Abstract
The necessity of supplementation of non-nutritive feed additives (NNFA) at 2 g/kg diet of mannan oligosaccharides (MOS), hydrated sodium calcium aluminosilicate (HSCAS) and Lacobacillus acidophilus (Lac) to block the negative impacts of aflatoxin (AF) on broilers performance and physiological traits throughout the withdrawal period from days 21 to 42 of age following AF feeding during 1–21 days of age was studied herein. There were two main experimental groups during 1–21 days of age in which of two concentrations of AF were supplemented at 0 and 200 ppb, and four subgroups of NNFA within diet without AF supplementation (unsupplemented control, MOS, HSCAS and Lac, groups 1, 2, 3 and 4, respectively) and with AF supplementation (unsupplemented control, MOS, HSCAS and Lac, groups 5, 6, 7 and 8, respectively), thereby there were eight experimental treatments. During 21–42 days of age period, 210 broilers chickens left from the first part of this study (1–21 days of age) were used for studying the residual effects of AF during days 21–42 of age after AF removal from the diet at day 21 of age. Each group supplemented with MOS, HSCAS or Lac during 1–21 days of age was divided into two subgroups during the recovery period (21–42 days of age). One subgroup continued to receive the same NNFA it had received during 1–21 days of age. The other received only the basal diet without AF and NNFA supplementation. The positive control group (group 1) that had received basal diet without AF supplementation during 1–21 days of age continued on the same treatment during 21–42 days of age. The negative control group (group 5) that had received AF alone during 1–21 days of age received the basal diet without AF supplementation during the recovery period. AF had a chronic negative effect on productive performance of broilers during the recovery period and this was further confirmed by increasing liver enzyme and histology of liver. MOS at 2 g/kg diet induced the best recovery in growth performance of AF groups and continued of MOS seems to be essential to block the carry over effect of AF during the withdrawal period. However, growth performance of the non-AF group supplemented with Lac was the best.
1. Introduction
Aflatoxins (AF) are a group of heterocyclic metabolites produced by fungi of the genus Aspergillus particularly Aspergillus flavus and Aspergillus parasiticus. There were 18 AF groups of which only AF B1, B2, G1 and G2 have been detected as natural contaminants of feeds and feedstuffs (Smith & Ross Citation1991). Chickens consumed AF exhibited a disease called aflatoxosis which induces severe economic losses in the poultry producers (Council for Agricultural Science and Technology Citation2003). The liver is the target organ for the toxic action of AF (Leeson et al. 1995). The negative effects of AF on poultry performance have been reported even after withdrawal of AF (Abdel-Hamid et al. Citation1995a, Citation1995b).
Hydrated sodium calcium aluminosilicate (HSCAS) supplementation to AF polluted diets significantly reduced the negative effect of the toxin on meat and egg production (Leeson et al. Citation1995) by immobilizing the toxin in the gut. HSCAS supplementation to AF contaminated diet of 2-week-old chicks decreased residues of AF B1 in the liver and meat compared with control diet (Hassan Citation2006). HSCAS decreased the negative impact of AF in the performance, and the relative weight of organs also increase protein levels (Arab Abousadi et al. Citation2007). In addition, a significant increase in body weight (BW) and feed conversion ratio (FCR) of broiler fed a diet with 5 g HSCAS/kg diet and no significant effect on blood traits and organs per cent except a decrease in spleen and proventriculus relative weight (Prvulovic et al. Citation2008).
Mannan oligosaccharides (MOS), a component of Saccharomyces cerevisiae cell wall, widely used as prebiotic in animal nutrition (Bovera et al. Citation2010a, Citation2010b, Citation2012; Piccolo et al. Citation2013; Abudabos & Yehia Citation2013) has been found to bind 80–90% of AF (Mahesh & Devegowda Citation1996; Gallo & Masoero Citation2010), and it has been the preferred non-nutritive feed additives (NNFA) for AF detoxification. Devegowda et al. (Citation1998) reported that mycosorb a natural product contained 10% MOS compound extract from S. cerevisiae at 0.1% and 0.2% to diets containing up to 200 ppb AF resulted in improved growth performance and serum total proteins. Parlat et al. (Citation2001) found a significant increase in body weight gain (BWG) and FCR of Japanese quail fed diet with 2.5 mg/kg AF with added glucomannan 1 g/kg diet. Schatzmayr (Citation2008) has also observed that Trichosporon mycotoxinivorans (yeast strain) is also able to block the negative influences of mycotoxins by degrading them or biotransforming them in the gastrointestinal tract.
The application of probiotic to detoxification of AF is rare in the literature, for example, Diaz et al. (Citation2005) demonstrated that the negative impact of 2 mg T-2 toxin/kg diet on BWG and FCR was entirely overcome by dietary supplementation of 2.0 kg/ton diet of the mycotoxin deactivator contained Eubacterium BBSH 797. In addition, Vicente et al. (Citation2007) reported that lactobacillus spp.-based culture improved FCR and growth but not in significant levels and significantly reduced mortality of broilers. Kalavathy et al. (Citation2008) found a significant increase in BWG and FCR when used lactobacillus cultures besides a decrease in serum triglycerides and lipids. Although many studies have addressed the efficiency of different feed supplements on performance of broilers, the necessity of these additives after AF removal is rare in the literature. Thus, this study aimed to observe the residual influence of AF in broilers after AF removal at day 21 of age and to determine if continued feeding of NNFA such as HSCAS, MOS and Lacobacillus acidophilus (Lac) is essential to reduce the adverse effects of AF on productive performance, physiological traits and liver histology of broilers during the recovery period (21–42 days of age).
2. Materials and methods
2.1. Chicks and experimental design
To study the effect of AF removal at 21 day of age and the essential of removal or continue of NNFA, 210 unsexed Cobb broilers were used during the recovery period (21–42 days of age). The experimental setup during the 1–21 days and production of AF were previously cited by Attia et al. (Citation2013). In addition, the experimental design during 1–21 days of age and 21–42 days of age are shown in . The broiler chickens during 1–21 days of age were divided into two main experimental groups and fed basal diets () contained either 0 or 200 ppb AF B. In brief, each main experimental group was subdivided into four subgroups and fed diet supplemented with or without MOS (2 g/kg diet BioMos®), a nutritional supplement manufactured by Alltech, Inc. (Nicholasville, KY, USA), HSCAS (2 g/kg diet NovaSil, Engelhard Corp., Beechwood, OH, USA) and Lac (2 g/kg diet Laceol Laboratory, Houdan, France). Thus, during 1–21 days of age, the experimental design was factorial with two concentrations of AF × 4 NNFA (unsupplemented, MOS, HSCAS and Lac), which resulted in eight experimental groups (). The eight experimental groups were the control group (group 1, the positive control) received the basal diet without supplementation, group 2 received the basal diet supplemented with MOS at 2 g/kg diet, group 3 received the basal diet supplemented with HSCAS at 2 g/kg diet, group 4 received the basal diet supplemented with Lac at 2 g/kg diet. Groups 5 received basal diet supplemented with AF B at 200 ppb and used as negative control, whereas groups 6, 7 and 8 received the same diet fed to group 5, but supplemented with the abovementioned dose of MOS, HSCAS and Lac, respectively.
Table 1. The experimental design used during 1–21 and 12–42 days of age.
At day 21 of age, AF was removed from the experimental diets () to determine whether or not supplementation with NNFA is essential for complete recovery from the toxic effects of AF. During 21–42 days of age, the positive and the negative control were kept as controls. In addition, each of the groups fed NNFA during 1–21 days of age were divided into two subgroups. One subgroup continued to receive the same NNFA it had received during 1–21 days of age, whereas the other subgroup received the basal diet without supplementation of NNFA (). Thus, there were 14 experimental groups (2 AF concentrations × 3 NNFA (MOS, HSCAS and Lac) × 2 treatments (continue vs. removal of supplementations), plus the positive and the negative controls.
Table 2. Composition of the experimental diets fed to broilers from days 1 to 42 of age (as-fed basis).
Group 1 consisted of broilers that received only the basal diet without AF and NNFA supplementations during days 1–21 of age (positive control). Group 2 received the basal diet supplemented with MOS at 2 g/kg diet, group 3 received the basal diet in which MOS was removed at 21 days of age. Group 4 received the basal diet supplemented with HSCAS at 2 g/kg diet and group 5 received the basal diet in which HSCAS was removed at 21 days of age. Group 6 received the basal diet supplemented with Lac at 2 g/kg diet and group 7 received the basal diet in which Lac was removed at 21 days of age. The groups 8, 9, 10, 11, 12 and 14 received the basal diet that had been supplemented AF during 1–21 days of age and removed during 21–42 days of age and fed diets that not supplemented with NNFA (group 8, negative control) or supplemented with MOS, HSCAS and Lac, respectively, as abovementioned for groups 2, 3, 4, 5, 6 and 7. Each treatment contained three replicates of five chickens each.
2.2. Husbandry
Chicks were maintained on 23 hours of light/day throughout the whole experimental period and were housed in the floor pens (10 birds/m2) furnished with wheat straw as a litter. Water and mash diets were offered ad libitum. The temperature within the house was 28°C ± 5°C.
2.3. Collection of data
Birds were individually weighed to calculate the BWG, whereas feed intake (FI) was estimated based on replicate measurements. FCR was estimated as kg feed consumed/kg BWG. Data for productive performance were estimated at weekly interval.
At day 42 of age, five chickens from each treatment were randomly and individually weighed and slaughtered according to Islamic method. Carcass, liver, intestine, heart, spleen, bursa of Fabricius, pancreas and abdominal fat were removed and individually weighed to the nearest 0.01 gram. Data were expressed as a per cent of live BW.
Ten blood samples as five heparinized and five unheparinized were collected from the wing vein of each treatment at 42 days of age. Blood was centrifugated at 1500 × g for 20 minutes to obtain plasma and serum which were stored at −18ºC. The counts of red blood cells (RBCs), white blood cells (WBCs), haemoglobin (Hgb), packed cell volume (PCV, %) and differential leukocyte were measured as previously cited by Attia et al. (Citation2013). Total serum protein (g/100 ml), albumin (g/100 ml), serum globulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglyceride and alkaline phosphatase (ALP) were obtained as previously cited by Attia et al. (Citation2013).
Liver specimens were collected from all groups on day 42. They were rapidly fixed in 10% neutral buffered formalin solution for at least 24 hours. The fixed specimens were processed through the conventional paraffin embedding technique. Paraffin blocks were prepared from which 5-micron thick sections were obtained and stained by Mayer's haematoxylin and eosin (H&E) according to Culling (Citation1983). The sections (n, 3 samples per treatment and 5 sections per sample) were examined using a light microscope equipped with a digital camera using 1000 × power.
2.4. Statistical analysis
Data were statistically analysed by SAS (Citation1994) software program using a two-way factorial design. Mean differences were detected by student Newman Keuls test when significant P values were obtained. All percentage was transferred to their analogue arc sin before running the analyses.
3. Results
3.1. Growth performance
Growth of broiler chickens was still negatively affected (P < 0.0001) even after 7 days of AF withdrawal (). From days 28 of age and onwards, there was no significant influence of AF on BWG of broilers. However, BWG for the entire period was negatively affected (P < 0.0006) by AF from days 1 to 21 of age, showing a negative carry over effect of AF even after 21 days of AF removal.
Table 3. Effect of AF withdrawal and/or non-nutritive feed additives on BWG and FCR of broiler chicks from days 21 to 42 of age.
NNFA did not significantly affect BWG of broiler chickens from days 21 to 28, 36 to 42, and from 21 to 42 of age although Lac significantly increased BWG of broilers during days 29–35 of age compared to the other NNFA and the control groups (). For the entire period, this effect was continued although differences were not significant.
A significant interaction between AF withdrawal and NNFA was shown only from days 21 to 28 and from days 36 to 42 of age (). During days 21 to 28 of age, the lowest BWG was from AF group fed diet without or with MOS while the highest BWG was from group fed diet without AF and supplemented with MOS. The difference between these groups was significant. Other experimental groups exhibited intermediate values.
During days 36–42 of age, there were no significant differences in BWG within chickens consumed AF diets with or without MOS, HSCAS and Lac. However, within groups fed diets without AF from day 1 of age, withdrawal of HSCAS yields significantly better BWG than HSCAS continuation up to day 42 of age. BWG of the control groups fed diets without HSCAS and Lac was higher than that of the positive control group.
There was no significant difference in FCR due to AF and NNFA during all experimental periods (). A significant interaction between AF withdrawal and NNFA was observed for FCR in each experimental period but not for the whole experimental period (). After 7 days of AF withdrawal, FCR was significantly worse than that of the control group. During the following periods, the difference between the abovementioned groups was diminished. Within AF withdrawal groups, continued MOS significantly improved FCR during days 21–28 of age compared to withdrawal of the MOS. Difference between these two groups was diminished during the following periods.
Continuation of Lac significantly improved the FCR only during days 21–28 of age compared to the removal of Lac. However, the difference between these two groups was not significant during the following periods.
Within the groups fed a diet without AF from days 1 to 42 of age, continuation of MOS significantly impaired the FCR during only days 21–28 of age compared to removal of the MOS from day 21 of age on. Differences between these groups were not significant during the following periods.
Continuation of Lac significantly improved the FCR during only days 29–35 of age compared to its counterpart control but had no significant effect at the other experimental periods.
HSCAS did not significantly affect FCR of AF withdrawal groups and the control groups during the recovery period. For the entire, there was no significant effect due to AF and/or NNFA on FCR.
3.2. Dressing, abdominal fat and inner organ weights
There was no significant effect due to AF withdrawal at 21 day of age on the dressed carcass percentage, abdominal fat and pancreas (). Withdrawal of AF significantly decreased relative weight of the liver and the spleen compared to the control group, but increased relative weight of the heart.
Table 4. Effect of AF withdrawal and/or non-nutritive feed additives on the dressing percentage and percentage of internal organs and abdominal fat of 42-day-old broiler chicks.
NNFA did not significantly affect the dressed carcass per cent, the internal organs or the abdominal fat at the end of the recovery period (). A significant interaction between AF and NNFA was found on only the per cent dressed carcass and the intestine (). Within AF withdrawal groups or those fed diet without AF from days 1 to 42 of age, Lac continuation significantly decreased dressing percentage. Removal of HSCAS and Lac within AF significantly decreased intestinal per cent compared to removal of AF from the negative control from days 21 of age. Within groups fed a diet without AF from day 1 of age, removal of MOS significantly increased intestinal per cent compared to HSCAS and Lac groups.
3.3. Blood constituents
At the end of the recovery period, withdrawal of AF group still had a significant negative effect on serum AST, ALP and triglyceride levels compared to the control group (). AF withdrawal at day 21 of age did not significantly affect ALT, total protein, albumin and globulin at day 42 of age. A significant influence due to NNFA or the interaction was not shown on all serum biochemical constituents at day 42 of age ().
Table 5. Effect of AF withdrawal and/or non-nutritive feed additives on blood serum biochemical constituents of broiler chicks at day 42 of age.
Most of the haematological measurements, except for a decrease in the percentage of eosinophils, were normal by the end of the recovery period ( and ). Eosinophil percentage was significantly decreased from 9.82% in the control group to 8.86% in the groups recovering from AF feeding.
Table 6. Effect of AF withdrawal and/or non-nutritive feed additives on haematological constituents of broiler chicks at day 42 of age.
Table 7. Effect of AF withdrawal and/or non-nutritive feed additives on the WBC differential count of broiler chicks at day 42 of age.
Hgb concentrations and the PCV were both significantly higher in the group fed Lac during the recovery period than the group that received no NNFA (). Groups fed the other NNFA had intermediate levels of both.
A significant interaction between AF and NNFA withdrawal was observed on the heterophil to lymphocyte ratio (H/L) ratio, and on the per cent lymphocyte, basophil and heterophil (). Within AF withdrawal groups, MOS continuation up to day 42 significantly decreased the H/L ratio, whereas increasing lymphocyte and the basophil compared to removal of MOS ().
Within groups fed the diet without AF from day 1 of age, MOS continuation significantly increased H/L ratio while decreasing lymphocyte (%) compared to removal of MOS (). HSCAS up to day 42 significantly increased H/L ratio, but decreased the lymphocyte and the basophil (). However, withdrawal of Lac at day 21 of age did not affect any of haematological criteria of groups fed diets with or without AF from day 1 of age or not.
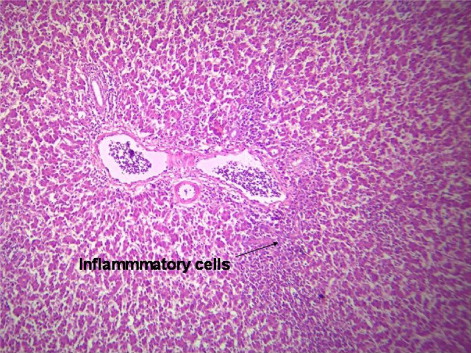
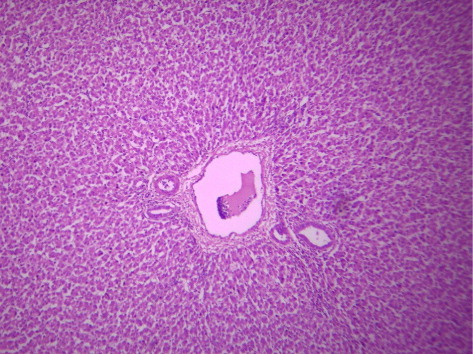
3.4. Histological picture of liver
indicates the histological status of the chicken's liver at the end of experiment (42 days of age) as affected by withdrawal of dietary AF and/or NNFA supplementation. Histological changes in the liver showed normal liver morphology of the control group (), while liver of AF fed birds were still exhibit histological changes at the end of the recovery period ( and ), revealing portal inflammatory, cell infiltration, portal fibrosis and biliary hyperplasia. Continuation of MOS () resulted in better recovery than removal of it (). Continued feeding of HSCAS within AF groups induced the most favourable effect on the hepatic tissues although removal of HSCAS caused mild portal inflammatory and cells infiltration ().
Table 8. Histological status of the chicken’s liver at the end of experiment (42 days) as affected by withdrawal of dietary AF and/or non-nutritive feed additives supplementation.
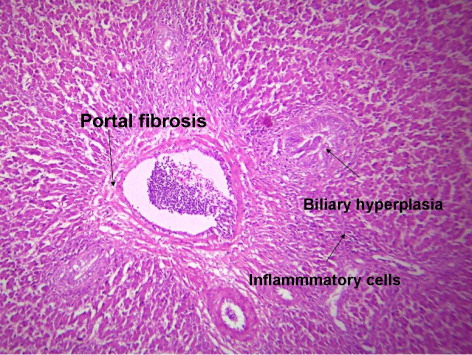
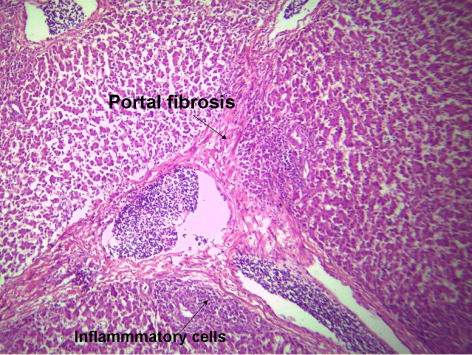
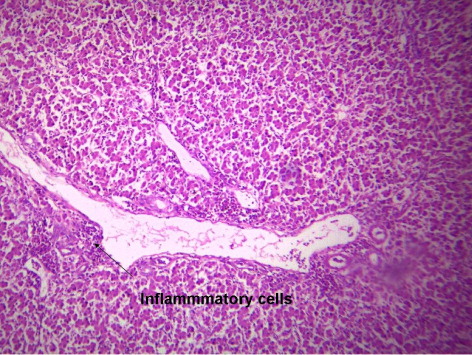
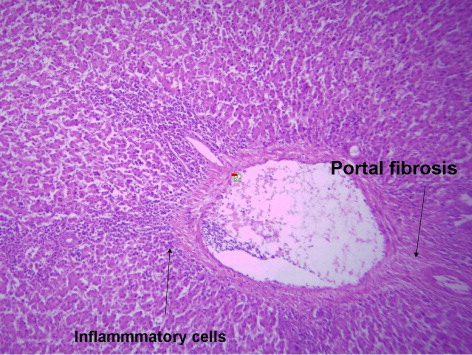
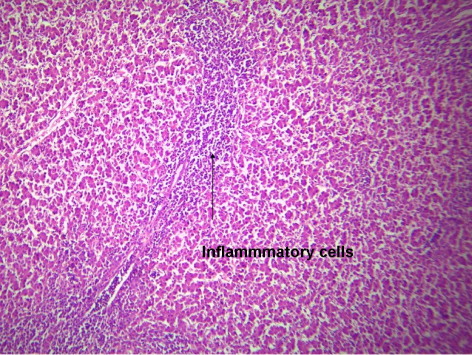
Continuing or removing of Lac within AF group showed the lowest recovery effect as liver showed a mild portal inflammatory, cells infiltration, portal fibrosis and biliary hyperplasia (). Within non-AF groups, continuation or removal of NNFA had no effects on liver pathology except for mild portal inflammation and cellular infiltration when Lac continued up to 42 days of age (). HSCAS induced the most favourable recovery effect on the hepatic tissues showing a decrease in the destructive effect of AF on the hepatic cells. The other NNFA had lesser effects.
4. Discussion
The negative impacts of AF on performance and physiological traits of broilers are documented in the first part of this work (Attia et al. Citation2013). In addition to its effects on growth and FCR shown in the earlier paper, there were adverse effects on the liver and organs of the immune system. Numerous physiological factors were shown to be affected, probably as a result of the toxins effects on the liver, thymus, spleen and bursa of Fabricius. Some of these effects were lessened by the simultaneous feeding of one or more of the NNFA.
Some of the effects of AF fed during the first 3 weeks of life persisted in the following weeks after its removal from the diet. Important among these are its depressive effects on BWG which amounted to 13.4% and 5.9% at days 28 and 42 of age, respectively, showing progressive decrease with prolonging of the recovery period. Besides the growth depression effect of AF on broilers performance during the recovery period, FCR was still depressed during the first week after AF removal from the diet. The effect on FCR, however, was no longer exists by the end of the 3-week withdrawal period. Similarly, Abdel-Hamid et al. (1Citation995a) found that AF negatively affected broilers performance even during the withdrawal period. Similar to the diminishing of the significant negative effect of AF with progress of withdrawal time, Hassan (Citation2005) reported that growth of broilers fed a diet with 1 mg AF/kg was gradually improved through withdrawal time; however, this improvement was still underlie the other treatments. In addition, Resanovic and Sinovec (Citation2008) mentioned that the removal of AF contaminated feed could alleviate its adverse effects on broilers performances.
None of the NNFA showed consistent effects on growth or FCR during the 3-week period after removal of AF from the diet. Similarly, Abdel-Hamid et al. (Citation1995a) found that most of the AF groups had less FCR than the control even during the withdrawal period despite the little improvement in FCR by the late withdrawal period. There was a recovery in FCR of the AF group with progressing of the recovery period, which could be attributed to the decrease in the FI (6.25%) and the increase in the BWG (4.26%). Also, Hassan (Citation2005) reported that AF impaired FCR of broilers during both AF and withdrawal period and negative influence of AF on economic traits, e.g. BWG, FI and FCR were gradually diminished during the recovery period.
The adverse impact of AF on growth performance during the recovery period could be ascribed to the chronic effect of AF on body organs e.g. spleen, liver, heart and hepatic function e.g. AST and ALT enzymes as well as the negative impact of AF on nutrient utilization by broilers (Osborne & Hamilton Citation1981; Miazzo et al. Citation2000). The histological findings of liver confirmed the chronic effects of AF at day 42 of age showing a negative change in liver cells revealing portal inflammatory, cells infiltration, portal fibrosis and biliary hyperplasia in AF group. Some aspects of liver function had returned to normal by day 42 of age. As examples are serum protein, albumin and globulin and the liver enzyme ALT. In contrast, not all of the effects of AF had returned to normal by 42 days of age. Among these were the enzymes AST, ALP and serum triglyceride. Various NNFA did not affect these characteristics nor did they affect most aspects of the RBCs or WBCs picture, indicating chronic effect of AF. Similarly, Hassan (Citation2005, Citation2006) reported that ALP, AST, ALT did not recover after the AF withdrawal from broiler diet. Furthermore, the decrease in the liver and spleen percentage after 21 days of AF removal was coincided with an increase in albumin/globulin ratio showing a negative change in the secondary lymph organs. Also, Abdel-Hamid et al. (1Citation995b) found an enlargement in the liver, heart and spleen percentage in AF contaminated groups either in the treated period or the withdrawal period.
Lac improved FCR and growth of group fed diet without AF from day 21 of age, and this is in agreement with those cited by Higgins et al. (Citation2010) who showed that the improvement in growth performance of Lac group could be ascribed to the alteration in ecology of gastrointestinal towards lactic acid bacteria and its role in improving in animal health. These enhancements were coincided with increasing Hgb (16.5%) and PCV (15.9%) compared to the control showing improved health of broilers during the recovery period (Kalavathy et al. Citation2008).
The importance of MOS continuation to AF group was demonstrated by the improvement in FCR observed during days 21–42 of age compared to withdrawal of MOS from the AF groups. The favourite impact of MOS on economic traits of AF group could be ascribed to its role as an adsorbent of immunosuppressive mycotoxins (Cotter et al. Citation2002; Gallo & Masoero Citation2010) and binding both zearalenone and AF B1 (Zaghini et al. Citation2005). Additionally, Hooge (Citation2004) indicated that the mode of action of MOS included its capability to adsorb enteropathogenic bacteria, to enhance gut health (Attia et al. Citation2014a) and finally its capability to improve immune response (Attia et al. Citation2014a, Citation2014b). Moreover, withdrawal of MOS of AF groups resulted in significantly higher H/L compared to non-AF groups showing improved humoral immunity. The influence of NNFA depends on AF presence as the response to MOS was different between both groups (with or without AF) in lymphocyte, heterophil and H/L ratio. In this regard, Kocher et al. (Citation2004) reported that MOS can affect nutrients utilization by the intestines and was able to improve gut ecology that enhance fibre fermentation with a decrease in bacterial populations utilizing starch and sugar.
5. Conclusion
AF induced a chronic adverse impact on productive performance of broilers during the recovery period as confirmed by increasing liver enzyme and histology of liver. Supplementation of MOS at 2 g/kg diet induced the best recovery in growth performance of AF groups. This suggested that MOS continuation seems to be essential to block the carry over effect of AF during the withdrawal period. However, growth performance of the non-AF group supplemented with Lac was the best.
Acknowledgement
To worldwide poultry producers.
References
- Abdel-Hamid AM, El-Dorra TM, Arief HSM. 1995a. Effect of some dietary supplements to aflatoxic diets of chickens. I. on the performance. J Agric Sci Mansoura Univ. 20:3208–3226.
- Abdel-Hamid AM, Hala SM, Arief HSM, El-keraby F, El-Dorra TM. 1995b. Effect of some dietary supplements to aflatoxic diets of chickens. II. on tissues analysis. J Agric Sci Mansoura Univ. 20:3227–3241.
- Abudabos AM, Yehia HM. 2013. Effect of dietary mannan oligosaccharide from Saccharomyces cerevisiae on live performance of broilers under Clostridium perfringens challenge. Ital J Anim Sci. 12:231–235.
- Arab Abousadi M, Rowghani E, Ebrahimi Honarmand M. 2007. The efficacy of various additives to reduce the toxicity of aflatoxin B1 in broiler chicks. Iran J Vet Res. 8(2), ser No 19.
- Attia YA, Allakany HF, Abd Al-Hamid AE, Al-Saffar AA, Hassan RA, Mohamed NA. 2013. Capability of different non-nutritive feed additives on improving productive and physiological traits of broiler chicks fed diets with or without aflatoxin during the first 3 weeks of life. J Anim Physiol Anim Nutr. 97(4):754–772. 10.1111/j.1439-0396.2012.01317.x
- Attia YA, Abd Al-Hamid AEA, Ibrahim MS, Al-Harthi MA, Bovera F, Elnaggar ASh. 2014a. Productive performance, biochemical and hematological traits of broiler chickens supplemented with propolis, bee pollen, and mannanoligosaccharides continuously or intermittently. Livest Sci. 164:87–95. 10.1016/j.livsci.2014.03.005
- Attia YA, Hamed RS, Abd El-Hamid AE, Shahba HA, Bovera F. 2014b. Effect of inulin and mannan-oligosaccharides compared with zinc-bacitracin on growing performance, nutrient digestibility and hematological profiles of growing rabbits. Anim Prod Sci (online), http://dx.doi.org/10.1071/AN13286
- Bovera F, Lestingi A, Iannaccone F, Tateo A, Nizza A. 2012. Use of dietary mannanoligosaccharides during rabbit fattening period: effects on growth performance, feed nutrient digestibility, carcass traits, and meat quality. J Anim Sci. 11:145–148.
- Bovera F, Marono S, Di Meo C, Piccolo G, Iannaccone , Nizza A. 2010a. Effect of mannanoligosaccharides supplementation on caecal microbial activity of rabbits. Animal 4:1522–1527. 10.1017/S1751731110000558
- Bovera F, Nizza S, Marono S, Mallardo K, Piccolo G, Tudisco R, De Martino L, Nizza A. 2010b. Effect of mannanoligosaccharides on rabbit performance, digestibility and rectal bacterial anaerobic populations during an episodi of epizootic rabbit enteropathy. World Rabbit Sci. 18(1):9–16. 10.4995/WRS.2010.18.02
- Cotter PF, Sefton AE, Liburn, MS. 2002. Manipulating the immune system of layers and breeders: novel applications of mannan oligosaccharides. In: Jacques KA, Lyons TP, editors. Proceedings of Alltech's 18th Annual Symposium. Lexington (KY): Nottingham University Press; p. 21–28.
- Council for Agricultural Science and Technology. 2003. Mycotoxins: risk in plant, animal and human systems. Ames, Iowa, USA. Task Force Report no. 139, January 2003, 199 p.
- Culling CF. 1983. Handbook of histopathological and histochemical staining. 3rd ed. London: Butterworth.
- Devegowda G, Raju MLVLN, Afzali N, Swamy HVLN. 1998. Mycotoxins picture worldwide: novel solutions for their counteraction. In: Lyons T, Jacques KA, editors. Biotechnology in the feed industry, Proceedings of Alltech’s 14 the Annual symposium. Nottingham; p. 241–255.
- Diaz GJ, Cortes A, Roldan L. 2005. Evaluation of four additives against the adverse effects of T-2 toxin in growing broiler chickens. J Appl Poult Res. 14:226–231. 10.1093/japr/14.2.226
- Gallo A, Masoero F. 2010. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital J Anim Sci. 9:e21. 10.4081/ijas.2010.e21
- Hassan RA, 2005. Capability of mannan oligosaccharide Bio-Mos® organic selenium and hydrated sodium calcium aluminaosilicate to detoxify aflatoxosis for growing local chickens. 1- Performance, some internal organs and blood constituents. Proceedings of 2nd Conference of Animal Production Research Institute, Sakha 27–29 September 2005: 559–574.
- Hassan RA. 2006. Capability of mannan oligosaccharide Bio-Mos® organic selenium and hydrated sodium calcium aluminaosilicate to detoxify aflatoxosis for growing local chickens. 2- Lymphoid organs, immune response and residues in tissues. Egypt Poult Sci J. 26:495–511.
- Higgins JP, Higgins SE, Wolfenden AD, Henderson SN, Torres-Rodriguez A, Vicente JL, Hargis BM, Tellez G. 2010. Effect of lactic acid bacteria probiotic culture treatment timing on salmonella enteritidis in neonatal broilers. Poult Sci. 89:243–247. 10.3382/ps.2009-00436
- Hooge DM. 2004. Turkey pen trials with dietary mannan oligosaccharides: meta-analysis, 1993–2003. Int J Poult Sci. 3(3):179–188. 10.3923/ijps.2004.179.188
- Kalavathy R, Abdullah N, Jalaludin S, Wong CMVL, Ho YW. 2008. Effect of lacobacillus culture and oxytetracyclin on the growth performance and serum lipids of chickens. Int J Poult Sci. 7:385–389. 10.3923/ijps.2008.385.389
- Kocher A, Canolly A, Zawadzki J, Gallet D. 2004. The challenge of finding alternatives to antibiotic growth promoters. Proceedings of the International Society for Animal Hygiene; 2004; St Malo, France: International Society for Animal Hygiene. 227–229.
- Leeson S, Diaz GJ, Summers JD. 1995. Poultry metabolic disorders and mycotoxins. Ontario: Guelph; p. 190–216.
- Mahesh BK, Devegowda G. 1996. Ability of aflatoxin binders to bind aflatoxin in contaminated poultry feed – an in vitro study. Proceedings of the 20th World's Poultry Congress. New Delhi, India; Vol. 4, p. 296.
- Miazzo R, Rosa CAR, De Queiroz Carvalho EC, Magnoli C, Chicchiera SM, Palacio G, Saenz M, Kikot A, Basaldella E, Dalceros A. 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult Sci. 79(1):1–6. 10.1093/ps/79.1.1
- Osborne DJ, Hamilton PB. 1981. Steatorrhea during aflatoxosis in chickens. Poult Sci. 60:1398–1402. 10.3382/ps.0601398
- Parlat SS, özcan M, Oguz H. 2001. Biological suppression of aflatoxosis in Japanese quail (coturnix conturnix japonica) by dietary addition of yeast (Sacchaomyces cerevisiae). Res Vet Sci. 71:207–211. 10.1053/rvsc.2001.0512
- Piccolo G, Centoducati G, Bovera F, Marrone R, Nizza A. 2013. Effects of mannan oligosaccharide and inulin on sharpsnout seabream (Diplodus puntazzo) in the context of partial fish meal substitution by soybean meal. Ital J Anim Sci. 12(1):133–138. 10.4081/ijas.2013.e22
- Prvulovic D, Kojic D, Grubor–Lajsic G, Slavica Kosarcic S. 2008. The effect of dietary inclusion of hydrated aluminosilicate on performance and biochemical parameters of broiler chickens. Turk J Vet Anim Sci. 32:83–89.
- Resanovic R, Sinovec Z. 2008. Effects of limited feeding of aflatoxin B1 contaminated on the performance of broilers. Mycotoxin Res. 22:183–188.
- SAS Institute. 1994. SAS/STAT user's guide statistics. Cary, NC: SAS Institute Inc.
- Schatzmayr G. 2008. Latest innovations in the deactivation of mycotoxins. World’s Poultry Congress 2008, Brisbane 30 June–4 July 2008, Australia.
- Smith JE, Ross K. 1991. The toxigenic aspergilli. In Smith JE, Henderson ES, editors. Mycotoxin and animal foods. Boca Raton: CRC Press; p. 101–118.
- Vicente JL, Avina L, Torres-Rodriguez A, Hargis B, Tellez G. 2007. Effects of a lactobacillus spp-based probiotic culture product on broiler chickens performance under commercial condition. Int J Poult Sci. 6(3):154–156. 10.3923/ijps.2007.154.156
- Zaghini A, Martelli G, Roncada P, Simioli M, Rizzi L. 2005. Mannanoligosaccharides and aflatoxin B1 in feed for laying hens: effects on egg quality, aflatoxins B1 and M1 residues in eggs and aflatoxin levels in liver Poult Sci. 84:825–832. 10.1093/ps/84.6.825