Abstract
The effect of cysteine addition to the freezing extender on the progressive motility, viability, plasma membrane and DNA integrity of buffalo bull spermatozoa was studied. Semen from three Nili-Ravi buffalo bulls of similar age group was collected with artificial vagina. Qualifying ejaculates were split in three aliquots for dilution (50 × 106 spermatozoa ml–1) with tris-citric acid extender containing either 0.0, 0.5, 1.0 mM cysteine. Extended semen samples were cooled and equilibrated before cryopreservation. Progressive motility, plasma membrane integrity, viability and DNA integrity were assessed at 0, 2 and 4 hours post-thaw incubation (37°C). Sperm progressive motility and plasma membrane integrity of buffalo bull spermatozoa were higher (P < 0.05) in extender containing 1.0 mM cysteine than those with 0.5 mM or 0.0 mM at 0, 2 and 4 hours post-thaw. Sperm viability and DNA integrity were higher (P < 0.05) in extender containing 0.5 mM and 1.0 mM cysteine than those with 0.0 mM cysteine at 0, 2 and 4 hours post-thaw. The in vivo fertility rates were similar (P > 0.5) with semen cryopreserved in extender containing cysteine (1.0 mM) compared to control. It is concluded that addition of 1.0 mM cysteine to the tris-citric acid extender improved the post-thaw in vitro quality of buffalo bull spermatozoa.
1. Introduction
Cryopreservation is associated with oxidative stress that reduces the viability and fertility of buffalo bull semen (Kumar et al. Citation2011). Oxidative stress during freeze–thawing process accelerates the production of reactive oxygen species (ROS) due to higher lipid peroxidation levels of plasma membrane (Sansone et al. Citation2000). Sperm membrane of buffalo bull has higher levels of polyunsaturated phospholipids compared to bull spermatozoa that make it more sensitive to freeze–thawing stress (Andrabi Citation2009). ROS molecules produced during cryopreservation damage motility, plasma membrane integrity, viability, acrosome and DNA integrity of buffalo semen (Kadirvel et al. Citation2009).
Buffalo semen has its endogenous protection system consisting of enzymatic (catalase, glutathione peroxidase, superoxide dismutase) and non-enzymatic (vitamin C, E, glutathione, cysteine) antioxidants against ROS molecules (Andrabi Citation2009). During cryopreservation higher concentrations of ROS molecules are produced and this system becomes insufficient for the protection of buffalo sperm (Kadirvel et al. Citation2009). It is also known that freeze–thawing cycle reduces the level of endogenous antioxidants of the mammalian semen (Stradaioli et al. Citation2007). This is the reason, supplementation of suitable antioxidants is being explored for improving quality and fertility of cryopreserved buffalo semen (Ansari et al. Citation2010, Citation2011).
Cysteine is a sulphur-containing amino acid, naturally found in seminal plasma and sperm nucleic acid, that maintains the integrity of the DNA and also acts as an antioxidant directly and/or indirectly through intracellular antioxidants that protect from ROS-mediated deleterious effects (Perumal et al. Citation2011; Topraggaleh et al. Citation2014). Being a precursor of intracellular glutathione biosynthesis, cysteine increases glutathione level. It is relevant to mention that, a significant reduction in the level of endogenous antioxidants during freeze–thawing of the bovine semen has been reported (Bilodeau et al. Citation2000; Stradaioli et al. Citation2007; Beheshti et al. Citation2011). Therefore, the present study was designed to study the effect of cysteine addition to the freezing extender on progressive motility, plasma membrane integrity, viability and acrosome status, DNA integrity and fertility of Nili-Ravi buffalo bull spermatozoa.
2. Materials and methods
2.1. Experimental semen extenders
The stock extender consisted of 1.56% citric acid (Fisher Scientific, UK), 3.0% tris– (hydroxymethyl)-aminomethane (Research Organics, USA), 0.2% fructose (Scharlau, Spain), 7% glycerol (Riedel-deHaen, Germany) and 20% egg yolk in distilled water. The pH of the extender was 7.0 and the osmotic pressure was ~320 mOsmol kg–1. Three experimental extenders were prepared by adding cysteine (Sigma, ST Louis USA No. C-8755) at the concentration of 0.0, 0.5 and 1.0 mM in stock extender.
2.2. Collection and initial evaluation of semen
Two consecutive semen ejaculates were collected from three Nili-Ravi buffalo breeding bulls (Bubalus bubalis) with artificial vagina at weekly intervals for three weeks (three replicates), and immediately transferred to the laboratory for initial evaluation (volume, motility, concentration). Sperm motility was assessed (200X) with phase contrast microscope. Sperm concentration was measured with bovine photometer ACCUCELL® (IMV, France). Qualifying semen ejaculates (motility > 60%, volume > 3.0mL, concentration > 0.5 × 109/mL) were split in three aliquots for dilution with different experimental extenders at 37°C.
2.3. Cryopreservation of semen
Semen aliquots were diluted with one of three experimental extenders to obtain concentration 50 × 106 mL–1 of motile spermatozoa. The diluted semen was cooled to 4°C within 2 hours and equilibrated for 4 hours at the same temperature. After equilibration, semen was filled in 0.5mL French straws with suction pump at 4°C in the cold cabinet and kept on liquid nitrogen vapours for 10 minutes 5 cm above the level of liquid nitrogen. Then straws were plunged into liquid nitrogen for storage. Straws were thawed at 37°C for 30 seconds in water bath and maintained at 37°C for up to 4 hours. Progressive motility, plasma membrane integrity, viability & acrosome status and DNA integrity assays were performed at 0, 2 and 4 hours of incubation (37°C).
2.4. Sperm motility
Sperm motility was assessed by placing a drop of semen on pre-warmed (37°C) glass slide, by phase contrast microscopy (400X).
2.5. Sperm plasma membrane integrity
Sperm plasma membrane (functional and structural) integrity of buffalo spermatozoa was assessed with supravital hypo-osmotic swelling test (HOST). The assay was performed by mixing 50 µL of the semen samples with 500 µL of the HOS solution (37°C) and incubated at 37°C for 30–40 minutes. The HOST solution (osmotic pressure ~190 mOsm/kg) contained sodium citrate (Merck, Germany) 0.735 g and fructose (Riedel-DeHaen, Switzerland) 1.351 g dissolved in 100 mL of distilled water. After incubation, an aliquot (5 μL) of the HOST-semen solution was placed on a warm slide and a droplet (5 μL) of Eosin [0.5% (w/v) in sodium citrate 2.9%] was mixed for 10 seconds. A cover slip was placed on the mixture and evaluated with phase contrast microscopy (400X). A total of 100 spermatozoa per preparation were observed in at least five different fields (Ansari et al. Citation2010, Citation2011). Clear heads and tails and swollen tails indicated intact, biochemically active sperm membranes, while pink heads and tails and unswollen tails indicated disrupted, inactive sperm membranes (Tartaglionea & Ritta Citation2004).
2.6. Sperm viability and acrosome status
Sperm viability (live sperm with intact acrosome) of buffalo bull spermatozoa was assessed by dual staining procedure with Trypan blue-Giemsa stain as described by Kovács and Foote (Citation1992). The dual staining procedure includes the use of the supravital stain Trypan blue to distinguish live and dead spermatozoa and Giemsa to evaluate the integrity of the acrosomal membrane. Equal drops of Trypan-blue and semen samples were placed on a glass slide and quickly mixed. Smears were air-dried, and slides were fixed with formaldehyde-neutral red (86 mL 1M HCl + 14 mL 37% formaldehyde + 0.2 g neutral red) for 5 minutes. Then slides were rinsed with distilled water, and 7.5% Giemsa stain was applied for 4 hours. After rinsing and air-drying, cover slips were mounted with Balsam of Canada and 100 spermatozoa were evaluated in at least five different fields in each smear by phase contrast microscope at 1000X. Trypan-blue penetrates non-viable, dead spermatozoa with disrupted membrane, which appeared stained in blue, while live spermatozoa with intact membrane appeared unstained. Giemsa accumulates in spermatozoa with an intact acrosome, staining the acrosome region in purple (Tartaglionea & Ritta Citation2004).
2.7. Sperm DNA integrity
Sperm DNA integrity was assessed by acridine orange assay described by Tejada et al. (Citation1984) and Martins et al. (Citation2007). Smears were prepared on glass slides from each semen sample and air-dried. The smears were fixed overnight in Carnoy's solution (freshly prepared, methanol and glacial acetic acid; 3:1). The slides were air-dried again, and incubated in buffer solution (80 mM citric acid and 15 mM Na2HPO4, pH 2.5) at 75°C for 5 minutes. Then slides were stained with acridine orange (0.2 mg/mL). Stained slides were washed with water to remove background staining; while still wet, the slides were covered with cover slips and evaluated with an epifluorescence microscope (480/550 nm excitation/barrier filter). One hundred cells were analysed of each semen sample cryopreserved in different experimental extenders. Sperm with normal DNA content presented green whereas those with an abnormal DNA content presented fluorescence that varied from yellow-green to red in spectrum.
2.8. In vivo fertility rate
Semen from two buffalo bulls was cryopreserved in extender containing cysteine 1.0 mM and 0.0 mM as described in earlier section. The inseminations were performed in two months during the peak of breeding season under field conditions, approximately 24 hours after onset of heat in buffaloes with previous normal parturition. Fertility rate was recorded by examining 200 artificially inseminated animals through rectal palpation at least 60 days post-insemination.
2.9. Data analysis
The data on the effect of cysteine addition to the extender on the progressive motility, plasma membrane integrity, viability and acrosome status DNA integrity were analysed by the analysis of variance in two-factor factorial (treatment × hour) randomized complete block design. When the F-ratio found significant (P < 0.05), Duncan multiple range test was used, to compare different treatment means. The data on in vivo fertility rate were analysed using chi-square test (MINITAB® Release 12.22, 1998).
3. Results
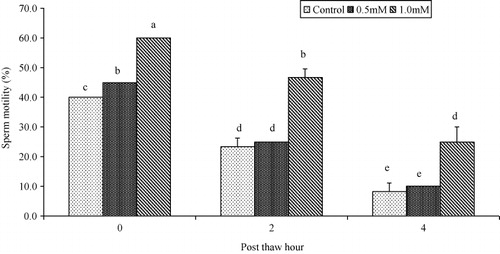
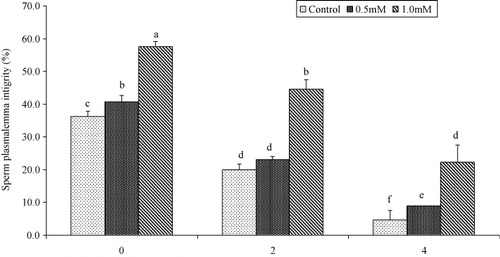
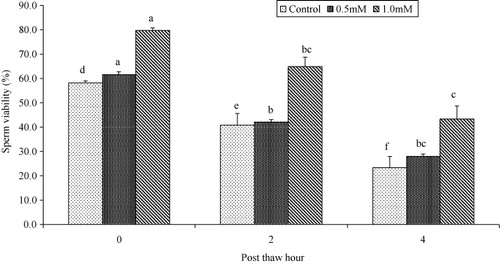
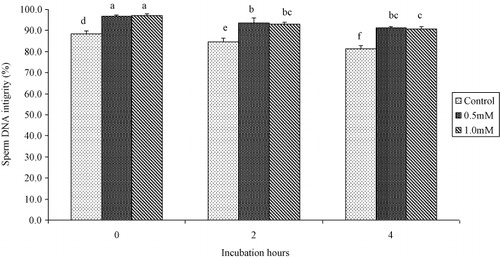
Progressive motility and plasma membrane integrity of buffalo bull spermatozoa were higher (P < 0.05) in extender containing 1.0 mM cysteine than those with 0.5 mM or 0.0 mM at 0, 2 and 4 hours post-thaw at 37°C (as shown in and ). Sperm viability was higher (P < 0.05) in extender containing 0.5 mM and 1.0 mM cysteine than those with 0.0 mM cysteine at 0, 2 and 4 hours post-thaw at 37°C (as shown in ). Sperm DNA integrity of buffalo bull spermatozoa was higher (P < 0.05) in extender containing 0.5 mM and 1.0 mM cysteine than those with 0.0 mM at 0, 2 and 4 hours post-thaw at 37°C (as shown in ).
The fertility rate was higher in extender containing cysteine 1.0 mM compared to the control (). However, improvement in fertility rate did not get statistical level of significance in Bull 1 (P = 0.089) and 2 (P = 0.119).
Table 1. Effect of cysteine addition in extender on in vivo fertility rate of buffalo bull semen.
4. Discussion
This study was designed to determine the effect of cysteine addition in freezing extender on the progressive motility, viability plasma membrane and DNA integrity of buffalo bull spermatozoa. It is known that in vitro assessment of longevity is made to predict the survivability of spermatozoa in female reproductive tract (Ansari et al. Citation2010, Citation2011). Therefore, the semen quality tests in this study were performed after thawing at 0, 2 and 4 hours of incubation at 37°C to determine the longevity of semen cryopreserved in different experimental extenders.
In the present study, progressive motility of buffalo bull sperm was high when the extender was supplemented with 1.0 mM cysteine than those with 0.0 mM and 0.5 mM cysteine at 0, 2 and 4 hours post-thaw. Our results are inline with the previous studies on cryopreserved bovine semen which reported higher sperm progressive motility with extender containing 1.0 mM cysteine to reduce oxidative stress (Bilodeau et al. Citation2001). In a recent study on Nili-Ravi buffalo semen an improvement in progressive motility has been observed after the addition of 1.0 mM cysteine in tris-citric acid extender (Ansari et al. Citation2011). It is believed that cysteine improves the progressive motility of buffalo bull spermatozoa by reducing the ROS levels (Alvarez & Storey Citation1983) in the semen-extender complex (El-Sheshtawy et al. Citation2008), which are responsible for inducing lipid peroxidation of bio-membrane system that is associated with sperm motility during the freeze–thawing cycle (Urata et al. Citation2001).
The sperm plasma membrane has a critical role in the fertilization process. In the present study, the percentage of plasma membrane integrity of buffalo bull spermatozoa was higher in extender containing 0.5 mM and 1.0 mM cysteine than those containing cysteine 0.0 mM at 0, 2 and 4 hours post-thaw. El-Sheshtawy et al. (Citation2008) reported a significantly higher percentage of sperm with intact plasma membrane after freeze–thawing with tris-citric acid diluent supplemented with cysteine (5 mM) in Egyptian buffalo semen. Similarly, higher percentage of sperm with functional plasma membrane was observed in bovine (Sariözkan et al. Citation2009) and ovine (Uysal & Bucak Citation2007) semen frozen-thawed in Bioxcell® and tris-based diluent added with 2.0 mM and 5.0 mM cysteine. It is suggested that cysteine supplementation in extender protects the membrane integrity by scavenging the ROS molecules (Alvarez & Storey Citation1983), directly and/or indirectly in the semen-extender complex, which can destroy the sperm membrane during the cryopreservation process (Bucak et al. Citation2008).
Sperm viability and intact acrosome are essential to achieve capacitation, acrosomal reaction and finally fertilization (Kovács & Foote Citation1992; Tartaglionea & Ritta Citation2004). In the present study, viability of buffalo bull spermatozoa was higher in samples cryopreserved in tris-citric acid extender containing cysteine 0.5 mM and 1.0 mM compared with the control at 0, 2 and 4 hours post-thaw. In a recent study, higher intact acrosome percentage were found in cryopreserved buffalo bull sperm due to addition of cysteine 1.0 mM in tris-citric acid extender (Ansari et al. Citation2011). An improvement in the percentage of viable spermatozoa has been observed in ovine semen after addition of 5.0 mM cysteine in tris-citric acid extender (Bucak et al. Citation2008). Also an increase in the semen catalase activity (842.40 ± 90.42 vs. 738.81 ± 39.69 kU/I) was found (Bucak et al. Citation2008). It is suggested that cysteine 1.0 mM maintains the viability of buffalo bull spermatozoa by coping with ROS levels through increasing the intracellular activity of antioxidants (Bucak et al. Citation2008, Citation2010).
Sperm DNA integrity of buffalo bull semen was higher in extender containing cysteine 0.5 and 1.0 mM compared with the control at 0, 2 and 4 hours post-thaw. It is known that freeze–thawing induces DNA fragmentation in buffalo semen (Kumar et al. Citation2011) and results in reduced total antioxidant capacity. It is to believe that antioxidants have a protective role for sperm DNA during the process of freezing and thawing (Bucak et al. Citation2010) by reducing oxidative stress.
In the present study, non-significant results were obtained for the fertility rate of buffalo cows inseminated with semen cryopreserved in tris-citric acid extender containing cysteine 1.0 mM and 0.0 mM. In other study, similar (P > 0.05) fertility rates were recorded in cows inseminated with semen extended in tris-egg yolk and Bioxcell® containing 5.0 mM cysteine hydrochloride and 2.0 mM cysteine compared to control (Sariözkan et al. Citation2009; Perumal et al. Citation2011). Similarly, extender supplementation with 5.0 mM cysteine in Laiciphos® extender did not improve the conception rate in bovine (Tuncer et al. Citation2010).
5. Conclusion
It is concluded that addition of 1.0 mM cysteine to the tris-citric acid extender improved the in vitro quality of buffalo bull spermatozoa post-thawing.
Acknowledgements
The authors thank the Higher Education Commission of Pakistan for financial assistance under the scheme ‘Indigenous 5000 PhD Fellowship Program’.
References
- Alvarez JG, Storey BT. 1983. Role of superoxide dismutase in protecting rabbit spermatozoa from O2 toxicity due to lipid peroxidation. Biol Reprod. 28:1129–1136. 10.1095/biolreprod28.5.1129
- Andrabi SMH. 2009. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domestic Anim. 44:552–569. 10.1111/j.1439-0531.2008.01240.x
- Ansari MS, Rakha BA, Ullah N, Andrabi SMH, Iqbal S, Khalid M, Akhter S. 2010. Effect of exogenous glutathione in extender on the freezability of Nili-Ravi buffalo (Bubalus bubalis) bull spermatozoa. Anim Sci Pap Rep. 28:235–244.
- Ansari MS, Rakha BA, Ullah N, Andrabi SMH, Khalid M, Akhter S. 2011. Effect of L-cysteine in tris-citric egg yolk extender on post-thaw quality of Nili-Ravi buffalo (Bubalus bubalis) bull spermatozoa. Pak J Zool. 43:41–47.
- Bilodeau JF, Blanchette S, Gagnon C, Sirad MA. 2000. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev. 55:282–288. 10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7
- Bilodeau JF, Blanchette S, Gagnon C, Siard MA. 2001. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology. 56:275–286. 10.1016/S0093-691X(01)00562-3
- Beheshti R, Asadi A, Eshratkhah A, Ghalekandi JG, Ghorbani A. 2011. The effect cysteine on post-thawed buffalo bull (Bubalus bubali) sperm parameters. Adv Environ Biol. 5:1260–1263.
- Bucak MN, Atessahin A, Yüce A. 2008. Effect of anti-oxidants and oxidative stress parameters on ram semen after the freeze–thawing process. Small Ruminant Res. 75:128–134. 10.1016/j.smallrumres.2007.09.002
- Bucak MN, Tuncer PB, Sarıözkan S, Başpınar N, Taşpınar M, Çoyan K, Bilgili A, Akalın PP, Büyükleblebici S, Aydos S, et al. 2010. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: antioxidants protect DNA integrity against cryodamage. Cryobiology. 61:248–253. 10.1016/j.cryobiol.2010.09.001
- El-Sheshtawy RI, El-Sisy GA, El-Nattat WS. 2008. Use of selected amino acids to improves buffalo bull semen cryopreservation. Global Vetrinaria. 2:146–150.
- Kadirvel G, Satish K, Kumaresan A. 2009. Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim Reprod Sci. 114:125–134. 10.1016/j.anireprosci.2008.10.002
- Kovács A, Foote RH. 1992. Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotech Histochem. 67:119–24. 10.3109/10520299209110020
- Kumar R, Mohanarao GJ, Arvind, Atreja SK. 2011. Freeze–thaw induced genotoxicity in buffalo (Bubalus bubalis) spermatozoa in relation to total antioxidant status. Mol Biol Rep. 38:1499–1506. 10.1007/s11033-010-0257-1
- Martins CF, Báo SN, Dode MN, Correa GA, Rumpf R. 2007. Effects of freeze-drying on cytology, ultrastructure, DNA fragmentation, and fertilizing ability of bovine sperm. Theriogenology. 67:1307–1315. 10.1016/j.theriogenology.2007.01.015
- Perumal P, Selvaraju S, Selvakumar S, Barik A, Mohanty D, Das S, Das R, Mishra P. 2011. Effect of pre-freeze addition of cysteine hydrochloride and reduced glutathione in semen of crossbred Jersey bulls on sperm parameters and conception rates. Reprod Domestic Anim. 46:636–641. 10.1111/j.1439-0531.2010.01719.x
- Sansone G, Nastri MJF, Fabbrocini A. 2000. Storage of buffalo (Bubalus bubalis) semen. Anim Reprod Sci. 62:55–76. 10.1016/S0378-4320(00)00154-8
- Sariözkan S, Bucak MN, Tuncer BP, Ulutaş PA, Bilgen A. 2009. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology. 58:1334–1338.
- Stradaioli G, Noro T, Sylla L, Monaci M. 2007. Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: comparison between two extenders. Theriogenology. 67:1249–1255. 10.1016/j.theriogenology.2007.01.009
- Tartaglionea CM, Ritta MN. 2004. Prognostic value of spermatological parameters as predictors of in vitro fertility of frozen-thawed bull semen. Theriogenology. 62:1245–1252. 10.1016/j.theriogenology.2004.01.012
- Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S. 1984. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Sterility. 42:87–91.
- Topraggaleh, TR, Shahverdi A, Rastegarnia A, Ebrahimi B, Shafiepour V, Sharbatoghli M, Esmaeili V, Janzamin E. 2014. Effect of cysteine and glutamine added to extender on post-thaw sperm functional parameters of buffalo bull. Andrologia. 46:777–783. 10.1111/and.12148
- Tuncer PB, Bucak MN, Büyükleblebici S, Sarıözkan S, Yeni D, Eken A, Akalın PP, Kinet H, Avdatek F, Fidan AF, Gündogan M. 2010. The effect of cysteine and glutathione on sperm and oxidative stress parameters of post-thawed bull semen. Cryobiology. 61:303–307. 10.1016/j.cryobiol.2010.09.009
- Urata K, Narahara H, Tanakay E, Gashiru T, Takayama E, Miyakaw I. 2001. Effect of endotoxin-induced reactive oxygen species on sperm motility. Fertil Sterility. 76:163–166. 10.1016/S0015-0282(01)01850-7
- Uysal O, Bucak MN. 2007. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Veterinaria Brno. 76:383–390. 10.2754/avb200776030383
