Abstract
A four-year-old Akkaraman ewe was presented to the Firat University Veterinary Teaching Hospital for circling, dullness, weakness and sternal recumbency. On physical examination, opisthotonus, mydriasis in the right eye and myosis in the left eye, absence of the pupillary reflex, ruminal tympany were detected. Serum pituitary hormone analyses indicated the hypopituitarism characterized by thyroid stimulating hormone (TSH) < 0.05 IU/L, follicle stimulating hormone (FSH) < 0.13 IU/L, luteinizing hormone (LH) < 0.10 IU/L and prolactin (PRL) < 0.50 ng/ml. On the basis of clinical presentation and hypopituitarism, tentative diagnosis of pituitary abscess was made. The animal was euthanized due to the poor prognosis. Morphological examination revealed coexistence of hypophyseal-carotid rete abscess and hyaline thrombosis in carotid rete, heteropic ossification in carotid rete and tuberal muscles. Microbiologically; Arcanobacterium pyogenes was isolated in blood agar culture from the hypophysis. Decreased serum pituitary hormone levels including TSH, FSH, LH and PRL in conjunction with the clinical findings were a useful diagnostic tool for antemortem diagnosis of pituitary abscess in the sheep.
1. Introduction
Abscess in the pituitary fossa has been reported as a rare and sporadic entity in ruminants (Taylor & Meads Citation1963; Morikawi et al. Citation1973; Espersen & Moller Citation1975; Perl et al. Citation1978; Fernandes et al. Citation2000; Yeruham et al. Citation2002; Rech et al. Citation2006; Guarana et al. Citation2009). The pathogenesis of the pituitary abscess formation is uncertain. The pituitary abscess may arise from haematogenous or lymphatic spread, particularly from a primary source of infection or by direct extension of adjacent infection (Espersen & Moller Citation1975; Fernandes et al. Citation2000; Dutta et al. Citation2006; Camara et al. Citation2009; Guarana et al. Citation2009). The possibility of haematogenous spread is postulated as more common in ruminants and pigs due to the carotid rete surrounding the hypophysis consisting of the complex mesh of arterial and capillary system (Taylor & Meads Citation1963; Espersen & Moller Citation1975; Yeruham et al. Citation2002; Rech et al. Citation2006). History and clinical presentation of pituitary abscess are highly variable and there is no laboratory test (i.e. serum pituitary hormone levels) which will be able to aid in antemortem diagnosis of the disease in ruminants. Consequently, definitive diagnosis is usually made at necropsy (Morikawi et al. Citation1973; Yeruham et al. Citation2002; Rech et al. Citation2006). The aim of this case presentation is to report some serum pituitary hormone levels in a sheep with pituitary abscess.
2. Materials and methods
A four-year-old Akkaraman ewe was referred to the Internal Medicine Clinic, Veterinary Teaching Hospital, Firat University. History revealed that the ewe had been circling around herself for about the past six or seven days prior and at the same day, she was down and unable to rise. The ewe was on pasture with approximately 100 other sheep. None of the other sheep had been affected noticeably.
Blood samples were collected from the jugular vein of the ewe into a tube with ethylenediamine tetraacetic acid (EDTA) and a plain tube without anticoagulant for haematological, biochemical and hormonal analyses, respectively. Sera were obtained by centrifugation of at 2000g at 4°C for 10 minutes of the plain blood tube. The biochemical analysis was conducted using an automated analyzer (Dimension ARX, Dade Behring). Serum pituitary hormone levels including thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), luteinizing hormone (LH) and prolactin (PRL) were determined by using Biomerieux Mini VIDAS automated system.
The ewe was euthanized due to poor prognosis and the request of animal owner. Following the gross examination, the representative tissue samples from the hypophysis, brain, carotid rete, cranial nerves, eyes and visceral organs were fixed in 10% formalin solution, processed routinely, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin. Selected sections of the hypophysis were also stained with Gomori methenamine silver, periodic acid–Schiff, Gram and Ziehl–Neelsen stains.
3. Results
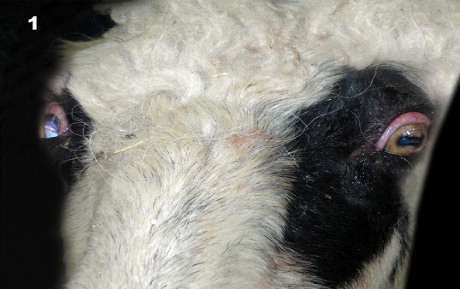
Clinical examination revealed the findings of sternal recumbency and ruminal tympany, absence of the pupillary reflex in eyes, opisthotonus and mydriasis in the right eye and myosis in the left eye (). Body temperature was 36.9°C, pulse-rate was 88 beats/min and ruminal pH was 7.0.
The results of haematological and biochemical examinations were as follows: white blood cells (×103/µL) 19 (reference range: 4–12), red blood cells (×106/µL) 11 (reference range: 9–15), haemoglobin (g/dL) 12 (reference range: 9–15), packed cell volume (%) 38 (reference range: 27–45), and fibrinogen (g/dL) 0.4 (reference range: 0.1–0.5), glucose (mg/dL) 110 (reference range: 50.0–80.0), blood urea nitrogen (mg/dL) 7 (reference range: 10.3–26.0), creatine kinase U/L 493 (reference range: 7.7–101.0), aspartate aminotransferase U/L 95 (reference range: 49.0–123.3), alanine aminotransferase U/L 23 (reference range: 14.8–43.8), alkaline phosphatase U/L 44 (reference range: 26.9–156.1), gamma glutamyl transferase U/L 93 (reference range: 19.6–44.1), total proteing/dL 7.2 (reference range: 5.9–7.8), total bilirubin (mg/dL) 0.4 (reference range: 0.1–0.5) and direct bilirubin (mg/dL) 0.1 (reference range: 0–0.2; Franks Citation1998).
The results of serum pituitary hormone levels were as follows: TSH < 0.05 IU/L (reference range: 0.11–0.14 IU/L), FSH < 0.13 IU/L (reference range: 0.58–0.72 IU/L), LH < 0.10 IU/L (reference range: 0.20–0.63 IU/L) and PRL < 0.50 ng/ml (reference range: 50–500 ng/ml; Kaneko et al. Citation2008).
On the basis of the above-mentioned clinical and laboratory findings, hypopituitarism was diagnosed. A tentative clinical diagnosis of pituitary abscess was also made.
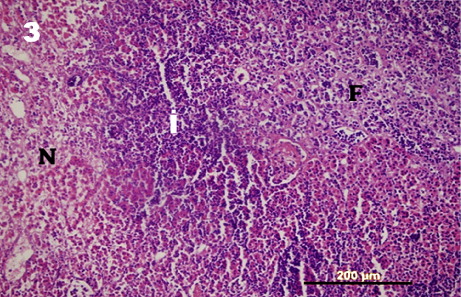
The post-mortem examination revealed that at the base of the skull yellow gelatinous edema accumulation and a swollen appearance of the upper surface of the duramater covering the hypophysis was discovered (). Upon cutting the dorsal view of the diaphragmatic aperture, there was about 5 ml of slightly turbid, brown-tinged exudate accumulation and hypophysis was embedded in the exudate. The parahorizontal cut surfaces of the gland had multifocal, haphazardly distributed gray to white, pinpoint sized foci of abscesses in parenchymal substance. The swabs sampling from inflammatory exudate on dorsal surface of the aperture applied into blood agar plates. There were no remarkable gross lesions in other organs or tissues or sinuses except for mild nasal conchal hyperemia.
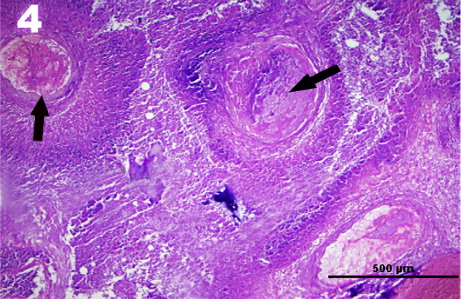
Histological findings showed that there was a small percentage of intact adenohypophyseal tissue remaining and most of the gland margins were mostly covered by a pyogenic membrane measuring from 100 to 1200 μm. The membrane also contained mild, focal mononuclear cell infiltrations and capillary proliferations. Most of the adenohypophyseal tissue showed the evidence of abscess formation, chronic suppurative hypophysitis, diffuse coagulation necrosis and fibrotic areas (). The abscess contained a mixture of neutrophils and lymphoplasmacytic infiltrations, and the pituitary gland also showed the areas of fibrosis and interspaced with small island of the normal gland. There was numerous intra-lesional cocoid, Gram-positive bacterial colonies that were haphazardly distributed throughout the necrotic areas. Neurohypophyseal tissue showed mild to moderate edema.
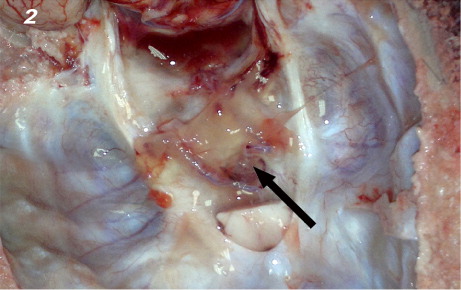
In carotid rete; there was severe thrombosis involving arteries, arterioles and capillaries (). Some of the thrombi were organized by fibrosis or hyalinized. The prominent perivascular mononuclear cell infiltrations and moderate to severe perivascular fibrosis were the other lesions detected.
There was a moderate focal lymphocytic neuritis and focally prominent disintegrations in the oculomotor nerves in longitudinal sections, whereas no other microscopic lesions were detected in other cranial nerves.
Microbiologically, Arcanobacterium pyogenes was isolated purely from inflammatory exudate from hypophysis in blood agar culture.
4. Discussion
The differential diagnosis for such clinical signs in sheep and goats includes pituitary abscess, listeriosis, peripheral vestibular lesion, polioencephalomalacia, coenurosis, encephalitis, meningitis, cerebral tumour and other brain abscess (Radostits et al. Citation1994; Constable Citation2004; Morin Citation2004). History and clinical presentation are highly variable to differentiate pituitary abscesses from these diseases. Also, there is no laboratory test (i.e. serum pituitary hormone levels) which will be able to aid in antemortem diagnosis of the disease in ruminants. Consequently, definitive diagnosis is usually made at necropsy (Morikawi et al. Citation1973; Yeruham et al. Citation2002; Morin Citation2004; Rech et al. Citation2006). In this case, decreased levels of TSH, FSH, LH and PRL allowed us to diagnose the hypopituitarism. Hypopituitarism was very helpful to exclude the pituitary abscess from the list of potential diagnoses when evaluated in conjunction with the clinical presentation in this case.
Concurrent occurrence of hypophyseal-carotid rete abscess formation and thrombosis in carotid rete were diagnosed based on the necropsy and histological results. The histological diagnosis of the abscess was based on the presence of pyogenic membrane, intra-lesional coccoid bacterial colonies and necro-suppurative changes. Microbiological isolation of A. pyogenes is consistent with those of earlier reports in the cows (Taylor & Meads Citation1963; Espersen & Moller Citation1975; Perl et al. Citation1978; Fernandes et al. Citation2000; Yeruham et al. Citation2002; Morin Citation2004; Dutta et al. Citation2006; Rech et al. Citation2006; Camara et al. Citation2009; Guarana et al. Citation2009), however the other micro-organisms including Corynebacterium sp. and Staphylococcus aureus were isolated in ovine cases (Guarana et al. Citation2009), and Corynebacterium pyogenes was isolated in hypophysis (Perl et al. Citation1978) and rete mirable (Morikawi et al. Citation1973) in cattle. High incidence of pituitary abscess with the use of a controlled suckling device to the nose in calves has been postulated by occurrence of rhinitis and secondary dissemination of the organisms, A. pyogenes, to the hypophysis (Fernandes et al. Citation2000). As abscess formation or suppurative inflammation was not present in other tissues examined, the primary source of the infection could not be determined in the present case as in some of the earlier reports in cattle (Espersen & Moller Citation1975; Perl et al. Citation1978; Dutta et al. Citation2006) and sheep (Guarana et al. Citation2009).
To the authors' knowledge, concurrent occurrence of the abscess in the carotid rete and hypophysis has not been reported earlier. The abscess together with the vascular thrombosis and presence of more chronic lesions in carotid rete rather support the earlier theory of bacterial embolism from other unidentified chronic source of infection. Furthermore, the isolation of A. pyogenes might also be indicative for hematogenous spread (Fernandes et al. Citation2000).
5. Conclusion
In conclusion, decreased serum pituitary hormone levels including TSH, FSH, LH and PRL in conjunction with the clinical findings were a useful diagnostic tool for antemortem diagnosis of pituitary abscess in the sheep.
References
- Camara ACL, Borges JRJ, Godoy RF, Moscardini ARC, Mustafa VS, Castro MB, Ximenes FHB, Paludo GR, Perecmanis S, Drummond VO. 2009. Pituitary abscess syndrome in calves from Mid-Western Brazil. Pesquisa Vet Brasil. 29:925–930.
- Constable PD. 2004. Clinical examination of the ruminant nervous system. Vet Clin N Am Food Anim Pract. 20:185–214. 10.1016/j.cvfa.2004.02.011
- Dutta P, Bhansali A, Singh P, Kotwal N, Pathak A, Kumar Y. 2006. Pituitary abscess: report of four cases and review of literature. Pituitary. 9:267–273. 10.1007/s11102-006-8327-z
- Espersen G, Moller T. 1975. A hypophysis-abscess-syndrome in cattle III. pathogenesis (author's transl). Nord Vet Med. 27:627–632.
- Fernandes CG, Schild AL, Riet-correa F. 2000. Pituitary abscess in young calves associated with the use of a controlled suckling device. J Vet Diagn Invest. 12:70–71. 10.1177/104063870001200114
- Franks PT. 1998. Referance guides. In: Aiello SE, editor. Merck veterinary manual. Philadelphia (PA): Merck and Co. Inc; p. 2188–2197.
- Guarana ELS, Junior PLJM, Rego RO. 2009. Pituitary abscess syndrome in sheep: case reports. Cienc Anim Brasil. 10:152–157.
- Kaneko JJ, Harvey JW, Bruss ML. 2008. Appendix. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. Amsterdam: Elsevier; p. 873–904.
- Morikawi M, Watase H, Fukumoto M, Hayashi IS. 1973. Exophthalmos due to rete mirabile abscess caused by infection with Corynebacterium pyogenes in cattle. Natl I Anim Health Q. 13:14–22.
- Morin DE. 2004. Brainstem and cranial nerve abnormalities: listeriosis, otitis media/interna, and pituitary abscess syndrome. Vet Clin N Am-Food Anim Pract. 20:243–273. 10.1016/j.cvfa.2004.02.007
- Perl S, Nyska A, Tromp A. 1978. Abscess in the hypophysis of a cow. Refuah Vet. 35:175–176.
- Radostits OM, Blood DC, Gay CC. 1994. Diseases of the nervous system. In: Radostits OM, Blood DC, Gay CC, editors. Veterinary medicine. London: Ballie´re Tindall; p. 458–505.
- Rech RR, Rissi DR, Silva MC, Inkelmann MA, Barros CSL. 2006. Histomorphology of gasserian ganglion, carotid rete mirable and pituitary gland in cattle: a study of 199 cases. Pesquisa Vet Brasil. 2:105–111.
- Taylor PA, Mead EB. 1963. Pituitary abscessation and a ‘Farcy Like’ condition in cattle due to Corynebacterium pyogenes. Can Vet J. 8:208–213.
- Yeruham I, Orgad U, Avidar Y, Elad D. 2002. Pituitary abscess and high urea concentration as causes of neurological signs in a cow. Rev Méd Vét. 153:829–831.