Abstract
This study was carried out with the aim of determining the effects of breeder age on incubation results, intestinal development during hatch window, chick quality and first week broiler performance. A total of 4800 eggs were obtained from commercial Cobb 500 broiler breeder flocks at 33 and 62 weeks old. Of these 33 and 62 weeks old flocks, 31.4% and 7.0% were hatched 24 h before pull time and 51.2% and 56.0% were hatched 12 h before pull time, respectively. Hatchability of fertile eggs and hatchability of total eggs were found higher in 33 weeks old flock than the other. The chick hatch weight was determined as 39.5 g and 41.4 g in 33 and 62 weeks old flocks, respectively. Chick weight/initial egg weight rate was found to be higher as 67.3% in the 33 weeks old flocks. On hatching day, chick length were also higher in 62 weeks old flock. Relative yolk-free chick weight was higher in 33 weeks old (85.0%) flock than the other (82.0%). Intestine weight rate was higher as 5.27% in chicks from 33 weeks old than the other (5.08%). At 1 week of age, the body weights and weight gains were 165.1 g and 156.0 g, and 125.6 g and 114.6 g in 33 and 62 weeks old flocks, respectively. Higher mortality ratio as 3.6% was observed in 62 weeks old flock. In conclusion, intestinal development during hatch window, incubation parameters, chick quality and first week broiler performance is affected by breeder age.
1. Introduction
Chick quality has great importance for broiler breeder producer who is paid based on number of saleable chicks and also broiler producer who wants high-quality, fast-growing and uniform broilers. The chick quality is affected by some factors, such as breeder age, egg size, hatching time and incubation conditions (Wilson Citation1991a, Citation1991b; Suarez et al. Citation1997). There is a complex relationship between these factors, for example, hatching time is influenced by breeder age, egg size and incubation conditions (Tona et al. Citation2003; Careghi et al. Citation2005; Vieira et al. Citation2005; Romanini et al. Citation2013). Wilson (Citation1991a) found a longer total incubation period and an alteration in hatching distribution for eggs from breeders at the onset of lay.
In commercial hatcheries, incubation periods lasts approximately 504–510 h (Vieira & Pophal Citation2000; Almeida et al. Citation2008). In hatchery practice, difference in the hatching times between the first and the last hatched chicks is expressed as the hatch window (Romanini et al. Citation2013). Decuypere et al. (Citation2001) reported a difference in hatch window of 24–48 h. In practice, many chicks hatch earlier, and therefore, they remain without food and water for the time from hatching to the time of placing broiler house (Noy & Sklan Citation1997; Dibner et al. Citation1998; Careghi et al. Citation2005).
Chick hatching weight is measured as a chick quality criteria, and at the time chicks are removed from the hatcher, their weight is determined by their weight at hatch and the amount of time they are held in the hatcher (Wyatt et al. Citation1985; Suarez et al. Citation1997). Wyatt et al. (Citation1985) found that chicks that remained in the hatcher for 14–32 h were 5–32% lighter than those remained for 7 h. Fanguy et al. (Citation1980) and Wyatt et al. (Citation1985) stated that the chicks that remained longer in the hatcher show worse live performance. The time period in hatcher after hatching is critical for the development of the gastrointestinal systems and nutrient absorption (Almeida et al. Citation2008; Yalcin et al. Citation2013). During the first days of the chick’s life, the small intestine grows five times faster than the rest of the body, and small intestine microvilli grow significantly faster in birds supplied with water and feed immediately after hatching (Dibner et al. Citation1998; Almeida et al. Citation2008).
It is known that there is a correlation between chick quality at hatch, broiler performance during the first week and at market age (Wilson Citation1991a; Nitsan Citation1995). After hatching, a delay in feed access affects negatively yolk sac absorption, development of intestine and growth rate during broiler growing period (Careghi et al. Citation2005; Mahmoud & Edens Citation2012). The aim of the current study was to determine the effects of breeder age on incubation results, intestinal development during hatch window, chick quality parameters and first week broiler performance. In literatures, there is lacking of experiments that investigate the intestinal development of early hatched and late hatched chicks during the hatching period. So in this study, to evaluate the development of intestine, samples were taken during hatch window, in times of 24 h and 12 h before pull and at hatch time (0 h).
2. Materials and methods
2.1. Animals and sampling
A total of 4800 eggs were obtained from commercial Cobb 500 broiler breeder parent stocks at 33 and 62 weeks old flocks. The breeder flocks received a broiler breeder diet with 2750 kcal ME/kg and 14.50% CP. The two flocks were kept under the same management conditions according to the breeding company’s recommendations. The eggs ranged from 55.0 g to 59.0 g in the 33 weeks of age group and from 60.0 g to 64.0 g in the 62 weeks of age group were weighed with ±0.1 precision one by one. To minimize the overheating and therefore the possible effects of overheating in eggs from 62 weeks of age flock, the egg weight range was chosen closer between 33 and 62 weeks of age broiler breeder flocks. The eggs from each flock age group were randomly placed into incubator trays consisting of 150 eggs (n = 16 trays/each breeder age). Eggs were stored at 18°C and 65% RH for 3 days and were then warmed to room temperature (21°C) for 8 h before setting. All eggs were incubated in same incubator (89,100 eggs capacity, multi-stage setters, fixed-shelf Chickmaster) at 37.5°C and a relative humidity of 55–60% during the first 18 days of incubation.
At day 18 of incubation, eggs were weighed to determine weight loss and then transferred to hatcher (87,480 eggs capacity, hatcher Chickmaster) with a temperature of 36.7°C and a relative humidity of 58%. Ideal incubation period is accepted as 510 h and counting of hatched chicks and samplings were performed according to the ideal incubation period (pull time). The hatched chicks in time of 486 h, 487–498 h, 499–510 h and 511–518 h were counted, and the rate of hatching chicks according to these times was calculated. In these times, after chicks were counted, chicks were transferred to another hatching baskets. Hatching distribution that is known as hatch window and total incubation period was determined:
During hatch window, to determine development of intestine, chicks (n = 20 chicks/sampling time/breeder age) were randomly sampled in the sampling times [24 h before pull time (−24 h), 12 h before pull (−12 h) and at hatch (0 h)]. In sampling times for −24 h and also −12 h before pull time, chicks were randomly sampled from hatcher. The sampled chicks were weighed and killed by cervical dislocation, and then the intestine was dissected. The intestine weight and length were measured, and the ratio of intestine weight to chick weight was calculated. The length of intestine was measured from the beginning of small intestine to the end of the cloaca.
After completing of hatching process, chicks were classified as saleable or cull chick (Tona et al. Citation2004; Molenaar et al. Citation2011). The percentage of saleable and cull chicks was expressed as a percentage of fertile eggs (Molenaar et al. Citation2011). Unhatched eggs were opened to macroscopically determine fertility and embryonic mortality (early, middle- and late-term embryonic mortality).
The chicks were weighed at feather dryness (approximately 2 h post-hatch) to determine the chick hatch weight and cloacal temperature. The cloacal temperatures of the chicks were also measured (to the nearest 0.01°C) using a thermocouple thermometer that was inserted into the cloaca. A total of 60 chicks from each breeder age group were randomly sampled to determine quantitative chick quality parameters. Then the chicks from each group were measured for chick weight and chick length and then killed by cervical dislocation to determine residual yolk sac weight and yolk-free chick weight. Chick length was measured from the tip of the beak to the tip of the longest toe by placing the chick face down on a flat surface and straightening the left leg (Hill Citation2001).
The chicks (n = 720) were randomly allocated into two groups (33 and 62 weeks of age). The chicks were placed in two pens in a same poultry house with a surface area of 2.0 × 2.0 m2 to provide 9 replicate pens and 40 chicks (20 males/20 females) per replicate. Wood shavings were used as litter material, which was laid at a thickness of 10 cm on the floors of the pens. The chicks received a standard pelleted broiler starter diet (22.5% CP and ME 12.8 MJ/kg of diet) between 1 and 7 days and were exposed to 24 h of light at the first week. Water was supplied to both groups ad libitum by constant refreshing. The temperature, humidity and other environmental factors were equal for each of the groups during the trial. The body weight gain values were monitored at the end of the first week; feed conversion ratios were calculated using the body weight gains and feed consumption values. The mortality was recorded during the first week.
2.2. Statistical analyses
Data were subjected to analysis of variance (ANOVA; SAS Institute Citation1998), utilizing ANOVA procedures for balanced data. Analysis for percentage data was conducted after an arcsine transformation of the data. Significant differences among treatment means were determined by Duncan’s multiple range test. Comparisons among the sampling times for the development of small intestine during hatch window were analysed with a mixed-model repeated measures ANOVA. The model included breeder age and sampling times and interaction between them as main effects. Mortality was analysed using chi-square tests, before analysis. Differences were considered significant at P < 0.05.
3. Results
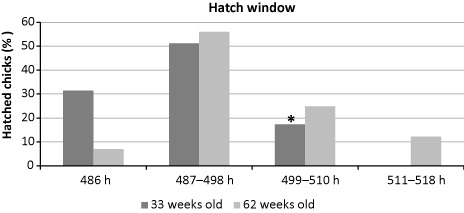
The hatch window results of 33 and 62 weeks old broiler breeder flocks are presented in . Hatch window was found that 31.4% and 7.0% of the chicks were hatched in the time of 486 h, 51.2% and 56.0% of the chicks between 487 h and 498 h, and 17.4% and 24.8% of the chicks between 499 h and 510 h in 33 and 62 weeks old broiler breeder flocks, respectively. Incubation period was completed in 510 h in 33 weeks old breeder flock, whereas it was completed in 518 h in 62 weeks old breeder flock. So, 12.2% of the chicks hatched between 511 h and 518 h in that flock.
*The hatching process was completed in 510 h in 33 weeks old age breeder flock.
The effects of breeder age on incubation results and cloacal temperature are presented in . The effects of breeder age on egg weight was found to be significant (P = 0.027). Egg weight was 58.7 g and 63.5 g in 33 and 62 weeks old flocks, respectively. Fertility was similar in breeder age groups, whereas hatchability of fertile eggs and hatchability of total eggs were found to be higher in 33 weeks old flock than the other (P = 0.001). Hatchability of fertile eggs and hatchability of total eggs were 92.7% and 88.3% in 33 weeks old flock, respectively. Early, middle- and late-term embryo dead and cull chick rate were found to be higher in 62 weeks old flock (P = 0.001). The cull chick rate was 0.85% and 1.8% in 33 and 62 weeks old flocks, respectively. Egg weight loss was found higher with a rate of 12.9% in 62 weeks old flock than the other (P = 0.032). Chick hatch weight was 39.5 g and 41.4 g in 33 and 62 weeks old flocks, respectively (P = 0.015). Chick weight/initial egg weight rate was found to be higher with a value of 67.3% in the 33 weeks old flock (P = 0.035). The cloacal temperature was found to be similar between flocks (P = 0.058).
Table 1. The effects of breeder age on incubation results (mean ± SEM).
The effects of breeder age on the development of intestine development during hatch window are presented in . The intestine weight rate was only found to be significant in breeder age groups. It was higher with a value of 5.27% in chicks from 33 weeks old flock than the other (5.08%; P = 0.046). During the hatch window, the chick weight, the weight and length of intestine and the intestine weight ratio were similar in sampling times.
Table 2. The effects of breeder age on intestinal development during hatch window.
The effects of breeder age on the chick weight, chick length, residual yolk sac weight, relative residual yolk sac weight, yolk-free chick weight and relative yolk-free chick weight are presented in . On hatching day, chick weight and length were found as 39.8 g and 41.5 g, and 18.1 cm and 19.6 cm in 33 and 62 weeks old flocks, respectively (P = 0.027; P = 0.032). Higher residual yolk sac weight and relative residual yolk sac weight were observed in 62 weeks old flock with a value of 7.5 g and 18.1% (P = 0.001). Although yolk-free chick weight was similar between flocks, relative yolk-free chick weight was higher in 33 weeks old flock (85.0%) than the other (82.0%; P = 0.001).
Table 3. Mean values of the chick weight (g), chick length (cm), residual yolk sac weight (g), relative residual yolk sac weight (%), yolk-free chick weight (g) and relative yolk-free chick weight (%) for breeder ages (mean ± SEM).
The effects of breeder age on the post-hatch first week broiler performance parameters are presented in . The initial body weight on day 1 was higher in the 62 weeks old flock (41.4 g) than the 33 weeks old flock (39.5 g; P = 0.024). At 1 week of age, the body weights and body weight gains were determined as 165.1 g and 156.0 g (P = 0.022), and 125.6 g and 114.6 g (P = 0.027) in 33 and 62 weeks old flocks, respectively. Feed consumption and feed conversion rate were similar between treatments for the first week. Higher mortality ratio as 3.6% was observed in 62 weeks old flock (χ 2 = 6.392, P = 0.011).
Table 4. Mean values of the post hatch first week broiler performance parameters for breeder ages (mean ± SEM).
4. Discussion
This study has investigated the effects of breeder age on incubation results, intestinal development during hatch window, chick quality parameters and first week broiler performance. In 62 weeks old flock, the actual hatching rate at −12 h and −24 h of pull time was lower than expected, so it caused longer time range between the first and last hatched chicks. Reis et al. (Citation1997) found that 56% of the eggs hatched at 485 h in 33 weeks old and at 490 h in 49 weeks old broiler breeder flocks. In other studies, hatch window range was found as 24–48 h (Decuypere et al. Citation2001), 28 h (Careghi et al. Citation2005) and 30 h (Van De Ven et al. Citation2011). Similarly, in another study, it was observed that eggs laid by 32-week-old breeders hatched 9 h early than those laid by 37-week-old breeders (Pedroso et al. Citation2005).
In this study, egg weight was 58.7 g and 63.5 g in 33 and 62 weeks flocks, respectively. Furthermore, it is consistent with the results of Vieira et al. (Citation2005), who observed that the time required for hatching increased in heavy eggs as compared to lighter eggs of older age breeders. In this study, the hatchability of fertile eggs and hatchability of total eggs of old breeders’ eggs were lower than those of young breeders, which were found significantly different. Similar to these results, in many of studies, it was found that older flocks tend to have the poorest hatchability (Lapao et al. Citation1999). Tona et al. (Citation2001) reported the highest total hatchability and the lowest total embryo mortality at 40 weeks of age (between flocks from 27 to 60 weeks of age), and also the lowest hatchability and highest rates of embryo mortality were observed towards the end of the study at older flock ages. In this study, higher ratio of embryo mortalities was observed in older flock, and these results are consistent with Wilson (1Citation991a), Reis et al. (Citation1997), Lapao et al. (Citation1999) and Hudson et al. (Citation2004).
In this study, rate of cull chicks (%) was found to be higher as 1.8% in 62 weeks old group. The reason of higher rate of cull chicks in 62 weeks old group could be attributed to longer time range between first and last hatched chicks in this group. Ar and Rahn (Citation1980) stated that the average egg weight loss should be between 12% and 14% to obtain the highest hatchability of chicken eggs. In this study, egg weight loss was higher as 12.9% in 62 weeks old flock. On the contrary, Almeida et al. (Citation2008) did not find differences for the relation between egg weight loss during incubation and breeder age, with an average rate of 11%. Chick hatch weight and chick weight/initial egg weight rate were found to be significant. Chick hatching weight was found higher in 62 weeks old flock, and chick weight/initial egg weight rate was affected by breeder age and was found lower as 65.2% in 62 weeks old flock. Chick weight to egg weight ratio was not affected by breeder age, with an average of 72–73% (Campos & Santos Citation2003; Vieira et al. Citation2005; Almeida et al. Citation2008).
This study was carried out with the eggs from 32 and 62 weeks of age broiler breeder flocks. It is known that an increase could be seen in metabolic heat production in heavy eggs from older flocks and therefore an increase also could be seen in eggshell and incubator temperatures. There was a difference of egg weight between 32 and 62 weeks of age flocks, therefore some small changes in eggshell temperature (EST) were observed (+0.5°C higher in EST in eggs of 62 weeks of age). Considering the results of the present study, it could be estimated that deviations from optimum temperatures especially higher temperatures during incubation of heavier eggs from older flocks are more hazardous for incubation parameters, chick quality and subsequently broiler performance. Therefore, in large-scale hatcheries, controlling the incubator temperature settings has crucial importance to prevent heating of eggs.
Embryo uses the nutrients from the yolk sac to initiate body growth (Chamblee et al. Citation1992; Murakami et al. Citation1992; Meijerhof Citation2009a), for development of the small intestine (Noy & Sklan Citation1999) and other organs. Residual yolk sac comprises approximately 14% of the chick’s body weight at the time of hatching (Mikec et al. Citation2006; Meijerhof Citation2009a). Before hatching, absorption of the yolk sac into the abdomen of the embryo provides nutrients for the chicks during the first few days of life. Chick weight is a combination of the real chick weight and the remaining yolk residual. In this study, on the hatching day, the residual yolk sac weight and relative residual yolk sac weight were found higher as 7.5 g and 18.1% in old flock, respectively. After subtracting yolk sac weight from chick weight, there were no differences between groups. Relative yolk-free chick weight was found to be higher in young flock.
The quality of the day-old chick is important for a good start of the chick and also for the final performance of broiler (Meijerhof Citation2009b). Breeder age, egg weight, egg age, climatic conditions of both hatchery spaces and incubators, and some other factors predominantly affect hatch window and therefore chick quality (Decuypere & Bruggeman Citation2007, Vargas et al. Citation2009). The weight of chicks at placement to broiler house is affected by their weight at hatch and the amount of time they are held in the hatcher, as chicks that have to wait in the incubator for prolonged periods suffer dehydration and weight loss. Careghi et al. (Citation2005) calculated this loss to amount to more than 8% of initial weight in 24 h. On hatching day, chick weight and length were found as 39.8 g and 41.5 g, and 18.1 cm and 19.6 cm in 33 and 62 weeks old groups, respectively. In this study, heavier and longer chicks in old flock were resulted from heavier egg weight. Wolanski et al. (Citation2004) showed that the length of the chicken is indicative for its development, and a positive correlation between chick length at day of hatch and broiler performance exists (Meijerhof Citation2009b). It was stated that chick length has a substantially higher positive correlation with broiler performance than day-old chick weight, especially when corrected for egg size (Wolanski et al. Citation2004; Meijerhof Citation2009b).
As the initial body weights were found significantly, it could be attributed to difference in egg weight. Although initial body weight was lower in young flock, after 1 week post-hatch, body weight and body weight gain were found higher than old flock. It could be explained by yolk sac absorption, and development of intestine of the chicks in this group was higher than the other. Palo et al. (Citation1995) reported that the development of gastrointestinal tract has a major role in chick growth during the post-hatch early growing period. In parallel with this, the rate of intestine weight was heavier in chicks from young than from old flock in this study.
Feed consumption and feed conversion rate were not influenced by breeder age. The mortality during first week was found significantly higher as 3.6% in 62 weeks old flock. This result could be explained by findings of Gonzales et al. (Citation2003) who stated that after 48 h of fasting, early mortality increases. In 62 weeks old flock, the actual hatching rate at −12 h and −24 h of pull was lower than expected. Otherwise, the time range after hatching is vital for the development of immune and gastrointestinal systems. While early term chick feeding stimulated the development of bursa and production of lymphocytes (Bigot et al. Citation2001), long fasting times stimulate corticosterone production that has a strong inhibitor effect on immune cells. These cause a decrease in growth rate (Decuypere et al. Citation2001) and an increase in early term chick mortalities (Hamdy et al. Citation1991).
5. Conclusion
In conclusion, incubation parameters, intestinal development during hatch window, some chick quality parameters and post-hatch first week broiler performance are affected by breeder age. Hatch window is one of the most important effecting factors for yolk sac absorption, intestinal development during hatching period and subsequently chick quality. It is also crucial for broiler performance in the way of chick growth during the post-hatch early period and first week chick mortalities. Knowing approximate total incubation period and hatch window range for different breeder age and also egg weight may improve chick quality by preventing overheating and dehydration in the hatcher. This study showed that breeder age affected intestine weight and subsequently first week chick performance. Therefore, the effects of breeder age on these parameters, especially intestinal development, could be studied more in detail.
References
- Almeida JG, Vieira SL, Reis RN, Berres J, Barros R, Ferreira AK, Furtado, FVK. 2008. Hatching distrubution and embryo mortality of eggs laid by broiler breeders of different ages. Brazilian J Poultry Sci. 10:89–96.
- Ar A, Rahn H. 1980. Water in the avian egg: overall budget of incubation. Am Zool. 20:373–384.
- Bigot K, Tesseraud S, Taouis M. 2001. The relation of egg weight to chick weight at hatching. Prod Anim. 14:219–230.
- Campos EJ, Santos JEC. 2003. O efeito de linhagens sobre o desenvolvimento embrionário [The effects of different strains on embryonic development]. In: Macari M, Gonzales E, editores. Manejo da incubação. 2nd ed. Jaboticabal: FACTA; p. 353–361.
- Careghi C, Tona K, Onagbesan O, Buyse J, Decuypere E, Bruggeman V. 2005. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poultry Sci. 84:1314–1320.
- Chamblee TN, Brake JD, Schultz CD, Thaxton JP. 1992. Yolk sac absorption and initiation of growth in broilers. Poultry Sci. 71:1811–1816.
- Decuypere E, Bruggeman V. 2007. The endocrine interface of environmental and egg factors affecting chick quality. Poultry Sci. 86:1037–1042.
- Decuypere E, Tona K, Bruggeman V, Bamelis E. 2001. The day-old chick: a crucial hinge between breeders and broilers. World’s Poultry Sci J. 57:127–138.
- Dibner JJ, Knight CD, Kitchell ML, Atwell CA, Downs AC, Ivey FJ. 1998. Early feeding and development of the immune system in neonatal poultry. J Appl Poultry Res. 7:425–436.
- Fanguy RC, Misra LK, Vo, KV. 1980. Effect of delayed placement on growth performance of commercial broilers. Poultry Sci. 59:1215–1220.
- Gonzales E, Kondo N, Saldanha ESPB, Loddy MM, Careghi C, Decuypere E. 2003. Performance and physiological parameters of broilers chickens subjected to fasting on the neonatal period. Poultry Sci. 82:1250–1256.
- Hamdy AM, Henken AM, Van Der Wel H, Galal AG, Abdelmoty AK. 1991. Effects of incubation humidity and hatching time on heat tolerance of neonatal chicks: growth performance after heat exposure. Poultry Sci. 70:1507–1515.
- Hill D. 2001. Chick length uniformity profiles as a field measurement of chick quality? Avian Poultry Biol Rev. 12:188.
- Hudson BP, Fairchild BD, Wilson JL, Dozier WA, Buhr RJ. 2004. Breeder age and zinc source in broiler breeder hen diets on progeny characteristics at hatching. J Appl Poultry Res. 13:55–64.
- Lapao C, Gama LT, Soares, MC. 1999. Effects of broiler breeder age and lengh of egg storage on albumen characteristics and hatchability. Poultry Sci. 78:640–645.
- Mahmoud KZ, Edens FW. 2012. Breeder age affects small intestine development of broiler chicks with immediate or delayed access to feed. Brit Poultry Sci. 53:32–41.
- Meijerhof R. 2009a. The influence of incubation on chick quality and broiler performance. In: Proceedings of 20th Annual Australian Poultry Science Symposium, 2009 Feb 9–11; Sidney (Australia): Poultry Research Foundation Faculty of Veterinary Science, University of Sydney Camden NSW; p. 167–170.
- Meijerhof R. 2009b. Incubation principles: what does the embryo expect from us? In: Proccedings of 20th Annual Australian Poultry Science Symposium, 2009 Feb 9–11; Sidney (Australia); p. 106–111.
- Mikec M, Bidin Z, Valentic A, Savic V, Zelenika TA, Raguz-Duric R, Novak IL, Balenovic M. 2006. Influence of environmental and nutritional stressors on yolk sac utilization, development of chicken gastrointestinal system and its immune status. World’s Poultry Sci J. 62:31–40.
- Molenaar R, Hulet R, Meijerhof R, Maatjens CM, Kemp B, Brand HVD. 2011. High eggshell temperatures during incubation decrease growth performance and increase the incidence of ascites in broiler chickens. Poultry Sci. 90:624–632.
- Murakami H, Akiba Y, Horiguchi M. 1992. Growth and utilisation of nutrients in newly-hatched chick with or without removal of residual yolk. Growth Develop Aging 56:75–84.
- Nitsan Z. 1995. The development of digestive tract in posthached chicks. 10th European Symposium on Poultry Nutrition; 1995; Antalya. Turquia; p. 21–28.
- Noy Y, Sklan D. 1997. Posthatch development in poultry. J Appl Poultry Res. 6:344–354.
- Noy Y, Sklan D. 1999. Energy Utilization in Newly Hatched Chicks. Poultry Sci. 78:1750–1756.
- Palo PE, Sell JL, Piquer FJ. 1995. Effect of early nutrient restriction on broiler chickens. 1. Performance and development of the gastrointestinal tract. Poultry Sci. 74:88–101.
- Pedroso AA, Stringhini JH, Leandro NSM, Xavier AS, Lima FG, Barbosa CE. 2005. Desempenho e biometria de órgãos digestórios de frangos provenientes de matrizes jovens após diferentes intervalos de alojamento [Interval between removal to hatchery and lodgment of chicks with different weights provenient from young breeders]. Revista Brasileira de Ciência Avícola 3(supl):5.
- Reis LH, Gama LT, Soares MC. 1997. Effects of short storage conditions and broiler age on hatchability, hatching time, and chick weights. Poultry Sci. 76:1459–1466.
- Romanini CEB, Exadaktylos V, Tong Q, McGonnel I, Demmers TGM, Bergoug H, Eterradossi N, Roulston N, Garain P, Bahr C, Berckmans D. 2013. Monitoring the hatch time of individual chicken embryos. Poultry Sci. 92:303–309.
- SAS Institute 1998. SAS user’s guide: A user's guide to SAS. Cary (NC): SAS Institute Inc.
- Suarez ME, Wilson HR, Mather FB, Wilcox CJ, McPherson BN. 1997. Effect of strain and age of the broiler breeder female on incubation time and chick weight. Poultry Sci. 76:1029–1036.
- Tona K, Bamelis F, Coucke W, Bruggeman V, Decuypere E. 2001. Relationship between broiler breeder’s age and egg weight loss and embryonic mortality during incubation in large-scale conditions. J Appl Poultry Res. 10:221–227.
- Tona K, Bamelis F, Ketelaere BD, Bruggeman V, Moraes VMB, Buyse J, Onagbesan O, Decuypere E. 2003. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poultry Sci. 82:736–741.
- Tona K, Onagbesan OM, Jego Y, Kamers B, Decuypere E, Bruggeman V. 2004. Comparison of embryo physiological parameters during incubation, chick quality, and growth performance of three lines of broiler breeders differing in genetic composition and growth rate. Poultry Sci. 83:507–513.
- Van De Ven LJF, Baller L, Vand Wagenberg AV, Kemp B, Vand Den Brand, H. 2011. Effects of egg position during late incubation on hatching parameters and chick quality. Poultry Sci. 90:2342–2347.
- Vargas FSC, Baratto TR, Magalhaes FR, Maiorka A, Santin E. 2009. Influences of breeder age and fasting after hatching on the performance of broilers. J Appl Poultry Res. 18:18–24.
- Vieira SL, Almeida JG, Lima AR, Conde ORA, Oimos, AR. 2005. Hatching distribution of eggs varying in weight and breeder age. Braz J Poultry Sci. 7:73–78.
- Vieira SL, Pophal S. 2000. Nutrição pós-eclosão em frangos de corte [Post-hatch nutrition of broiler chickens]. Revista Brasileira Ciência Avícola 2:189–286.
- Wilson HR. 1991a. Effect of egg size on hatchability, chick size, and posthatching growth. In: Tullett, SG, editor. Avian incubation. Surrey: Butterworth-Heinemann; p. 279–283.
- Wilson HR. 1991b. Interrelationship of egg size, chick size, posthatching growth, and hatchability. World’s Poultry Sci J. 47:5–20.
- Wolanski NJ, Luiten EJ, Meijerhof R, Vereijken ALJ. 2004. Yolk utilisation and chick length as parameters for embryo development. Avian Poultry Biol Rev. 15:233–234.
- Wyatt CL, Weaver WDJ, Beane WL. 1985. Influence of egg size, eggshell quality, and posthatching holding time on broiler performance. Poultry Sci. 64:2049–2055.
- Yalcin S, Izzetoglu GT, Aktas A. 2013. Effects of breeder age and egg weight on morphological changes in the small intestine of chicks during the hatch window. Brit Poultry Sci. 54:810–817.
