Abstract
The main objective of this study was to investigate the effect of incremental levels of degummed crude canola oil (DCCO) supplementation to pasture-dominant diets of grazing, primiparous, Holstein-Friesian cows on lactation performance, milk composition and liveweight traits. We tested the hypothesis that supplementing primiparous Holstein-Friesian cows with DCCO in a pasture-based dairy system will increase milk yield, fat and protein contents, but decrease cow body condition score (BCS) and liveweight. A random allocation of 20 primiparous Holstein-Friesian cows into four treatments was utilized in an eight-week feeding trial after two weeks of adjustment. The experimental treatments included a wheat-based pellet without DCCO (control), wheat-based pellet with DCCO added at 25 mL/kg on dry matter (DM) basis (low), 35 mL/kg on DM basis (medium) and 50 mL/kg on DM basis (high). Treatment and week (duration) of supplementation were significant sources of variation influencing milk yield (P = 0.0042), fat (P = 0.0118) and protein (P = 0.0002). Cows in the high treatment group had the greatest milk yield (168.7 ± 3.5 kg/week) and lower fat (3.3 ± 0.1%) and protein (3.0 ± 0.09%) percentages than cows in the control group (milk yield of 157.1 ± 3.5 kg/week, 4.0 ± 0.2% fat and 3.1 ± 0.0% protein). With the exception of somatic cell count and yield, the week (duration) of supplementation significantly influenced all milk composition traits. We concluded that supplementation of grazing dairy cows with DCCO had no negative impact on BCS and body weight gain. DCCO can be used to enhance milk yield, but at the expense of milk fat and protein.
1. Introduction
Pasture is the main feed source in South Eastern Australia where dairy farms are mostly concentrated (Dairy Australia Citation2011). In seasons where rainfall is below average, barley and wheat supplements are partially used in pasture-based systems to increase the energy intake and milk production of lactating cows (Akbaridoust et al. Citation2014). On a typical pasture-based dairy farm, primiparous cows are the most energy-challenged animals because they are at the bottom of the social hierarchy (Moran & McLean Citation2001).
In spite of previous studies in other parts of the world suggesting that dietary fat supplements can increase milk yield (Griinari & Bauman Citation2006; Bernal-Santos et al. Citation2010), such supplements are generally not very popular within the Australian dairy system mainly because of the associated depression of milk fat and protein content (Khorasani et al. Citation1991; Wu & Huber Citation1994; He & Armentano Citation2011). The specific mechanism by which supplementary fat affects lactation traits is still largely unknown (Griinari & Bauman Citation2006). Therefore, further studies in different dairy production systems are required to enable informed choices and tailored decisions when feeding lactating cows with specific dietary fat supplements, hence the need for the current study in a typical Australian pasture-based production system.
The effects of canola oil supplementation on primiparous Holstein-Friesian cows in the published literature are inconsistent and limited, particularly in pasture-based dairy systems. Therefore, we hypothesized that supplementing primiparous, Holstein-Friesian cows in a pasture-based dairy system with degummed crude canola oil (DCCO) will increase milk yield, fat and protein contents, but decrease cow body condition score (BCS) and liveweight traits. The major objective of this study was to investigate the effect of the dietary inclusion of incremental levels of DCCO for eight weeks to pasture-dominant diets of grazing, primiparous Holstein-Friesian cows on lactation performance, milk composition and liveweight traits.
2. Materials and methods
All experimental procedures were in accordance with the University of Tasmania Animal Ethics Committee guidelines, the 1993 Tasmania Animal Welfare Act and the 2004 Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
2.1. Site and climatic conditions
The experiment was carried out at the University of Tasmania’s Dairy Research Centre, Tasmanian Institute of Agriculture Elliot Dairy Research Farm in Somerset, North-Western Tasmania, Australia, from September to November 2012. Tasmania is Australia’s smallest state with a land size of 68,000 km2 and located within the cool, temperate, climatic zone at latitude 42° South and longitude 42°145 East. It is characterized by four distinct seasons – winter, autumn, spring and summer. The experiment was carried out in spring when the mean annual rainfall and humidity were 2500 mm and 60%, respectively.
2.2. Animals and treatments
The condition and energy status of the experimental cows were visually assessed based on BCS on a scale of 1–5 (Stockdale Citation2001; DPI Citation2003). Twenty primiparous, spring-calving, purebred, Holstein-Friesian cows (average liveweight of 400 ± 40 kg, BCS 4 ± 1 and 40 ± 8 days in milk), were randomly allocated into one of four treatments of wheat-based pellet without DCCO (control), wheat-based pellet with DCCO added at 25 mL/kg on dry matter (DM) basis (low), 35 mL/kg on DM basis (medium) and 50 mL/kg on DM basis (high). All the experimental animals were kept as a single herd in a fenced paddock under the same grazing management with access to 3000 kgDMha–1 of a mixture of ryegrass (Lolium perenne), cocksfoot (Dactylis glomerata) and white clover (Trifolium repens) pasture. Water was offered ad libitum. Each cow received 6 kg of the pelleted supplements daily for eight weeks, after two weeks of adjustment. Supplements were offered to cows in two splits during morning (3 kg) and evening (3 kg) milking sessions at 05:00 h and 15:00 h. There was no residual feed left over from any of the treatment groups. The chemical compositions of treatment, control and basal feeds are presented in
Table 1. Chemical composition of experimental and basal feeds.
2.3. Feed chemical composition and analysis
DMcontents of the basal and experimental diets were determined by drying samples to a constant temperature at 65°C in a fan-forced oven, finely ground to pass through a 2 mm sieve using Laboratory Mill (Thomas Model 4 Wiley® Mill; Thomas Scientific), and further drying at 105°C for 24 h. The DM was computed as the difference between the initial and final weights of samples. Ash content was determined by combusting samples in a furnace at 600°C for 8 h. Neutral (NDF) and acid (ADF) detergent fibre contents were measured using an Ankom fibre analyser (ANKOM220; ANKOM Technology, USA). Nitrogen was determined using a Thermo Finnigan EA 1112 Series Flash Elemental Analyser (Del Galdo et al. Citation2006) and the values multiplied by 6.25 to give the crude protein percentage. Ether extract was determined using an Ankom fat/oil extractor (ANKOMXT15; ANKOM Technology, USA). Metabolisable energy (ME) was calculated as per Weiss (Citation1993).
2.4. Milk sampling and analysis
Weekly milk samples were bulked from daily consecutive milkings at 05:00 h and 15:00 h for 8 weeks (2240 samples in total). Representative aliquots of fresh milk samples from each cow were collected using the Milking Point Controller (MPC 680) fitted to a De Laval herringbone milking machine into labelled plastic vials containing bronopol blue milk preservative and stored at –20°C until further analysis (Kroger Citation1985). No experimental cow suffered mastitis before, during or after the feeding trial period.
Fat, protein, lactose, solids-non-fat and somatic cell count (SCC) analyses were carried out at TasHerd Pty Ltd Hadspen, Tasmania, the officially contracted herd recording and milk testing agency using the Fourier Transformed Infrared spectrometry technology (Bentley Fourier Transform Spectrometer). The weekly milk yield from each cow was recorded using De Laval’s Alpro Herd Management System software (Alpro Windows 7.00 version 7.00.00, 2011). Fat-corrected milk (FCM) was computed using the equation below (Beever & Doyle Citation2007):
2.5. Liveweight
Weekly liveweight of the cows was automatically recorded as they passed through the De Laval auto drafter (De Laval Automatic Weigh System AWS100). These weights were used to calculate the specific growth rate (SGR) = 100 × [(lnW 1) – (lnW 0)] × D – 1, where W 0 and W 1 represent initial and final weights, and D is the duration of the experiment in days (Amirkolaie et al. Citation2005). Subjective assessment of BCS on a scale of 1–8 was also recorded weekly by the same assessor (DPI Citation2003).
2.6. Statistical analyses
Initially, summary statistics by level and week of DCCO supplementation were computed to give means, standard deviations standard error, variance, minimum and maximum values that were scrutinized for any data entry errors. Linear, cubic and quadratic orthogonal contrasts were tested by regressing the dependent on explanatory variables using PROC REG (SAS Citation2009), but found to be inconsequential. Therefore, repeated measures analysis of variance in PROC MIXED (SAS Citation2009) was employed fitting the fixed effects and second-order interactions of treatment and week of lactation, while base line milk values and cows were fitted as random effects. The degrees of freedom utilized in testing for significance and mean separation were estimated by the Satterthwaite method (SAS Citation2009). Significant differences and mean separations at the P < 0.05 threshold were carried out using Tukey’s probability pairwise comparison tests (SAS Citation2009) and presented as pooled Least square means and standard error means (LSM ± SEM).
3. Results
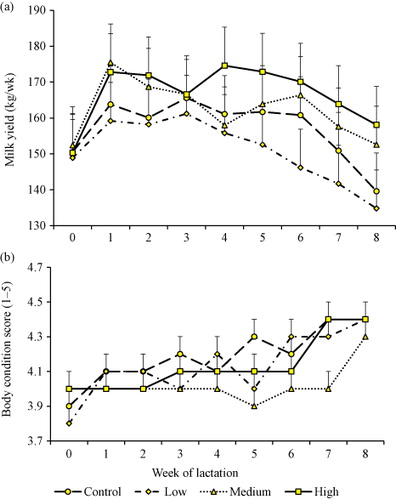
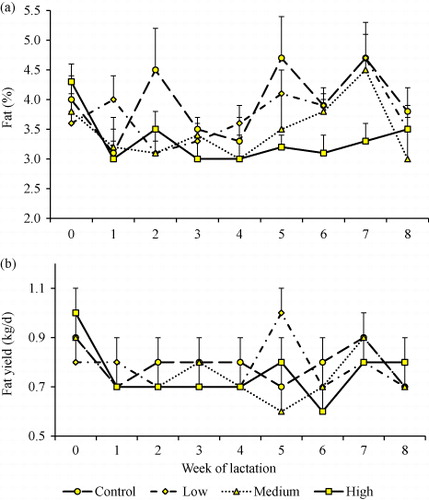
It was evident that DCCO supplementation significantly influenced milk yield (P = 0.0042), protein (P = 0.0002), fat (P = 0.0118) and solids-non-fat (P = 0.0136) percentages (). Cows in the high treatment group receiving 50 mL/kgDM of DCCO produced greater milk yield (168.7 ± 3.4 vs. 157.1 ± 3.7 kg/wk), but lower fat percentage (3.3 ± 0.1 vs. 4.0 ± 0.2%) than unsupplemented cows in the control group (0 mL/kgDM; ). Protein percentage was lower in the medium (35 mL/kgDM) treatment group than in the control and low treatment groups. Both treatment and week (duration) of supplementation had significant impacts on liveweight traits (BCS and SGR). Week of supplementation was a significant factor influencing almost all the lactation traits apart from SCC (P = 0.4718), milk yield (P = 0.1204) and protein percentage (P = 0.0768). The interaction between treatment and week of supplementation produced no significant effects on lactation and liveweight traits with the exception of BCS ().
Table 2. Least square means and standard error means (LSM ± SEM) of lactation and liveweight traits.
3.1. Weekly trends for lactation and liveweight traits
As the level of DCCO increased in the diet, milk yield also increased (). The experiment started in spring when lush pasture was available to the experimental cows. The summer season commenced in Week 6 of the experiment with reduction in pasture growth with declining rainfall and DM intakes. The ME provided by pasture was 3999.2 KJ/100 gDM while oil supplement was at 4083.3 KJ/100 gDM. Therefore, the close proximity between the medium and the high groups would have come about due to pasture availability. BCS for all the supplemented cows showed continuous increase throughout the duration of the feeding trial, although cows in the medium supplementation group had the least BCS ().
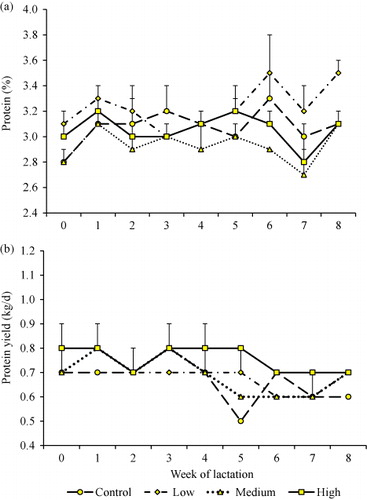
There was a concomitant decrease in milk fat percentage as DCCO content increased (). Cows in the medium and high treatment groups had the least fat compared to the control group (). Cows in the low DCCO group consistently had the greatest protein percentage that rose from 3.1% at the commencement of the feeding trial to 3.5% in Week 8 (). However, both supplemented and unsupplemented cows had similar protein yields throughout the duration of supplementation ().
4. Discussion
Dietary supplementation of dairy cows with oils to increase milk production has been trialled mostly in confined systems, where cows are fed total mixed rations, but there are wide variations in outcome between the different studies (Gagliostro & Chilliard Citation1992; Schroeder et al. Citation2004; Rabiee et al. Citation2012). Variation in results from a few pasture-based systems has also been observed (Kay et al. Citation2007; Hutchinson et al. Citation2011). The findings in this current study agree with previous authors who reported an increase in milk yield (Khorasani et al. Citation1991; Chilliard et al. Citation2001; Bobe et al. Citation2009; Hutchinson et al. Citation2012), but contrasts with others who reported either a decrease or no effect on milk production (Bayourthe et al. Citation2000; Chichlowski et al. Citation2005; Bernal-Santos et al. Citation2010; Caroprese et al. Citation2010; He & Armentano Citation2011). Our finding in this study also concurs with the assertion of increased milk production in cows supplemented with DCCO. The increase in milk production could possibly be attributed to the greater energy density of the feed and better efficiency of energy utilization (Jenkins & McGuire Citation2006; Kay et al. Citation2007; Odens et al. Citation2007; He & Armentano Citation2011). In addition, fat has the capacity to reduce heat and energy loss in urine, which potentially increases the efficiency of energy utilization and partitioning of absorbed nutrients for milk production and storage of excess energy in the adipose tissue of a lactating cow (Jenkins Citation1993; Schroeder et al. Citation2004; Kay et al. Citation2007; Odens et al. Citation2007).
Our finding in this study is also in agreement with other reports that fat supplementation leads to a decrease in milk fat (Tackett et al. Citation1996; Bauman & Griinari Citation2001, Citation2003; Peterson et al. Citation2003; Chichlowski et al. Citation2005; Griinari & Bauman Citation2006; Hutchinson et al. Citation2012). Previous studies had linked differences in milk fat to protein ratio to genetic variation (Malau-Aduli & Anlade Citation2002; Buttchereit et al. Citation2011, Citation2012; Negussie et al. Citation2013). However, this needs further elucidation. Among other likely mechanisms involved in depressed milk fat and protein contents are the negative effects of fat supplementation on fibre digestion in the rumen leading to a reduction in the proportions of acetate to butyrate ratio, the main precursors of milk fat production (Schroeder et al. Citation2004). Second, fat percentage is influenced more by lipolytic processes that tend to change the fat to protein ratio in the milk depending on energy intake and rate of microbial protein synthesis (Negussie et al. Citation2013). Third, a potent inhibitor of milk fat depression has been identified as trans-10 cis-12 CLA, which upon concentration in vivo, causes coordinated reduction of mRNA in the mammary gland responsible for activating primary enzymes for fat synthesis (Baumgard et al. Citation2000, Citation2002). Furthermore, milk fat yield is also largely dependent on, and intrinsically determined by, milk yield because of the well-known negative correlation between milk and fat yields (DePeters & Cant Citation1992; He & Armentano Citation2011). DCCO has shown the potential to depress milk fat content, which at the same time, could be utilized effectively in post-partum grazing cows to improve cow body condition and energy status where DM intake is limited.
Milk fat and protein are the most economically important components of milk because of their contribution to total milk solids upon which dairy farmers are paid, but a negative relationship has been established between milk yield and fat (Chichlowski et al. Citation2005). Supplementation of cows with DCCO in this study marginally decreased milk protein concentration. Previous studies have reported decreased milk protein when fat was supplemented to dairy cows (Jahreis & Richter Citation1994; Delbecchi et al. Citation2001; Larsen et al. Citation2012). The purported mechanism behind milk protein synthesis is associated with glucose deficit (Schroeder et al. Citation2004). Glucose provides the necessary energy rumen microbes need to drive the process of amino acid production necessary for milk protein synthesis (Wu & Huber Citation1994). However, the physiological mechanism of how fat supplementation affects protein synthesis eludes us and warrants further investigation.
Liveweight traits are regularly used in the dairy industry to estimate the energy status of dairy cows during lactation (Malau-Aduli & Abubakar Citation1992; Stockdale Citation2001; Roche et al. Citation2009). In the present study, the similar influence of treatment and week of measurement on liveweight traits suggests that supplementation of grazing dairy cows with DCCO had no negative impacts on BCS and body weight gain. Second, it also suggests the maintenance of a positive energy balance with limited depot fat remobilization from adipose tissues.
5. Conclusion
This study showed that supplementing grazing primiparous Holstein-Friesian dairy cows with DCCO increased milk yield and depressed milk fat, without any negative impacts on BCS and liveweight gain. Depressed milk fat production could be useful in pasture-based systems where energy is limiting, to improve the energy status of cows for milk production. There is the need for further investigation into the underlying mechanisms with regard to circulating plasma metabolites and gene expression of supplemented cows to provide a better understanding of DCCO’s role as a dietary fat supplement for lactating cows.
Acknowledgements
We acknowledge the supervisory role of Peter Nichols, the collaborative research support from Coprice Feeds Pty Limited, Cobden, Victoria and TasHerd Pty Limited, Hadspen, Tasmania for the pelleted feeds and milk composition analysis, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akbaridoust G, Plozza T, Trenerry V, Wales W, Auldist M, Dunshea F, Ajlouni S. 2014. Influence of different systems for feeding supplements to grazing dairy cows on milk fatty acid composition. J Dairy Res. 81:156–163.
- Amirkolaie AK, Leenhouwers JI, Verreth JA, Schrama JW. 2005. Type of dietary fibre (soluble versus insoluble) influences digestion, faeces characteristics and faecal waste production in Nile tilapia (Oreochromis niloticus L.). Aquac Res. 36:1157–1166. 10.1111/j.1365-2109.2005.01330.x
- Bauman DE, Griinari JM. 2001. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livest Prod Sci. 70:15–29. 10.1016/S0301-6226(01)00195-6
- Bauman DE, Griinari JM. 2003. Nutritional regulation of milk fat synthesis. Annu Rev Nutr. 23:203–227. 10.1146/annurev.nutr.23.011702.073408
- Baumgard LH, Corl BA, Dwyer DA, Sæbø A, Bauman DE. 2000. Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am J Physiol-Reg I. 278:179–184.
- Baumgard LH, Matitashvili E, Corl B, Dwyer D, Bauman D. 2002. trans-10, cis-12 Conjugated linoleic acid decreases lipogenic rates and expression of genes involved in milk lipid synthesis in dairy cows. J Dairy Sci. 85:2155–2163. 10.3168/jds.S0022-0302(02)74294-X
- Bayourthe C, Enjalbert F, Moncoulon R. 2000. Effects of different forms of canola oil fatty acids plus canola meal on milk composition and physical properties of butter. J Dairy Sci. 83:690–696. 10.3168/jds.S0022-0302(00)74930-7
- Beever D, Doyle P. 2007 Feed conversion efficiency as a key determinant of dairy herd performance: a Review. Anim Prod Sci. 47:645–657. 10.1071/EA06048
- Bernal-Santos G, O’Donnell A, Vicini J, Hartnell G, Bauman D. 2010. Hot topic: enhancing omega-3 fatty acids in milk fat of dairy cows by using stearidonic acid-enriched soybean oil from genetically modified soybeans. J Dairy Sci. 93:32–37. 10.3168/jds.2009-2711
- Bobe G, Lindberg G, Reutzel L, Hanigan M. 2009. Effects of lipid supplementation on the yield and composition of milk from cows with different ß-lactoglobulin phenotypes. J Dairy Sci. 92:197–203. 10.3168/jds.2008-1252
- Buttchereit N, Stamer E, Junge W, Thaller G. 2011. Short communication: genetic relationships among daily energy balance, feed intake, body condition score, and fat to protein ratio of milk in dairy cows. J Dairy Sci. 94:1586–1591. 10.3168/jds.2010-3396
- Buttchereit N, Stamer E, Junge W, Thaller G. 2012. Genetic parameters for energy balance, fat /protein ratio, body condition score and disease traits in German Holstein cows. J Anim Breed Genet. 129:280–288. 10.1111/j.1439-0388.2011.00976.x
- Caroprese M, Marzano A, Marino R, Gliatta G, Muscio A, Sevi A. 2010. Flaxseed supplementation improves fatty acid profile of cow milk. J Dairy Sci. 93:2580–2588. 10.3168/jds.2008-2003
- Chichlowski MW, Schroeder JW, Park CS, Keller WL, Schimek DE. 2005. Altering the fatty acids in milk fat by including canola seed in dairy cattle diets. J Dairy Sci. 88:3084–3094. 10.3168/jds.S0022-0302(05)72990-8
- Chilliard Y, Ferlay A, Doreau M. 2001. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest Prod Sci. 70:31–48. 10.1016/S0301-6226(01)00196-8
- Dairy Australia. 2011. Situation and outlook. [ cited 2012 Dec 1]. Available from: http://www.dairyaustralia.com.au
- Del Galdo I, Oechel WC, Francesca Cotrufo M. 2006. Effects of past, present and future atmospheric CO2 concentrations on soil organic matter dynamics in a chaparral ecosystem. Soil Biol Biochem. 38:3235–3244. 10.1016/j.soilbio.2006.04.012
- Delbecchi L, Ahnadi C, Kennelly J, Lacasse P. 2001. Milk fatty acid composition and mammary lipid metabolism in Holstein cows fed protected or unprotected canola seeds. J Dairy Sci. 84:1375–1381. 10.3168/jds.S0022-0302(01)70168-3
- DePeters EJ, Cant JP. 1992. Nutritional factors influencing the nitrogen composition of bovine milk: a review. J Dairy Sci. 75:2043–2070. 10.3168/jds.S0022-0302(92)77964-8
- DPI. 2003. The Condition Magician. Body condition scoring in dairy herds. Seasonal/split and year round calving. 2nd. ed. Melbourne: Department of Primary Industry.
- Gagliostro GA, Chilliard Y. 1992. Utilización de lípidos protegidos en la nutrición de vacas lecheras I Efectos sobre la producción y la composición de la leche, y sobre la ingestión de materia seca y energía [Using protected lipid nutrition of dairy cows. Effects on production and milk composition, and on dry matter intake and energy]. Rev Argent Prod Anim. 12:1–15.
- Griinari J, Bauman D. 2006. Milk fat depression: concepts, mechanisms and management applications. Ruminant physiology digestion, metabolism and impact of nutrition on gene expression, immunology and stress.Wageningen: Wageningen Academic Publishers; p. 389–409.
- He M, Armentano L. 2011. Effect of fatty acid profile in vegetable oils and antioxidant supplementation on dairy cattle performance and milk fat depression. J Dairy Sci. 94:2481–2491. 10.3168/jds.2010-3755
- Hutchinson I, de Veth MJ, Stanton C, Dewhurst RJ, Lonergan P, Evans AC, Butler ST. 2011. Effects of lipid-encapsulated conjugated linoleic acid supplementation on milk production, bioenergetic status and indicators of reproductive performance in lactating dairy cows. J Dairy Res. 78:308–317. 10.1017/S0022029911000422
- Hutchinson I, Hennessy A, Dewhurst R, Evans A, Lonergan P, Butler S. 2012. The effect of strategic supplementation with trans-10, cis-12 conjugated linoleic acid on the milk production, estrous cycle characteristics, and reproductive performance of lactating dairy cattle. J Dairy Sci. 95:2442–2451. 10.3168/jds.2011-4632
- Jahreis G, Richter G. 1994. The effect of feeding rapeseed on the fatty‐acid composition of milk lipids and on the concentration of metabolites and hormones in the serum of dairy cows. J Anim Physiol An N. 72:71–79. 10.1111/j.1439-0396.1994.tb00373.x
- Jenkins TC. 1993. Lipid metabolism in the rumen. J Dairy Sci. 76:3851–3863. 10.3168/jds.S0022-0302(93)77727-9
- Jenkins TC, McGuire M. 2006. Major advances in nutrition: impact on milk composition. J Dairy Sci. 89:1302–1310. 10.3168/jds.S0022-0302(06)72198-1
- Kay J, Mackle T, Bauman D, Thomson N, Baumgard L. 2007. Effects of a supplement containing Trans-10, Cis-12 conjugated linoleic acid on bioenergetic and milk production parameters in grazing dairy cows offered ad libitum or restricted pasture. J Dairy Sci. 90:721–730. 10.3168/jds.S0022-0302(07)71556-4
- Khorasani GR, Robinson PH, De Boer G, Kennelly JJ. 1991. Influence of canola fat on yield, fat percentage, fatty acid profile, and nitrogen fractions in Holstein milk. J Dairy Sci. 74:1904–1911. 10.3168/jds.S0022-0302(91)78356-2
- Kroger M. 1985. Milk sample preservation. J Dairy Sci. 68:783–787. 10.3168/jds.S0022-0302(85)80889-4
- Larsen MK, Hymøller L, Brask-Pedersen DB, Weisbjerg MR. 2012. Milk fatty acid composition and production performance of Danish Holstein and Danish Jersey cows fed different amounts of linseed and rapeseed. J Dairy Sci. 95:3569–3578. 10.3168/jds.2011-5163
- Malau-Aduli AEO, Abubakar BY. 1992. Estimation of 305-day yield from total milk yields in Bunaji and Friesian-Bunaji crosses. Nigerian J Anim Prod. 19:141–145.
- Malau-Aduli AEO, Anlade YR. 2002. Comparative study of milk compositions of cattle, sheep and goats in Nigeria. Anim Sci J. 73:541–544. 10.1046/j.1344-3941.2002.00074.x
- Moran J, McLean D. 2001. Heifer rearing: a guide to rearing dairy replacement heifers in Australia. Melbourne, Australia: Bolwarrah Press.
- Negussie E, Stranden I, Mantysaari EA. 2013. Genetic associations of test-day fat: protein ratio with milk yield, fertility, and udder health traits in Nordic Red cattle. J Dairy Sci. 96:1237–1250. 10.3168/jds.2012-5720
- Odens LJ, Burgos R, Innocenti M, VanBaale MJ, Baumgard LH. 2007. Effects of varying doses of supplemental conjugated linoleic acid on production and energetic variables during the transition period. J Dairy Sci. 90:293–305. 10.3168/jds.S0022-0302(07)72630-9
- Peterson DG, Matitashvili EA, Bauman DE. 2003. Diet-induced milk fat depression in dairy cows results in increased trans-10, cis-12 CLA in milk fat and coordinate suppression of mRNA abundance for mammary enzymes involved in milk fat synthesis. J Nutr. 133:3098–3102.
- Rabiee A, Breinhild K, Scott W, Golder HM, Block E, Lean IJ. 2012. Effect of fat additions to diets of dairy cattle on milk production and components: a meta-analysis and meta-regression. J Dairy Sci. 95:3225–3247. 10.3168/jds.2011-4895
- Roche JR, Friggens NC, Kay JK, Fisher MW, Stafford KJ, Berry DP. 2009. Invited review: body condition score and its association with dairy cow productivity, health, and welfare. J Dairy Sci. 92:5769–5801. 10.3168/jds.2009-2431
- SAS. 2009. Statistical analysis system. Version 9.2. Cary (NC): Statistical Analysis System Institute.
- Schroeder G, Gagliostro GA, Bargo F, Delahoy J, Muller L. 2004. Effects of fat supplementation on milk production and composition by dairy cows on pasture: a review. Livest Prod Sci. 86:1–18. 10.1016/S0301-6226(03)00118-0
- Stockdale C. 2001. Body condition at calving and the performance of dairy cows in early lactation under Australian conditions: a review. Anim Prod Sci. 41:823–839.
- Tackett V, Bertrand J, Jenkins T, Pardue F, Grimes L. 1996. Interaction of dietary fat and acid detergent fiber diets of lactating dairy cows. J Dairy Sci. 79:270–275. 10.3168/jds.S0022-0302(96)76360-9
- Weiss WP. 1993. Predicting energy values of feeds. J Dairy Sci. 76:1802–1811. 10.3168/jds.S0022-0302(93)77512-8
- Wu Z, Huber J. 1994. Relationship between dietary fat supplementation and milk protein concentration in lactating cows: a review. Livest Prod Sci. 39:141–155. 10.1016/0301-6226(94)90180-5