEO Co, France) is used as a source of β-glucanase enzymes. Eight experimental dietary treatments consist of NO: Normal ME basal diets without enzyme, NM: Normal ME basal diet + 1g/kg Hemicell, NG: Normal ME basal diet + 1g/kg Rovabio, NMG: Normal ME basal diet + 1g/kg Rovabio + 1g/kg Hemicell, LO: Low ME basal diets without enzyme, LM: Low ME basal diet + 1g/kg Hemicell, LG: Low ME basal diet + 1g/kg Rovabio and LMG: Low ME basal diet + 1g/kg Rovabio + 1g/kg Hemicell. Experimental diets were randomly allocated to one of eight diet treatment groups for a 21-day trial period. Broilers received standard feed mixture ad libitum containing adequate requirements as presented in . Fresh feed offered to all the birds twice daily to prevent wastage. Water also was supplied ad libitum throughout the entire experiment. The temperature was maintained at 32°C for 5 days and was gradually reduced with normal brooding practices. Lighting was at 80 lx for 23 h/day.
Table 1. Ingredients and composition of the basal diets (g/kg) with low and normal ME levels.
2.2. Morphological examination
At the end of the experiment, on 21st day, eight birds from each treatment were killed and 5 cm segments were removed from the duodenum, jejunum and ileum as follows: (1) the apex of the duodenum, (2) midway between the point of entry of the bile ducts and Meckel’s diverticulum (jejunum) and (3) 15 cm proximal to the cecal junction (ileum). Samples were fixed in 10% neutral-buffered formalin solution and routinely processed to 5-mm hematoxylin eosin-stained sections. Intestinal samples were dehydrated, cleared and embedded in paraffin. Serial sections were cut at 7 μm and placed on glass slides. Sections were deparaffinized in xylene, rehydrated in a graded alcohol series and examined by light microscope (Uni et al. Citation1995, Citation1998). Morphometric indices were determined by computer analysis with Leica QWin image analysis software. The morphometric variables analyzed by computer included: villus length (from the tip of the villus to the villus crypt junction), villus width and crypts of Lieberkuhn depth. Values are calculated using means from 15 adjacent villus and only vertically oriented villus was measured ().
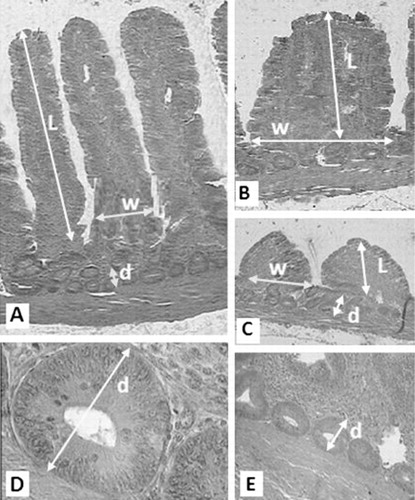
2.3. Statistical analysis
Results were analyzed using Proc GLM of SAS 9.1 (SAS Institute, version 9.1, Citation2002, Cary, NC, USA). The results were expressed as Lsmean ± SEM. The Tukey’s test was used to compare least squares means. All data were checked for normal distribution by Proc univariate and Shapiro–Wilk test.
3. Results
Effects of treatments on duodenal morphology of 21-day old broilers are summarized in . Morphologic measurements in the duodenum displayed significant effects of G, M and ME × M × G interaction on villus Length. There was a significant effect of M, ME × G interaction and ME × M interaction on duodenal villus width. Also, duodenum crypt depths were significantly affected by M supplementation and ME × G × M interaction.
Table 2. Effects of ME and enzymes levels and all their interactions on duodenal morphology of 21-day-old male broiler intestines (LSM ± SEM).
Morphologic measurements of the jejunal villus are presented in . Histology indicated that the jejunal villus length was significantly affected by ME × G interaction. There were no significant effects of enzymes, ME levels or their interactions on jejunal villus width. Also, the jejunal crypt depths were significantly affected by ME levels, M, ME × G interaction and ME × M × G interaction.
Table 3. Effects of ME and enzymes levels and all their interactions on jejunal morphology of 21-day-old male broiler intestines (LSM ± SEM).
Ileal morphometric data are presented in . As shown in this table, ileal villus length was significantly affected by G, M and ME × G interaction. Ileal villus widths were significantly affected by ME × M interaction and ME × G × M interaction. Also, ileal crypt depths were significantly affected by ME levels, M, ME × G interaction and ME × G × M interaction.
Table 4. Effects of ME and enzymes levels and all their interactions on ileal morphology of 21-day-old male broiler intestines (LSM ± SEM).
4. Discussion
Some previous researches suggest that the addition of exogenous enzyme results in an increase in dietary energy values by 2% on average which is due to hydrolysis of NSPs, allowing digestive enzymes access to substrates such as protein and starch with a consequent improvement in the digestibility of nutrients (Choct & Annison Citation1990; Fuente et al. Citation1995; Nian et al. Citation2011). Our hypothesis in the present study was illumination of endogenous enzymes and effects of ME levels and their effects of interaction on small intestine morphological status in male broiler chickens.
In the barley-based diets, injury to the small intestine may be caused by the viscous characteristics of NSPs (Stanogias & Pearce Citation1985). The improved performance of poultries fed NSP-rich diets by exogenous enzyme, such as β-glucanase supplementation, is not due to release of simple sugars, but rather due to the ability of the enzymes to prevent the formation of viscous digesta. By adding enzymes into a diet, the viscosity of the content is reduced and nutrient uptake and animal performance are improved (Yang et al. Citation2009). The addition of exogenous enzymes is necessary to decrease the anti-nutritive effects of this viscous diet (Choct & Annison Citation1992b). It has been reported that decrease in digesta viscosity after exogenous enzyme addition to the broiler diets is associated with an improvement in small intestine morphology (Yasar & Forbes Citation2000). The structure of the intestinal mucosa can elucidate some information on gastrointestinal tract (GIT) health. Presence of stressors in the digesta can lead to rapid changes in the intestinal mucosa due to the close proximity of the mucosal surface to the intestinal content (Xu et al. Citation2003). Changes in intestinal morphology, such as shorter villus and deeper crypts, have been associated with the presence of toxins (Yason et al. Citation1987). The increase in villus height suggests an increase in the absorptive surface area and greater absorption of available nutrients (Zulkifli et al. Citation2009). In addition, because the jejunum is recognized as the major site of absorption in the small intestine, the increase in villus height could represent an attempt to increase intestinal surface area to maximize absorption (Thompson & Appegate Citation2006). In the present study, villus length was similar in duodenum, jejunum or ileum among diets containing low or normal ME levels, but duodenal and ileal villus length was positively affected only by β-mannanase or β-glucanase enzymes. We found some interactions for villus length such as interaction between dietary ME levels and glucanase enzyme in jejunum and ileum. Hence, we observed that β-mannanase or xylanase (without interfering the ME effects) can change the intestinal villus length but when this chicken was treated with different ME levels, the villus length is returned to normal situation. However, we observed that duodenal villus width has been affected by β-mannanase enzyme. Also, it has been observed some interactions for villi width such as interaction between dietary ME levels and β-mannanase in duodenum or interaction between dietary ME levels and β-mannanase enzyme in ileum. These data can be a good evidence for beneficial effects of glucanase and β-mannanase on the intestinal feature. Also, it seems that interaction effects between these two enzymes and ME levels can prevent apoptosis in the GIT. Although, we did not check the apoptosis feature in different segments of small intestine. Solomon and Tullett (Citation1988) concluded that ileal villus with taller, narrower and regular shape provides greater surface for absorption. However, results of the present study showed that duodenal crypt depth was affected by β-mannanase enzyme but jejunal and ileal crypt depth was affected by dietary ME levels and β-mannanase enzyme. It has been observed some significant interaction affects crypt depth such as interaction between dietary ME levels and glucanase enzyme in ileum. The crypt can be regarded as the villi factory; a large crypt indicates fast tissue turnover and a normal demand for new tissue. Increasing of crypt depth may propose a more normal proliferation (Iji et al. Citation2001). Therefore, in the present study, increasing of duodenal crypt depth in the group supplemented by β-mannanase may be due to normal proliferation or an unusual demand for cell proliferation and tissue renewal. However, increase in tissue turnover will increase the amount of nutrients required for maintenance and will lower the efficiency of the animal.
In conclusion, duodenal and ileal villus length was significantly changed by addition of β-glucanase to the male broiler’s diets. The length and width of villus and crypts depth in duodenum, crypts depth in jejunum and length of villus and crypts depth in ileum were significantly changed by addition of β-mannanase to the broilers diet. However, it seems that these two exogenous enzymes can improve morphological changes in small intestine of male broiler chicks. ME levels have different effects on intestinal morphology rather than β-mannanase and β-glucanase enzymes and therefore, we concluded that the positive effects of these two exogenous enzymes on small intestinal morphology were not due to releasing of ME from diet ingredients in the broiler GIT.
Acknowledgements
The authors would like to acknowledge all of the personnel who work in the animal farm of Faculty of Agriculture, Islamic Azad University, Varamin-Pishva branch and the Animal Science Laboratory, Islamic Azad University, Science and research campus, for their support and contribution to this study. We also thank Mr Soheil Molayi and Mohamad Amanlou (graduate students of Islamic Azad University, Varamin-Pishva branch) for providing technical supports.
References
- Al-marzooqi W, Leeson S. 2000. Effect of dietary lipase enzyme on gut morphology, gastric motility, and long-term performance of broiler chicks. Poult Sci. 79:956–960. 10.1093/ps/79.7.956
- Amerah AM, Ravindran V, Lentle RG, Thomas DG. 2007a. Influence of feed particle size and feed form on the performance, energy utilization, digestive tract development, and digesta parameters of broiler starters. Poult Sci. 86:2615–2623. 10.3382/ps.2007-00212
- Amerah AM, Ravindran V, Lentle RG, Thomas DG. 2007b. Feed particle size: implications on the digestion and performance of poultry. World’s Poult Sci J. 63:439–455. 10.1017/S0043933907001560
- Batal AB, Parsons CM. 2002. Effects of age on development of digestive organs and performance of chicks fed a corn-soybean meal versus a crystalline amino acid diet. Poult Sci. 81:1338–1341. 10.1093/ps/81.9.1338
- Buliga GS, Brant DA, Fincher GB. 1986. The sequence statistics and solution conformation of a barley (1 -3, 1 -4)-b – D -glucan. Carbohyd Res. 157:139–156. 10.1016/0008-6215(86)85065-0
- Choct M, Annison G. 1990. Antinutritive activity of heat pentosans in broiler diets. Brit J Nutr. 31:811–821.
- Choct M, Annison G. 1992a. The inhibition of nutrient digestion by wheat pentosans. Br J Nutr. 67:123–132. 10.1079/BJN19920014
- Choct M, Annison G. 1992b. Anti-nutritive effect of wheat pentosans in broiler chickens: roles of viscosity and gut microflora. Br J Nutr. 33:821–834.
- Fuente JM, De Ayala P, Villamide MJ. 1995. Effect of dietary enzyme on metabolizable energy of diets with increasing levels of barley fed to broilers at different ages. Anim Feed Sci Technol. 56:45–53. 10.1016/0377-8401(95)00815-5
- Ijipa PA, Saki A, Tivey DR. 2001. Intestinal structure and function of broiler chickens on diet supplemented with a mannan oligosaccharide. J Sci Food Agric. 81:1186–1192. 10.1002/jsfa.925
- Nian F, Guo YM, Ru YJ, Li FD, Peron A. 2011. Effect of exogenous xylanase supplementation on the performance, net energy and gut microflora of broiler chickens fed wheat-based diets. Asian-Aust J Anim Sci. 24:400–406. 10.5713/ajas.2011.10273
- SAS. 2002. SAS user's guide: statistics, version 9.1. Cary (NC): SAS Institute.
- Solomon SE, Tullett SG. 1988. The effect of virginiamycin on the ileum of the domestic fowl. 1. Light and scanning electron microscope observations. Anim Technol. 39:157–160.
- Stanogias G, Pearce GR. 1985. The digestion of fiber by pig. 2. Volatile fatty acid concentration in large intestine digesta. Br J Nutr. 53:513–536. 10.1079/BJN19850061
- Tarachai P, Yamauchi K. 2000. Effects of luminal nutrient absorption, intraluminal physical stimulation, and intravenous parenteral alimentation on the recovery responses of duodenal villus morphology following feed withdrawal in chickens. Poult Sci. 79:1578–1585. 10.1093/ps/79.11.1578
- Thompson KL, Appegate TJ. 2006. Feed withdrawal alters small intestinal morphology and mucus of broilers. Poult Sci. 85:1535–1540. 10.1093/ps/85.9.1535
- Uni Z, Gal-garber O, Geyra A, Sklan D, Yahav S. 2001. Changes in growth and function of chick small intestine epithelium due to early thermal conditioning. Poult Sci. 80:438–445. 10.1093/ps/80.4.438
- Uni Z, Ganot S, Sklan D. 1998. Posthatch development of mucosal function in the broiler small intestine. Poult Sci. 77:75–82. 10.1093/ps/77.1.75
- Uni Z, Noy Y, Sklan D. 1995. Posthatch changes in morphology and function of the small intestines in heavy- and light-strain chicks. Poult Sci. 74:1622–1629. 10.3382/ps.0741622
- Wang ZR, Qiao SY, Lu WQ, Li DF. 2005. Effects of enzyme supplementation on performance, nutrient digestibility, gastrointestinal morphology, and volatile fatty acid profiles in the hindgut of broilers fed wheat-based diets. Poult Sci. 84:875–881. 10.1093/ps/84.6.875
- Ward AT, Marquardt RR. 1987. Antinutritionalactivity of a water-soluble pentosan-rich fraction from rye grain. Poult Sci. 66:1655–1674.
- Wood PJ, Wiszz J, Beer MU, Newman CW, Newman RK. 2003. Structure of (1 -3) (1 -4)-b -D -glucan in waxy and nonwaxy barley. Cereal Chem. 80:329–332. 10.1094/CCHEM.2003.80.3.329
- Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. 2003. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 82:648–654. 10.1093/ps/82.4.648
- Yamauchi K, Isshiki Y. 1991. Scanning electron microscopic observations on the intestinal villus in growing White Leghorn and broiler chickens from 1 to 30 days of age. Br Poult Sci. 32:67–78. 10.1080/00071669108417328
- Yang Y, Iji PA, Choct M. 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics, composition, and nutritive value. World’s Poult Sci J. 65:97–114. 10.1017/S0043933909000087
- Yasar S, Forbes JM. 2000. Enzyme supplementation of dry and wet wheat-based feeds for broiler chickens: performance and gut responses. Br J Nutr. 84:297–307. 10.1017/S0007114500001574
- Yason CV, Summers BA, Schat KA. 1987. Pathogenesis of rotavirus infection in various age groups of chickens and turkeys: pathology. Am J Vet Res. 6:927–938.
- Zang JJ, Piao XS, Huang DS, Wang JJ, Ma X, Ma YX. 2009. Effects of feed particle size and feed form on growth performance, nutrient metabolizability and intestinal morphology in broiler chickens. Asian-Austr J Anim Sci. 22:107–112. 10.5713/ajas.2009.80352
- Zulkifli I, Iman Rahayu HS, Alimon AR, Vidyadaran MK, Babjee SA. 2009. Gut microflora and intestinal morphology of commercial broiler chickens and Red Jungle Fowl fed diets containing palm kernel meal. Archiv fur Geflugelkunde. 73:49–55.
