Abstract
14-3-3σ is a cell cycle regulator that has been designed as a ‘double-edged sword’ in the context of human cancers, in which in some it appears to function as a tumour suppressor with decreased expression contributing to tumourigenesis, while in others it may be involved in tissue invasion and metastasis. A variety of studies in both human and canine species have been focused in the immunohistochemical detection of 14-3-3σ, especially in neoplastic diseases. Different commercial available antibodies have been previously used to detect 14-3-3σ in canine tissues. This paper shows important differences regarding the pattern of expression between monoclonal and polyclonal antibodies against this protein and proposes the use of the former in future studies of 14-3-3σ in canine species.
1. Introduction
14-3-3σ protein belongs to a highly conserved family of acidic proteins, the 14-3-3 family, which consists of at least seven mammalian isoforms. Of the seven isoforms, 14-3-3σ is especially linked to cancer. Most of the isoforms are expressed in all tissues, whereas the σ isoform has been restricted to the epithelial cells (Wilker et al. Citation2005; Huang et al. Citation2014). 14-3-3σ is a protein kinase-dependent activator tyrosine and endogenous inhibitor of protein kinase C; it plays a negative role in the G2/M checkpoint, pausing the cell cycle and allowing DNA repair. Loss of 14-3-3σ expression results in malignant transformation in vitro and supports tumour formation in vivo, which suggests its role as a tumour suppressor gene (Holm et al. Citation2009).
Several studies in both human and canine species have used immunohistochemical techniques to detect 14-3-3σ, especially in neoplastic diseases. However, the immunohistochemical procedures and the antibodies employed in each work vary greatly between authors (Nakajima et al. Citation2003; Holm et al. Citation2009). In veterinary science, it has been recently investigated the expression of σ isoform in normal and neoplastic canine tissues, showing that this protein is an epithelial cell marker and that its expression is altered in canine tumours (Suárez-Bonnet et al. Citation2010, Citation2011). Since polyclonal and monoclonal antibodies have different sensitivity and specificity to detect a given antigen (Holm et al. Citation2009), and the 14-3-3 family has at least seven isoforms, it would be of interest to study the expression of σ isoform using different antibodies in the same tissues.
In the present study, we aimed to compare the expression of 14-3-3σ protein in normal canine tissues using two different commercial available antibodies, in order to elucidate possible differences in terms of immunoreaction, which allows to select the most reliable antibody against the σ isoform.
2. Materials and methods
2.1. Immunohistochemical study
Normal canine tissues from several organs () were obtained from two dogs (one male and one female) that were euthanized in a municipal kennel (for reasons unrelated with this study). For the immunohistochemical study, serial tissue sections, 3 mm thick, were placed on Vectabond-coated slides (Sigma Diagnostics), de-paraffinated, rehydrated in a graded series of alcohol and incubated with 3% hydrogen peroxidase in methanol for 30 min. The sections were then subjected to high-temperature antigen retrieval by placing in citrate buffer (pH 6.2) in a water bath at 95°C for 25 min. After cooling, the slides were covered with 10% normal rabbit serum in phosphate-buffered saline (PBS) for 30 min before incubation with the primary antibodies. A goat polyclonal antibody specific for the N-terminus of the 14-3-3σ isoform (N-14; Santa Cruz Biotechnology; 1:50; 16 h at 4°C) and a mouse monoclonal antibody raised against recombinant 14-3-3σ of human origin (clone 5D7, Santa Cruz Biotechnology; 1:50; 16 h at 4°C) were used to detect σ isoform in canine tissues. Antigen–antibody reactions were visualized by the avidin–biotin peroxidase complex (ABC) method (Vector Laboratories, Burlingame, CA). As chromogen, we used 0.5% 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemical Co, St. Louis, MO) diluted 1:10 in 0.05M Tris containing 0.3% hydrogen peroxide, which was applied to the slides for 1–2 min at room temperature. Slides were counterstained with Harris hematoxylin. In each assay, samples of human lung squamous cell carcinoma were used as positive controls. Negative controls were prepared by replacing the primary antibody with normal rabbit serum diluted 1:100 in PBS.
Table 1. Reactivity patterns of polyclonal (N14) and monoclonal (5D7) antibody to 14-3-3σ in normal canine tissues.
2.2. Western blot analysis
To demonstrate the antibodies’ specificity homogenates of mammary gland, urinary bladder, salivary gland, oesophagus, skin, kidney, cerebrum, cerebellum and lymph node were analysed by Western blotting. Samples were homogenized in 0.1% Triton X-100 plus protease inhibitors in 25 mM Tris (pH 7.5) using a dispersing instrument (Ultra Turrax, Rose Scientific Ltd.), and cell debris was removed by centrifugation. The Bradford method (BioRad) was used to determine protein concentrations. SDS-PAGE was used to extract 60–80 µg of proteins prior to gels being transferred to nitrocellulose membranes (Pall Life Sciences). Protein transfer was confirmed by Ponceau Red staining (Sigma Diagnostics). The membranes were then ‘blocked’ with 5% powdered milk in Tris-buffered saline Tween 20 (TBS-T) for 1 h at room temperature and then incubated with anti-14-3-3σ antibodies (N-14 and clone 5D7; Santa Cruz Biotechnology). After rinsing in TBS-T, the membranes were incubated with a sheep anti-goat and a rabbit anti-mouse antibodies for 1 h at room temperature. Finally, the blots were rinsed and visualized using Immun-Star WesternC and BioRad Chemi-Doc imaging system (BioRad).
3. Results and discussion
The immunohistochemical results are summarized in . The expression of 14-3-3σ protein was largely restricted to cells of epithelial origin with a tendency to be stronger in cells destined for squamous epithelium or differentiating towards squamous cells, as it has been previously reported (Nakajima et al. Citation2003; Suárez-Bonnet et al. Citation2010). However, differences in the immunohistochemical expression of the protein were observed with the two employed antibodies. Neuroendocrine cells of adrenal medulla, pancreatic islet and neurons of ganglia showed moderate to strong positivity with the polyclonal N14 antibody. The immunohistochemical staining patterns in neuroendocrine cells of the adrenal medulla ( and ) and those of the islet of Langerhans ( and ) were homogenously cytoplasmic with no membrane reinforcement neither staining of the nuclei. The specimens of adrenal gland also contained a sample of ganglia, in which the soma of all neurons showed diffuse and homogeneously cytoplasmic precipitation of chromogen. In contrast with this pattern of staining, monoclonal 5D7 antibody did not produce positive immunoreaction in the tissues described above.
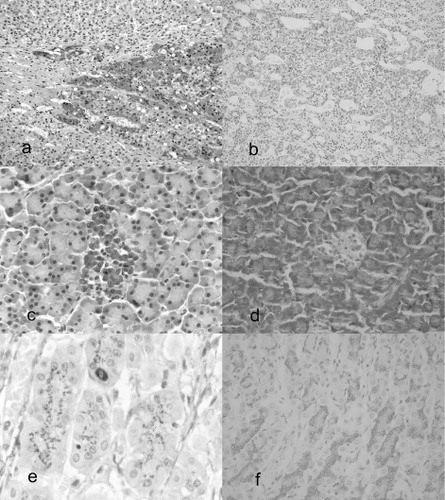
14-3-3σ is a specific epithelial cell marker in human and canine species (Nakajima et al. Citation2003; Suárez-Bonnet et al. Citation2010). Moreover, it is well known that 14-3-3 proteins are broadly expressed in the brain with modification of its distribution and expression during embryologic phases to mature and senescence (Humahara et al. Citation2011). The present study revealed that, when a polyclonal antibody (N14) is used to detect 14-3-3σ, a variety of cells with a nervous origin showed an unexpected positive immunostaining. It is possible that N14 cross-reacts with one or more isoforms of the 14-3-3 family, different from σ, that could be present in cells of nervous origin. It would be of potential interest to analyse the expression of the six remaining isoforms of 14-3-3 proteins, in order to determinate specifically their localization in canine tissues.
In the stomach, specifically in the oxyntic gland mucosa, N14 produced a supranuclear granular staining in both parietal and zymogenic cells. Besides, neuroendocrine cells located along the neck of the oxyntic gland mucosa showed a strong and homogeneous cytoplasmic stain ( and ). In small and large intestine, only neuroendocrine cells showed 14-3-3σ expression using the polyclonal N14 antibody, while the glandular tissue was negative. Monoclonal 5D7 did not produce immunostaining in stomach and small and large intestine. Correspondingly, N14 pattern in glandular cells of prostate gland displayed a supranuclear granular stain and a homogeneous cytoplasmic stain of ductal cells. In contrast, 5D7 showed mild and sporadic cytoplasmic expression in glandular cells with similar staining of duct cells. The stain of a supranuclear structure in glandular cells is a common and well-known non-specific cross-reaction with the Golgi apparatus (Ramos-Vara et al. Citation2008). In the current study, this type of chromogen precipitation produced by the polyclonal antibody N14 was abolished by the use of the mouse monoclonal antibody 5D7.
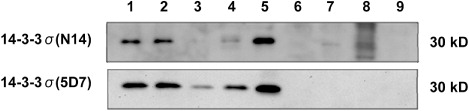
Western blot analysis revealed that both N14 and 5D7 antibodies recognized the 30 kDa polypeptide in mammary gland, urinary bladder, salivary gland, oesophagus and skin. N14 also produced a mild band with tissue of cerebellum. However, 5D7 produced a more defined and strong immunoreaction with a lesser background in all bands ().
4. Conclusions
The results of the present study highlight the need to validate human commercial antibodies for any particular veterinary immunohistochemical test. The differences observed between N14 and clone 5D7 should be taken into consideration in future studies about 14-3-3σ protein. In the current work, monoclonal 5D7 antibody has demonstrated a more accurate capability to detect 14-3-3σ, given that non-specific staining in non-epithelial cells was avoided and more defined bands were obtained in the Western blot analysis. We propose the use of clone 5D7 as the antibody of election in the detection of this protein in canine tissues.
Acknowledgement
The main author want to thank Dra. Elena Suárez-Bonnet for helping in the editing process.
References
- Holm R, Ali T, Svendsrud DH, Nesland JM, Kristensen GB, Lyng H. 2009. Expression of 14-3-3 sigma in cervical squamous cell carcinomas: relationship with clinical outcome. Oncol Rep. 22:11–15. 10.3892/or_00000399
- Huang HH, Chen CH, Huang SC, Yang CH, Hwang CF. 2014. Expression of 14-3-3 sigma, cyclin-dependent kinases 2 and 4, p16, and Epstein-Barr nuclear antigen 1 in nasopharyngeal carcinoma. J Laryngol Otol. 24:1–8.
- Nakajima T, Shimooka H, Weixa P, Segawa A, Motegi A, Jian Z, Masuda N, Ide M, Sano T, Oyama T, et al. 2003. Immunohistochemical demonstration of 14-3-3 sigma protein in normal human tissues and lung cancers, and the preponderance of its strong expression in epithelial cells of squamous cell lineage. Pathol Int. 53:353–360. 10.1046/j.1440-1827.2003.01481.x
- Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B, Chelack B, Czub S, Del Piero F, Dial S, Ehrhart EJ, Graham T, Manning L, Paulsen D, Valli VE, West K, American Association of Veterinary Laboratory Diagnosticians Subcommittee on Standardization of Immunohistochemistry 2008. Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagn Invest. 20:393–413.
- Suárez-Bonnet A, Herráez P, Mulas JM, Rodríguez F, Déniz JM, Monteros AE. 2011. Expression of 14-3-3σ protein in normal and neoplastic canine mammary gland. Vet J. 190:345–351.
- Suárez-Bonnet A, Martín de Las Mulas J, Herráez P, Rodríguez F, Espinosa de los Monteros A. 2010. Immunohistochemical localisation of 14-3-3 sigma protein in normal canine tissues. Vet J. 185:218–221.
- Umahara T, Uchihara T, Nakamura A, Iwamoto T. 2011. Differential expression of 14-3-3 protein isoforms in developing rat hippocampus, cortex, rostral migratory stream, olfactory bulb, and white matter. Brain Res. 1410:1–11. 10.1016/j.brainres.2011.06.036
- Wilker EW, Grant RA, Artim SC, Yaffe MB. 2005. A structural basis for 14-3-3 sigma functional specificity. J Biol Chem. 280:18891–18898. 10.1074/jbc.M500982200
